Research Article
Obesity Mediates the Relationship between Vitamin D and Accelerated Epigenetic Aging in Females with Knee Pain
1Pain Research and Intervention Center of Excellence (PRICE) at the University of Florida, Gainesville, Florida, USA
2Department of Community Dentistry and Behavioral Science, the University of Florida, Gainesville, Florida, USA
3Department of Biostatistics, the University of Florida, Gainesville, Florida, USA
4Department of Neuroscience, the University of Florida, Gainesville, Florida, USA
5Genetics and Genomics Program, the University of Florida, Gainesville, Florida, USA
6Department of Medicine, the University of Alabama at Birmingham, Birmingham, Alabama, USA
*denotes equal contributions and co-first authorship
*Corresponding author: Yenisel Cruz-Almeida, University of Florida, 1329 SW 16th Street, Suite 5108, Gainesville, FL, 32605, USA, E-mail: cryeni@ufl.edu
Received: March 17, 2022 Accepted: April 11, 2022 Published: April 18, 2022
Citation: Strath LJ, Peterson JA, Meng L, Rani A, Johnson AJ, Huo Z, Foster TC, Edberg JC, Fillingim RB, Cruz-Almeida Y. Obesity Mediates the Relationship between Vitamin D and Accelerated Epigenetic Aging in Females with Knee Pain. Int J Obes Nutr Sci. 2022; 3(1): 34-40. doi: 10.18689/ijons-1000106
Copyright: © 2022 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective: Epigenetic clocks have been associated with a variety of health outcomes and is significantly associated with pain outcomes that can be influenced by varying nutrient statuses, such as vitamin D. It is important to explore the factors that may influence vitamin Ds effects. Here, we sought to examine the mediating effects of obesity on the association between vitamin D and epigenetic aging among adults with and without chronic knee pain.
Methods: Self-reported pain, Waist-to-hip ratio, and blood samples to quantify vitamin D and epigenetic aging were collected.
Results: After controlling for covariates, there was no complete or indirect mediation of the vitamin D association with the epigenetic clock DNAGrimAgevia Waist-to-hip Ratio (WHR) of obesity in the pain group. However, upon adding sex into a moderated-mediation (sex moderating the relationship between vitamin D and WHR), there was a significant indirect effect of vitamin D status on AgeAccelGrim through WHR in females (ab=-0.0286; CI [-0.055, -0.009]), but not males (ab=-0.0067; CI [-0.0451, 0.0216]) in the pain group.
Conclusions: While novel, the data from this study highlight the important role that vitamin D plays within the genomic environment, as well as how the contribution of obesity in a sex-dependent manner may potentially influence this relationship.
Keywords: Vitamin D, Chronic Pain, Epigenetic Aging, Obesity, Fat Distribution Abbreviations: Waist-to-hip ratio (WHR)
Introduction
Variability in aging phenotypes emphasizes that chronological age alone, is a poor predictor of functional decline. Rather, defining functional or physiological age by biological biomarkers (i.e., biological age), provides a better estimate of the trajectory of successful or unsuccessful aging[1]. Epigenetic clocks utilize the methylation status of specific DNA sites, across the genome, to calculate biological age. Recent data show that, relative to chronological age, epigenetic clocks provide better estimates health outcomes, such as physical function [2], obesity [3], diabetes [4], and even chronic pain [5].
We have recently demonstrated that 1,25-dihydroxyvitamin D (vitamin D) levels are related to accelerated biological aging using the epigenetic clock DNAmGrimAge [6]. Vitamin D has been historically associated with both pain and epigenetic outcomes [7]. Through its interactions with the vitamin D receptor (VDR), it is hypothesized that vitamin D has the ability to influence gene expression through the activation of transcription factors, as well as upregulate or downregulate DNA methylation at certain points in the genome, which can affect subsequent regulation of biological processes including cell differentiation and genomic imprinting [8, 9]. While we have recently suggested that one of the ways vitamin D is associated with pain outcomes is through epigenetic aging [6], the factors influencing the mechanism whereby vitamin D regulates aging remains to be elucidated.
