Research Article
Effect of Harvesting Stage on Quality Losses of Persimmon Fruits during Storage
1State Key Laboratory of Crop Genetics and Germplasm Enhancement, College of Horticulture, Life Science Building, Nanjing Agricultural University, P.R. China
2Department of Horticulture, The Agricultural University, Peshawar, Pakistan
3Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan
4Hainan Key Laboratory of Tropical Oil Crops Biology/Coconut Research Institute, Chinese Academy of Tropical Agricultural Science, Wenchang, China
5Department of Crop Sciences, College of Agriculture and Marine Science, Sultan Qaboos University, Sultanate of Oman
6Tea Research Institute, Nanjing Agricultural University, P.R. China
7Department of Agriculture, Abdul Wali Khan University Mardan, Pakistan
*Corresponding author: Amjad Iqbal, Hainan Key Laboratory of Tropical Oil Crops Biology/Coconut Research Institute, Chinese Academy of Tropical Agricultural Science, Wenchang, China, E-mail: amjadiqbal147@gmail.com
Received: March 8, 2017 Accepted: March 26, 2017 Published: April 1, 2017
Citation: Khan N, Ullah I, Khan WA, et al. Effect of Harvesting Stage on Quality Losses of Persimmon Fruits during Storage. Madridge J Food Technol. 2017; 2(1): 78-83. doi: 10.18689/mjft-1000112
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The influence of harvesting stage (yellow-orange, orange and orange-red stage) and storage duration (Control, 10, 20 and 30 days) has significantly affected the quality attributes of the persimmon. The fruits, which were harvested at orange-red stage, had significantly higher weight loss (28.67%), total soluble solids (13.23), electrical conductivity (4.85 S/m) and pH(6.57) among the various treatments. Besides, the persimmon fruits that were harvested at the orange-red stage had a significantly lower fruit firmness, ascorbic acid and titratable acidity. Moreover, the storage duration of 30 days had also affected the various quality parameters of the persimmon fruits (including, weight loss, total soluble solids, electrical conductivity, pH, disease incidence) quite drastically. Certainly, the interaction between harvesting stage and storage duration had a pronounced impact on weight loss, total soluble solids, electrical conductivity and pH of the fruits. In our study, we have found that the fruits, which were harvested at yellow stage and were stored for 20 days performed well among all treatments except control (fresh fruits). It is, therefore, concluded that to minimize the quality losses of persimmon, the fruits should be picked at yellow stage and stored for a maximum of 20 days.
Keywords: Persimmon; Harvesting stage; Storage duration; Electrical conductivity; Ascorbic acid.
Introduction
Persimmon (Diosphyrous kaki) belongs to the family Ebenaceae that originated from China [1,2]. In Pakistan, it is mostly cultivated in Malakand, Chakwal and Hazara regions. The persimmon fruit is a berry contains large seeds resembles to almond shape, although in new cultivar the seeds are absent [3,4]. The nutritional assessment of the fruit revealed that it is a good source of ascorbic acid, minerals, fibers and carotenoids [5]. On the other hand it is quite natural that persimmon fruits with high tannin contents in the flesh have an astringent mouth feel. There are two types of persimmon cultivars, i.e. astringent and non-astringent [6], now a day; consumers can easily find both cultivars in the retail outlets. Traditionally, astringent fruits were either treated with ethylene or left on trees to become over mature in order to become non-astringent [7]. Furthermore, maturity index is used as visual observation of the external color of the fruit [8] that allows the grower to harvest the fruits in time. Harvest maturity is the point at which the fruit has reached a minimum level of development [9]. The fruit at such level can withstand transportation and will ripe slowly in order to produce a fruit of good eating quality. Both qualitative and quantitative losses can occur in fruits between the harvesting stage and consumption by the consumers. Besides, fruits are living entities that continuously respire and can lead to high water losses. Unlike other climacteric fruit, persimmons at the early stages are not edible due to firm texture, astringency, low sugar and water contents [7]. The mature fruit on the other hand contains more sugar and water contents that can lead to fast deterioration. To minimize the postharvest losses and secure the economic value of the persimmon fruit, this study was designed to investigate the effect of different harvesting stages and storage conditions on the quality and shelf life of the persimmon fruit.
Materials and Methods
An experiment on influence of different harvesting stage and storage duration on the physico-chemical analysis of persimmon (Diosphyrous kaki) was managed in the post-harvest laboratory, Department of Horticulture, Agriculture University Peshawar during the year 2015. The fruit of the persimmon was harvested from the research farm at three different stages i.e. yellow-orange, orange and orange-red. The fruits were then stored for various intervals, i.e. control, 10, 20 and 30 days. For each harvesting stage, 120 fruits of uniform size and free from infection were picked for each treatment. After gathering the fruits in separate boxes the fruits were directly transferred to the laboratory and were stored at room temperature (18-22°C) and relative humidity of 65-70%.The experiment was carried out in completely randomized design (CRD) with two factors. The experimental data for various quality parameters was recorded in triplicate at every 10 days of interval during storage.
Weight loss (%): The weight loss was recorded with the help of digital weight balance during fresh (F) and after storage (S) by the following formula:
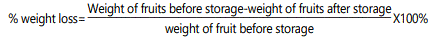
Fruit firmness (kg/cm2): The firmness was recorded with Wagner tester model FT-327 having 8 mm plunger tip.
Total soluble solids (0Brix): A hand refractive meter was used for reading the TSS (Kernco, Instruments Co. Texas).
Electrical conductivity (S/m): 10 fruit per replication were cut into a flat circular plate with the help of 10 mm sterilized cork and were thoroughly washed with distilled water. The fruit pieces were then poured into 100 ml conical flask containing 50 ml of distilled water and autoclaved for 25 minutes at 121°C. After cooling the solution, the volume of the contents in the flask was readjusted to 50 ml with the distilled water and the electrical conductivity (CT) was measured with digital conductivity meter.
Fruit pH: It was determined through using an electronic pH meter (Inolab pH level-I D 82362, Germany)
Titratable acidity: The persimmon titratable acidity was carried out by the method prescribed in AOAC [10].
Ascorbic acid (mg/100 ml): It was determined according to the well-established method of AOAC, [10]. Ten fruit were selected for each replication and ascorbic acid was recorded by using the following formula:
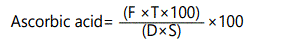
Where
F = factor of standardization = ml of ascorbic acid used/ml of dye used
T = ml of dye used for the sample - ml of dye used for blank
D = ml of sample taken for titration
S = ml of dilute sample taken for titration
Disease incidence (%): the persimmon fruit was visually observed every for infection and was calculated through following formula:
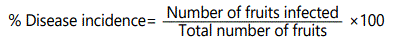
Statistical analysis
The experiment was carried out according to completely randomized design (CRD) with the help of two factors replicating three times. The statistical software SPSS-20 was used to compute the ANOVA at p=0.05.
Results
Weight Loss (%)
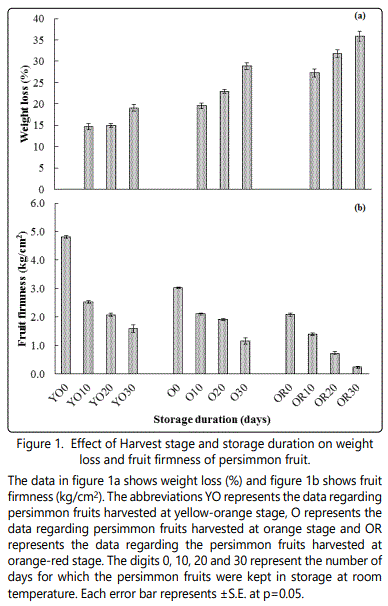
The data in figure-1a regarding weight loss (%) showed a significant difference among the fruits that were harvested at different stage. A maximum loss in weight was observed in fruits that were harvested at orange red stage (28.67%), while the minimum (14.83%) losses were observed in fruits that were harvested, when the colour of fruits were yellow-orange. The effect of different storage duration on weight loss was also significant; with maximum (26%) weight loss was recorded for fruits stored for 30 days compared to the control fruits. The interactive influence of different harvesting stage and storage duration revealed a maximum weight loss (35.82%) in persimmons with orange red colour, followed by the orange (28.83%), while minimum weight loss (19.07 %) was recorded for persimmon having yellow orange colour, stored for 30 days.