To better understand the relationship between vitamin D and epigenetic measures of aging, it is important to examine factors that may influence vitamin D status. As vitamin D is a fat-soluble vitamin, greater amounts of adipose tissue are associated with decreased serum-blood vitamin D levels due to its natural affinity to these types of environments [10]. Thus, when obesity is defined using BMI, clear evidence exists of an association with epigenetic aging [11-16]. However, a key limitation of BMI is that it treats all body tissue as homogenous and does not account for distribution of body weight. Waist-to-hip ratio (WHR) is another measure of obesity that provides an easily calculated proxy measure of fat distribution. Increased WHR reflects greater android fat distribution (i.e., increased fat around the waist) [17] and has been associated with cardiovascular disease [18] and all-cause mortality [19]. Further, there is a clear sex difference in fat distribution that must be accounted for, as males are more likely to deposit fat around their waist, whereas women are more likely to deposit fat around their hips [20]. The sex differences seen in body composition whereby females tend to have a higher fat mass percentage than males, as well as store adipose tissue differently are steadfast [20-23]. It is possible that these differences, in part, mediate the effects of vitamin D on epigenetic mechanisms, which may also impact sex differences in the relationship between epigenetics and pain [24]. Since we have shown a significant relationship between circulating vitamin D levels and accelerated epigenetic aging, this investigations ought to extend this work to examine the effects of obesity as measured by WHR on the association of serum vitamin D levels with epigenetic aging in individuals with and without chronic knee pain. Additionally, given the sex differences in obesity and fat deposition as well as pain prevalence, we considered sex as a potential moderator of these relationships. Specifically, we hypothesized that WHR mediates the association of vitamin D levels and epigenetic aging in a sex-dependent manner.
Methods
Participants
Participants were adults between the ages of 45-85 with and without symptomatic knee osteoarthritis (KOA) recruited from the University of Florida (UF; Gainesville, Florida, USA) and the University of Alabama at Birmingham (UAB; Birmingham, Alabama, USA). Individuals who self-identified as non-Hispanic and “African American/Black” (NHB) or nonHispanic and “White/Caucasian/European” (NHW) and English speaking, were eligible for inclusion. Individuals were excluded if they reported 1) significant surgery to the index (i.e., most painful) knee (e.g., total knee replacement surgery); 2) cardiovascular disease or history of acute myocardial infarction; 3) uncontrolled hypertension (blood pressure > 150/95 mmHg); 4) systemic rheumatic diseases (e.g., rheumatoid arthritis, systemic lupus erythematosus, and fibromyalgia); 5) neuropathy; 6) chronic opioid use; 7) serious psychiatric illness; 8) neurological disease (e.g., Parkinsonʼs, multiple sclerosis, stroke with loss of sensory or motor function, or uncontrolled seizures); 9) pregnant; 10) significantly greater pain in a body site other than the knee. All participants provided written informed consent and the study was IRB approved and conducted with accordance with the Declaration of Helsinki. Participants were recruited as part of a parent study that aimed to examine ethnic and race group differences in physical symptoms, psychosocial functioning, and pain-related central nervous system structure and function in KOA.
Demographic information including age, ethnicity/race, and sex were self-reported during initial phone screening. Eligible individuals were scheduled for a Health Assessment Session (HAS), at which informed consent was obtained prior to study procedures. A health history and pain history, and physical exam were conducted during the HAS. Approximately one week later, participants attended a second session at which blood samples were obtained and quantitative sensory testing (QST) was conducted. The present study is an ancillary investigation that aimed to determine the relationship of obesity, Vitamin D and cellular epigenetic aging, thus, only measures relevant to the study hypotheses are included and presented below.
Graded Chronic Pain Scale (GCPS)
The GCPS is a robust, validated [25] self-reported questionnaire that measures two dimensions of chronic pain severity: pain intensity and pain-related disability. The questionnaire consists of seven items, with six scored on an 11-point Likert scale asking participants to report their current, average and worst pain over the last six months (i.e., 0 = “no pain” to 10 = “pain as bad as it can be”), and how much pain has interfered with daily activities, recreation/social/family activities, and ability to work (i.e., 0 = “no interference” to 10 = “unable to carry out activities”). Scores are then calculated for the two subscales: characteristic pain intensity is calculated as the mean intensity ratings for the current, worst and average pain multiplied by 10; and the pain-related disability score, which is calculated as the mean rating for difficult performing daily, social and work-related activities multiplied by 10, with each score ranging from 0-100. One open-ended question asks participants to report “how many days in the last six months have you been kept from your usual activities because of pain”. Higher scores indicate greater pain and pain-related disability.
Pain group classification
Consistent with the Task Force for the Classification of Chronic Pain consensus for the 11th version of the International Classification of Diseases (ICD-11) of the World Health Organization (WHO) recommendations(26), incorporating both pain disability and its duration [27], individuals were categorized based on how limiting their pain in their daily life using the Graded Chronic Pain Scale (GCPS) [28]. Scores from the GCPS characteristic pain intensity scale and Disability points were then used to categorize participants according to a pain grade: Grade 0 = no reported pain intensity; Grade 1 = low disability (i.e., <3 disability points) and low pain intensity (i.e., <50); Grade 2 = low disability-high intensity pain (i.e., ≥50); Grade 3 = high disability-moderately limiting (i.e., 3-4 Disability Points), regardless of pain intensity; Grade 4 = high disability-severely limiting (i.e., 5-6 Disability Points), regardless of pain intensity [28]. Pain Groups were defined based on pain grade as follows: No chronic pain control group (i.e., Grade 0), present chronic pain (i.e., Grades 1-4).
Blood collection and processing
Blood samples were collected from the forearm or hand vein at the onset of the second session and included collection of a 10ml K2 EDTA tube and a 7ml Corvac Serum Separator tube that were subsequently used for DNA methylation and Vitamin D analyses, respectively.
DNA extraction and methylation analysis
The EDTA tube was centrifuged at 3000rpm for 10 minutes and the buffy coat was carefully extracted and transferred to a cryovial for -80-degree storage. The EDTA tube was centrifuged at 3000rpm for 10 minutes and the buffy coat was carefully extracted and transferred to a cryovial for -80-degree storage. To isolate genomic DNA, the frozen buffy coat samples were processed for DNA isolation using the GentraPuregene method according to the manufacturerʼs instructions (Qiagen). DNA was quantitated by both OD260 (Lunatic, Unchained Labs) and fluorescence based (Qubit, ThermoFisher) methods. Sodium Bisulfite conversion and EPIC methylation array was performed by Moffitt Cancer Center, Molecular Genomics Core located at 3011 Holly Dr., Tampa, FL 33612. Sodium Bisulfite conversion and EPIC methylation array was performed by Moffitt Cancer Center, Molecular Genomics Core located at 3011 Holly Dr., Tampa, FL 33612.
DNA methylation age calculation
The raw data generated by Illumina EPIC array (.idat files) underwent quality control and normalization prior to the calculation of DNAmGrimAge via an online calculator (https://dnamage.genetics.ucla.edu/home). The normalized beta values were obtained using ChAMP (Chip Analysis Methylation Pipeline for Illumina Human Methylation EPIC) protocol [5]. These normalized beta values were subset to sites required for the calculation of DNA Methylation Age and uploaded with a sample annotation file as per the protocol document that accompanies the online calculator. The age-adjusted AgeAccelGrim variable was calculated as the difference of DNAmGrimAge subtracted from chronological age.
Vitamin D assay
Blood was collected into a Corvactube and wrapped in foil to protect from light. After 30 minutes, samples were centrifuged at 1800G for 10 minutes and then transferred to a 0.5mL serum aliquot into an amber cryovial and stored at -80°C until processed for assays. Vitamin D was measured on a TOSOH Bioscience AIA-900 (South San Francisco, CA) using immunofluorescence.
Obesity-related anthropometric data
BMI was calculated by participantsʼ weight and height, which were assessed at the first study visit, based on the formula where BMI equals to kilograms per meters squared. Participantsʼ Waist-to-hip ratio (WHR) was calculated by the ratio of their waist around the navel to their hips at the widest point. The circumferences were measured to the nearest centimetre using a flexible tape with the participant standing. In women, the abdominal circumference (waist) was measured as the narrowest part of the body between chest and hips and in men it was measured at the level of the umbilicus. The participantʼs hip circumference was measured as the maximum circumference around the buttocks posteriorly at the level of greater trochanters. WHR was determined by dividing waist circumference by hip circumference.