Fruit firmness (kg/cm2)
On a general basis, the firmness of the persimmon fruits decreased sharply with time in all tested treatments (Figure 1b). Persimmon fruits at the growth stage with yellow-orange colour had maximum (2.61 kg/cm2) firmness, followed by orange (1.95 kg/cm2), whereas orange red colour persimmon had minimum firmness (1.01 kg/cm2). The influence of different storage duration had significantly affected the fruit firmness. In all tested treatments, fruits that were stored for 10 days were firm as compared to the fruits stored for 20 days and 30 days. From our study we observed the impact of harvesting stage and storage duration on fruit firmness, which was significantly different. The firmness of the fruits were reduced from yellow to orange stage (2.61 to 1.01 kg/cm2), similarly extended storage duration predominantly reduced the firmness of fresh fruits from 3.22 kg/cm2 to 0.80 kg/cm2, when stored for 30 days. The interactive influence of different harvesting stage and storage duration also resulted in significantly higher fruit firmness(1.59 kg/cm2) for yellow orange fruits stored for 30 days, while low firmness (0.24 kg/cm2) was recorded for orange red fruits stored or 30 days.
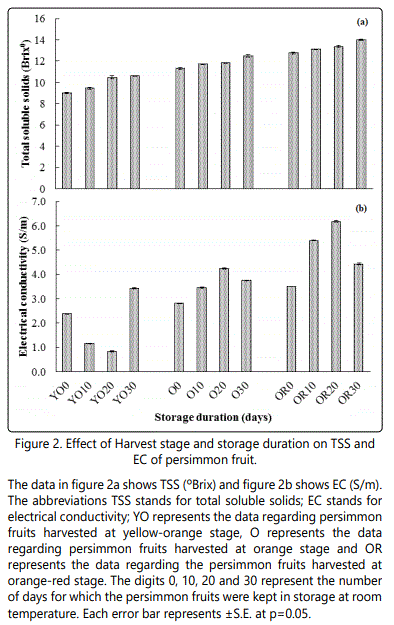
Total soluble solids (0Brix)
The change in total soluble solids (TSS) of different harvesting stage and storage is given in figure 2a. A maximum TSS (13.23) was observed at orange-red fruits, progressively followed by orange fruits (11.70) and yellow-orange fruits (9.78). Similarly, different storage duration had also influenced the TSS of the fruits significantly. The value of TSS was increased from 10.86 for fresh fruits to 12.21 for fruits stored for 30 days. The recorded TSS for fruits stored for 10 and 20 days were 11.36 and 11.84, respectively. The interaction of maturity stage and storage had significantly influenced the TSS contents of the fruits, which were recorded high in fruits picked when orange-red (13.98) and stored for 30 days as compared to the yellow-orange fruits (10.61).
Electrical conductivity (S/m)
The data in figure 2b concerning the electrical conductivity showed a significant difference among the fruits harvested at a different stage and stored for longer time. The fruits that were harvested after having orange red colour had a higher electrical conductivity (4.85 S/m) as compared to the fruits that were harvested at yellow-orange stage (1.91 S/m). Likewise, a significantly higher electrical conductivity (3.81 S/m) was recorded in fruits that were stored for 30 days, followed by 20 days (3.72 S/m) and 10 days (3.30 S/m) of storage. A very low electrical conductivity was recorded for the fresh fruits (2.88 S/m).The interaction effect of harvest stage and storage duration revealed a higher electrical conductivity (4.43 S/m) in fruits that were harvested at orangered stage and stored for 30 days, compared to the fruits that were harvested at yellow-orange stage (3.42 S/m).