Statistical analyses
Prior to running analyses, the data were cleaned so that only those with valid data for the variables of interest were used in the analyses. The age-adjusted AgeAccelGrim variable was calculated as the difference between chronological age and cellular DNAmGrimAge. Independent T-tests were employed to examine demographic characteristics of the Pain and No Pain groups. Next, linear regression-based mediation analyses were performed for each group to whether WHR mediated the Vitamin D-epigenetic age relationship, with Vitamin D as the independent variable (X), WHR as the mediator (M), and AgeAccelGrim dependent variable. Age, race, sex and study site were included as covariates due to their known association with the variables of interest (i.e., Vitamin D levels, epigenetic age, WHR). Finally, a moderated mediation analyses was employed to observe any effects of sex on the model, with Vitamin D as the independent variable (X), sex as the moderator (W; males as reference), WHR as the mediator (M), and AgeAccelGrim dependent variable (Y), with age, race and study site as the covariates.
Results
Participants
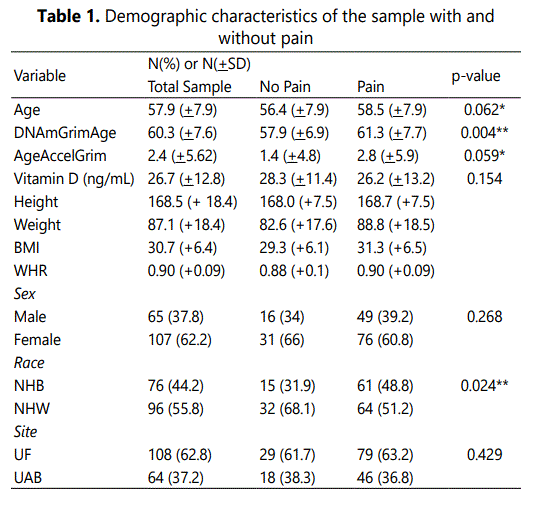
Of the 245 individuals who participated in the parent study, 172 individuals had complete epigenetic, vitamin D, WHR and all covariate data comprising the present study sample. Participant demographics have been previously reported [6], and are summarized below. Participants in the sample were mostly female (62.2%), were almost equal across races (non-Hispanic White; NHW; 55.8%) and had a mean age of 57.9 years (+7.9 years). As a whole, the sample had a mean Vitamin D serum level of 26.7ng/mL (+12.8 ng/mL). The mean AgeAccelGrim was 2.4 years (+5.6 years). There were no significant differences in Vitamin D levels between the sexes (p>0.05). There was a significant difference in Vitamin D based on study site (t(170)=2.464, p=0.02) whereby participants at UF had significantly higher serum levels of Vitamin D (UF = 28.6 ng/mL; UAB = 23.6 ng/mL). There was also a significant difference in Vitamin D based on race (t(170)=-2.66, p=0.009), whereby NHW participants had significantly higher serum levels of Vitamin D (NHW = 28.9 ng/mL; non-Hispanic Black (NHB) = 23.9 ng/mL). Sample demographic characteristics across the no pain vs. pain groups can be seen in Table 1.
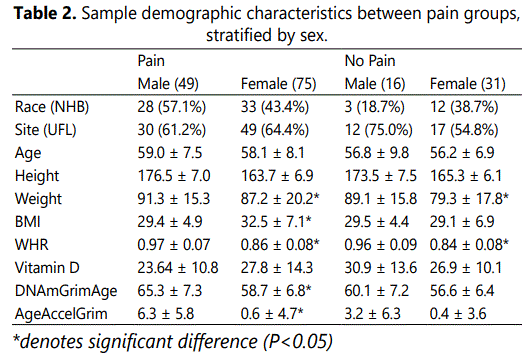
WHR Mediates the Association between Vitamin D Levels and Epigenetic Aging in Females
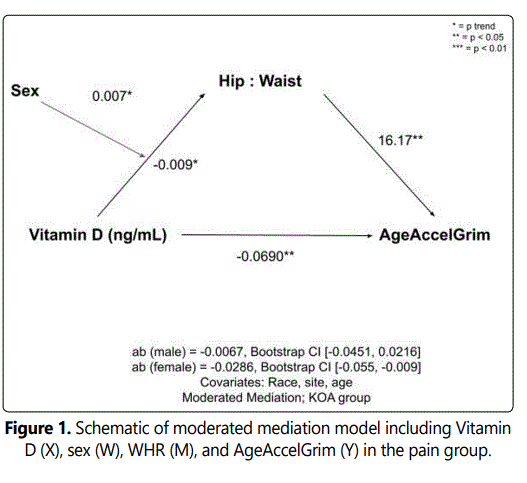
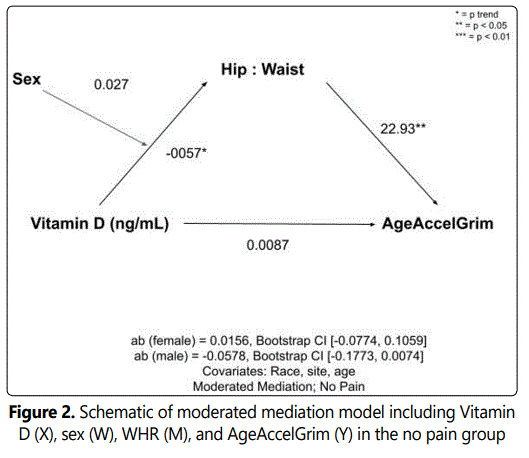
In the unadjusted analysis with AgeAccelGrim as the dependent variable, complete mediations of Vitamin D by WHR were observed. Indirect effects were calculated by multiplying the a and b pathways constituting the effect. After controlling for covariates (race, sex, site, and age) there was no complete or indirect mediation of vitamin D on AgeAccelGrim via WHR in the no pain or pain groups. However, upon adding sex as the moderator (W) in the pain group (whereby sex moderated the relationship between the independent X variable, Vitamin D and the mediating M variable, AgeAccelGrim), there was a significant indirect effect of Vitamin D status on AgeAccelGrim through WHR in females (ab=-0.0286; CI [-0.055, -0.009]), but not males (ab=-0.0067; CI [-0.0451, 0.0216]). After adding sex as the moderator in the no pain control group, the indirect effects of vitamin D through WHR did not reach statistical significance. Schematics of the moderated mediation analyses for the pain and no pain control groups can be seen in Figures 1 and 2, respectively.
Discussion
This study hypothesized that body fat distribution may play a role in the association of Vitamin D with epigenetic aging and subsequent pain. This hypothesis was tested in individuals with and without chronic knee pain. The association between Vitamin D and epigenetic aging in those with chronic pain was mediated in part by WHR, and this relationship was moderated by sex, whereby only females showed an association. These results demonstrate that android body fat distribution is associated with decreases in serum vitamin D and in turn, epigenetic aging, particularly among females with chronic knee pain.
Excess adiposity has been previously associated with increased pain severity and disability, mainly due to the fact that adipose cells release many pro-inflammatory cytokines that have the potential to cause pain [29]. However, excess visceral adipose tissue may also influence pain outcomes by interacting with nutrients that have been associated with the pain experience such as vitamin D. It has been noted in the literature that increased adiposity has the ability to influence the amount of active, circulating vitamin D. Particularly, those with increased android fat distribution are also at a higher risk of vitamin D deficiency [30]. Usually, andrioid fat patterning (i.e., fat around waist) favors males and gynoid fat patterning (i.e., fat around hips) favors females. However, after menopause, females are just as likely to deposit fat around the waist [20]. It is important to note, that on average in adults, females have a higher proportion of overall body fat than males [22]. Because vitamin D is naturally fat-soluble, the micronutrient will have an affinity for high fat environments, leading Vitamin D to concentrate in the visceral adipose tissue rather than the blood [31]. Previous research has shown that in both sexes, serum vitamin D concentrations were inversely related to BMI and fat mass percentage, further indicating a negative relationship between body fat and circulating Vitamin D [32]. However, data has also shown that females with vitamin D deficiency had higher FM % compared to males with vitamin D deficiency, suggesting a potential sex difference [33].