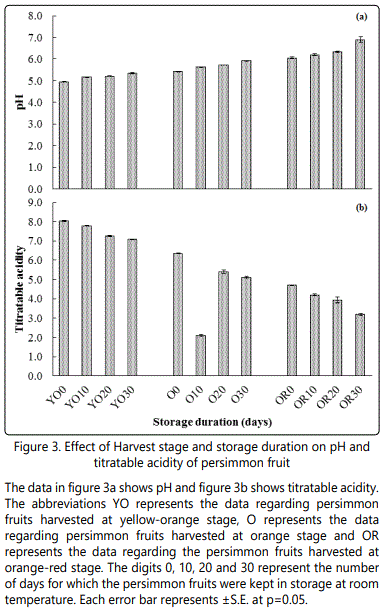
pH of the fruits
The fruits pH from all treatments was significantly increased over the time (Figure-3a). A maximum pH (6.22) were observed, when the fruits were orange red followed by the orange fruits (5.64) and yellow-orange fruits (5.15). Moreover, the pH of the fruits was increased with increasing storage duration. The pH of fresh fruits was 5.45 that were increased to 5.61, 5.71 and 5.91, when stored for 10, 20 and 30 days, respectively. The interaction of harvesting stage and storage duration has also influenced the pH of the fruits. A higher pH (6.89) was recorded for the fruits that were harvested at orange-red stage and stored for 30 days.
Titratable acidity
A maximum titratable acidity (7.51) were observed at yellow orange stage of the fruit (Figure-3b), whereas fruits harvested at orange-red stage had minimum titratable acidity (3.85). The interactive effect of different harvesting stage and storage duration on titratable acidity was significantly different. A maximum titratable acidity was observed at yellow orange stage of fruits, followed by orange fruits, while minimum titratable acidity was recorded for fruits that were harvested at orange red stage and stored for 30 days.
Ascorbic acid (mg/100 ml)
A significant difference in AA was existed among the fruits harvested and stored at different stages (Figure 4a). Significantly higher values of ascorbic acid (6.72 mg/100 ml) were observed in fruits that were picked at yellow orange stage, while lower values of ascorbic acid (3.60 mg/100 ml) were recorded in fruits that were harvested at orange red stage. The effect of different storage duration on ascorbic acid resulted in a decreasing trend in the concentration of ascorbic acid. The fruits that were stored for 30 days have the lowest concentrations of ascorbic acid (4.41 mg/100 ml) as compared to the freshly harvested fruits (5.73 mg/100 ml).The interaction between harvesting stage and storage duration had significantly influenced the concentration of ascorbic acid. A significantly higher concentration of ascorbic acid (5.94 mg/100 ml) was recorded in fruits that were harvested at yellow orange stage and stored for 30 days. While a minimum values for ascorbic acid (3.16 mg/100 ml) was observed in fruits that were harvested at orange-red stage and treated under similar conditions as other treatments during storage.
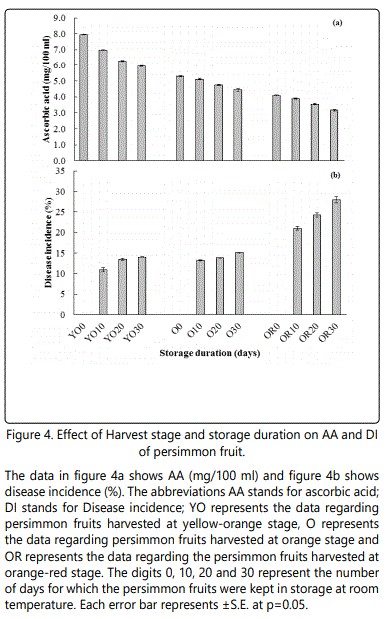
Disease incidence (%)
The effect of different harvesting stage on disease incidence resulted in significant losses due to diseases (Figure 4b). The fruits that were harvested at orange stage of the fruit were prone to diseases as compared to the fruits that were harvested at orange stage. The fruits that were harvested at yellow orange stage were found resistant to diseases. The data regarding storage duration revealed that the fruits losses its resistance to diseases with passage of time during storage. The incidence of disease were higher in fruits that were stored for 30 days, followed by the fruits that were stored for 20 days and days, whereas no incidence of disease were recorded in fresh persimmon fruit. The interaction effect of different harvesting stage and storage duration revealed higher chances of diseases in fruits that were harvested at orangered stage and stored for 30 days. On the other hand, minimum losses have been observed in fruits that were harvested at orange stage and stored for 30 days.