As previously stated, circulating levels of vitamin D have been associated with various pain outcomes. We have previously shown that vitamin D status is associated with pain severity and interference in pain conditions such as knee pain [34] and chronic low back pain [35]. Previous research has shown that vitamin D has the ability to influence gene expression through various epigenetic mechanisms [9], and we have shown that interactions with the epigenome mediates the relationship between vitamin D and pain outcomes [6]. By binding to the vitamin D receptor (VDR) found on the promoter regions of various genes, vitamin D can influence the ability of other transcription factors to bind to and transcribe DNA into mRNA [9]. Specifically, vitamin D and the VDR may be able to influence genes related epigenetic cellular aging [36]. DNA surrogates have recently been grouped together to make epigenetic clocks that are able to determine oneʼs cellular age [37]. The difference between oneʼs chronological age and epigenetic age (AgeAccelGrim) can help determine if an individualʼs cells are aging at an accelerated or slower rate than the time they have spent alive. One of these clocks, DNAGrimAge, has the largest associations with pain outcomes. Previously, we have shown that AgeAccelGrim mediates the effects of Vitamin D on characteristic pain intensity and pain related disability [6]. Additionally, some evidence suggests that vitamin D influences DNA methylation, as those with deficient levels of vitamin D show increased methylation on various genes, many of which are related to immune system function [38]. The relationship between immune system dysfunction and chronic pain has been well established [39]. Differences in the immune system modulation of pain between males and females is thought to be one of the key factors driving sex differences seen in pain responses whereby females often report greater pain severity and prevalence [24]. Interestingly, in the present study, we noted that the mediation between vitamin D, WHR and epigenetic changes in cellular aging was moderated by sex, such that WHR mediated this relationship only among females with chronic knee pain. Thus, it is plausible that sex differences in WHR may influence circulating levels of vitamin D and its subsequent ability to regulate gene expression, potentially contributing to sex differences in pain. Further research is needed to examine these associations.
We acknowledge that there were some limitations to our study, the first being the cross-sectional design. While the current study provides valuable information of the associations of vitamin D and obesity at a given point in time, the study does not effectively assess the relationship over time, nor does it provide any evidence of causality. Given that body composition and vitamin D levels can vary across days, months and years, future studies should aim to incorporate a longitudinal design with controlled manipulations in order to gain insight of effects across the lifespan. Additionally, future studies should aim to include a variety of nutrients that are also known to interact with the epigenome (e.g.vitamins A and E) in order to truly understand to what extent vitamin D alone or in combination is having an impact on gene expression and subsequent pain and disability outcomes [40, 41].
The data from this study highlight the important role that Vitamin D plays within the genomic environment, as well as how other biological factors such as WHR and sex may influence the relationship of vitamin D with epigenetic age. These data also add to the growing body of evidence of the importance of a nutrient-dense diet as well as healthy body fat levels in the potential acceleration of epigenetic aging as well as development of painful conditions. This growing body of literature can inform potential lifestyle interventions to help promote healthy aging, as well as prevent and treat pain. The benefits and outstanding safety profile of nutrition-based interventions provide important advantages over other alternatives, such as opioid medications. In the particular case of vitamin D, it would also be imperative to improve on occupational health protocols to ensure adequate sunlight exposure, as well as possibly explore vitamin D supplementation for those residing in sunlight-restricted geographic regions. Addressing obesity is also recommended in order to improve a variety of health outcomes, including those that may be influenced by vitamin D status. Continuing to understand the mechanisms of specific nutrients and the benefits of high-quality diet patterns, the factors influencing them such as obesity, has the potential to promote a longer and healthier life span.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgements
UPLOAD2 participants and study team; UAB National Center for Advancing Translational Sciences of the National Institutes.
Funding Source(s)
This work was supported by NIH/NIA Grants R01AG059809, R01AG067757 (YCA); UL1TR003096 (JDE); and R37AG033906 (RBF). A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratoryʼs Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and DMR-1644779 and the State of Florida.
References
- Barter JD, Foster TC. Aging in the Brain: New Roles of Epigenetics in Cognitive Decline. The Neuroscientist. 2018; 24(5): 516-25. doi: 10.1177/1073858418780971
- Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, Harris SE, et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. International Journal of Epidemiology. 2015; 44(4): 1388-1396. doi: 10.1093/ije/dyu277
- de Toro-Martín J, Guénard F, Tchernof A, Hould F-S, Lebel S, Julien F, et al. Body mass index is associated with epigenetic age acceleration in the visceral adipose tissue of subjects with severe obesity. Clinical epigenetics. 2019; 11(1): 172. doi: 10.1186/s13148-019-0754-6
- Grant CD, Jafari N, Hou L, Li Y, Stewart JD, Zhang G, et al. A longitudinal study of DNA methylation as a potential mediator of age-related diabetes risk. Geroscience. 2017; 39(5-6): 475-489. doi: 10.1007/s11357-017-0001-z
- Cruz-Almeida Y, Sinha P, Rani A, Huo Z, Fillingim RB, Foster T. Epigenetic aging is associated with clinical and experimental pain in communitydwelling older adults. Mol Pain. 2019; 15:1744806919871819. doi: 10.1177/1744806919871819
- Helde-Frankling M, Bjorkhem-Bergman L. Vitamin D in Pain Management. Int J Mol Sci. 2017; 18(10): 2170. doi: 10.3390/ijms18102170
- Chen L, Dong Y, Bhagatwala J, Raed A, Huang Y, Zhu H. Effects of Vitamin D3 Supplementation on Epigenetic Aging in Overweight and Obese African Americans With Suboptimal Vitamin D Status: A Randomized Clinical Trial. J Gerontol A Biol Sci Med Sci. 2019; 74(1): 91-98. doi: 10.1093/gerona/gly223
- Fetahu IS, Höbaus J, Kállay E. Vitamin D and the epigenome. Frontiers in Physiology. 2014; 5(164). doi: 10.3389/fphys.2014.00164
- Vanlint S. Vitamin D and Obesity. Nutrients. 2013; 5(3): 949-956. doi: 10.3390/nu5030949
- Cameron AJ, Magliano DJ, Dunstan DW, Zimmet PZ, Hesketh K, Peeters A, et al. A bi-directional relationship between obesity and health-related quality of life: evidence from the longitudinal AusDiab study. Int J Obes (Lond). 2012; 36(2): 295-303. doi: 10.1038/ijo.2011.103
- Bigand T, Wilson M, Bindler R, Daratha K. Examining Risk for Persistent Pain among Adults with Overweight Status. Pain Manag Nurs. 2018; 19(5): 549-556. doi: 10.1016/j.pmn.2018.02.066
- Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence Study. J Pain. 2007; 8(5): 430-436. doi: 10.1016/j.jpain.2006.12.003
- Majedi H, Amini MH, Yousefshahi F, Khazaeipour Z, Majedi M, Rahimi M, et al. Predicting Factors of Pain Duration in Patients with Chronic Pain: A Large Population-based Study. Anesth Pain Med. 2020; 10(1): e95776. doi: 10.5812/aapm.95776
- McCarthy LH, Bigal ME, Katz M, Derby C, Lipton RB. Chronic pain and obesity in elderly people: results from the Einstein aging study. J Am Geriatr Soc. 2009; 57(1): 115-119. doi: 10.1111/j.1532-5415.2008.02089.x
- Ray L, Lipton RB, Zimmerman ME, Katz MJ, Derby CA. Mechanisms of association between obesity and chronic pain in the elderly. Pain. 2011; 152(1): 53-59.
- Tichet J, Vol S, Balkau B, Le Clesiau H, DʼHour A. Android fat distribution by age and sex. The waist hip ratio. Diabete Metab. 1993; 19(2): 273-276.
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005; 366(9497): 1640-1649. doi: 10.1016/S0140-6736(05)67663-5
- Bigaard J, Frederiksen K, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, et al. Waist and hip circumferences and all-cause mortality: usefulness of the waist-to-hip ratio?. Int J Obes Relat Metab Disord. 2004; 28(6): 741-747. doi: 10.1038/sj.ijo.0802635
- Goodman-Gruen D, Barrett-Connor E. Sex differences in measures of body fat and body fat distribution in the elderly. American Journal of epidemiology. 1996; 143(9): 898-906.
- Lemieux S, Prudʼhomme D, Bouchard C, Tremblay A, Després J-P. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. The American journal of clinical nutrition. 1993; 58(4): 463-467. doi: 10.1093/ajcn/58.4.463
- Bredella MA. Sex differences in body composition. Adv Exp Med Biol. 2017; 1043: 9-27. doi: 10.1007/978-3-319-70178-3_2
- Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. British Journal of Nutrition. 2008; 99(5): 931-940. doi: 10.1017/S0007114507853347
- Sorge RE, Strath LJ. Sex differences in pain responses. Current Opinion in Physiology. 2018; 6: 75-81. doi: 10.1016/j.cophys.2018.05.006
- Elliott AM, Smith BH, Smith CW, Chambers AW. Changes in chronic pain severity over time: the Chronic Pain Grade as a valid measure. Pain. 2000; 88(3): 303-308.
- Nugraha B, Gutenbrunner C, Barke A, Karst M, Schiller J, Schäfer P, et al. The IASP classification of chronic pain for ICD-11: functioning properties of chronic pain. Pain. 2019; 160(1): 88-94. doi: 10.1097/j.pain.0000000000001433
- Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and Profile of High-Impact Chronic Pain in the United States. The Journal of Pain. 2019; 20(2): 146-60.
- Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992; 50(2): 133-149. doi: 10.1016/0304-3959(92)90154-4
- Andreozzi P, Verrusio W, Viscogliosi G, Summa ML, Gueli N, Cacciafesta M, et al. Relationship between vitamin D and body fat distribution evaluated by DXA in postmenopausal women. Nutrition. 2016; 32(6): 687-692. doi: 10.1016/j.nut.2015.12.029
- Oliai Araghi S, van Dijk SC, Ham AC, Brouwer-Brolsma EM, Enneman AW, Sohl E, et al. BMI and Body Fat Mass Is Inversely Associated with Vitamin D Levels in Older Individuals. J Nutr Health Aging. 2015; 19(10): 980-985. doi: 10.1007/s12603-015-0657-y
- Muscogiuri G, Barrea L, Somma CD, Laudisio D, Salzano C, Pugliese G, et al. Sex differences of vitamin D status across BMI classes: An observational prospective cohort study. Nutrients. 2019; 11(12): 3034.
- Han SS, Kim M, Lee SM, Lee JP, Kim S, Joo KW, et al. Association between body fat and vitamin D status in Korean adults. Asia Pac J Clin Nutr. 2014; 23(1): 65-75. doi: 10.6133/apjcn.2014.23.1.10
- Glover TL, Horgas AL, Fillingim RB, Goodin BR. Vitamin D status and pain sensitization in knee osteoarthritis: a critical review of the literature. Pain Manag. 2015; 5(6): 447-453. doi: 10.2217/pmt.15.43
- Panwar A, Valupadas C, Veeramalla M, Vishwas HN. Prevalence of vitamin D deficiency in chronic and subacute low back pain patients in India: a triple-arm controlled study. Clin Rheumatol. 2018; 37(5): 1367-74. doi: 10.1007/s10067-017-3798-z
- Vetter VM, Spira D, Banszerus VL, Demuth I. Epigenetic Clock and Leukocyte Telomere Length Are Associated with Vitamin D Status but not with Functional Assessments and Frailty in the Berlin Aging Study II. J Gerontol A Biol Sci Med Sci. 2020; 75(11): 2056-63. doi: 10.1093/gerona/glaa101
- Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013; 14(10): R115.
- Koivisto O, Hanel A, Carlberg C. Key Vitamin D Target Genes with Functions in the Immune System. Nutrients. 2020; 12(4). doi: 10.3390/nu12041140
- Totsch SK, Sorge RE. Immune System Involvement in Specific Pain Conditions. Mol Pain. 2017; 13:1744806917724559.
- Remely M, Ferk F, Sterneder S, Setayesh T, Kepcija T, Roth S, et al. Vitamin E Modifies High-Fat Diet-Induced Increase of DNA Strand Breaks, and Changes in Expression and DNA Methylation of Dnmt1 and MLH1 in C57BL/6J Male Mice. Nutrients. 2017; 9(6):607. doi: 10.3390/nu9060607
- Bar-El Dadon S, Reifen R. Vitamin A and the epigenome. Crit Rev Food Sci Nutr. 2017; 57(11): 2404-11. doi: 10.1080/10408398.2015.1060940