Discussion
It is obvious that biological systems can loss water over time, which is controllable by making changes in either extrinsic or intrinsic condition of the system. In our study, we observe a rapid weight loss in persimmon fruits during the extending storage duration that were harvested at orange-red stage. But, such losses can be greatly reduced by delaying harvesting till achieving a yellow orange colour of the persimmon fruit. Many researchers in the past had also found that a swift loss in weight of a fruit occurs beyond a certain point during storage of perishable fruits [11,12]. Rab et al. [12], reported that moisture losses in sweet oranges were significantly decreased during prolonged storage that mainly contributed towards the weight loss of fruits. Similarly, the firmness of the fruits was also falling off over the storage period. Such decline in firmness may be due to polygalacturonases that are associated with the cell walls of the intact cells [13]. The wall polymerase enzymes upon release from the cells can make specific cuts on the pectic backbone of the cell walls, which leads to the breakdown of pectic polysaccharide in to more simpler and soluble fractions. Similar observation has been reported by many researchers, for example Jan et al. [14], reported that firmness mostly depends on TSS and texture content of the apple fruit.
The increase in TSS contents of the fruits over the storage duration might be due to the continuous respiration of the fruits and loss of water contents. As freshly harvested fruits are living entities, which are able to respire and continue its physiological process, such as conversion of starches to organic acids. As, we know that TSS represents the °Brix of the system, so another possibility of such increase might be the breakdown of the polysaccharides in to the simpler sugar. The results of our work are in parallel to that of Khan et al. [15], and Rab et al. [12] , who found that TSS increases by extending the storage duration. Besides, a significant change in electrical conductivity has been observed in all tested treatments that might be attributed to the leakage of the electrolytes from the cells as a result of broken cell walls due to intrinsic and extrinsic enzymes. The prolonged storage might lead to the softening of the cell walls and membranes that ultimately cause breakage of cell walls, cell membranes, loss of water and leakage of electrolytes [16].
Persimmon fruitʼs pH was significantly increased during the storage of the fruits for 30 days in all tested treatments. The high pH value reflects on the utilization of organic acid into their respective metabolites. The results of our finding are analogous to Jan et al. [14], who reported that during storage pH value increases. On the contrary, titratable acidity decreased as a function of storage time in all tested fruits. The decrease in the acidity is often used as an indicator for maturity as organic acids (i.e. citric acid, malic acid and acetic acid) decrease during the ripening of fruit [17,18]. The results of our findings are in close agreement with the work of Ghafir et al. [11], who reported that during storage the organic acid can be consumed due to respiration and ripening processes thus decrease the acidity. According to Kazemi et al. [19] , fruit titratable acidity is the most important attribute and primarily its concentration specifically depends on organic acid.
Another quality parameter that was significantly affected during storage of persimmon fruits was ascorbic acid. The results of our work confirmed the work of Jung and Watkins, [20], who observed that during postharvest handling and storage the fruits can loss a significant amount of antioxidants including ascorbic acid. Similarly, Jan et al. [14], also reported a decrease in ascorbic acid contents of apple fruits during storage. Moreover, the increased rotting of the fruits due to late harvest can be attributed to the softening of the fruits. Upon the maturity, the cell wall of the fruits become loose that can easily be damaged. To minimize such damages the fruits may be harvested before it reaches to full maturity [21,22].
Conclusion
The influence of different harvesting stages (yellow orange, orange and orange red) and the storage duration significantly affected the quality attributes of the persimmon. It is, therefore, concluded from the present study that the persimmon fruits should be harvested at yellow-orange stage. The harvested fruits if stored at room temperature can then be kept for 20 days at most to restrict significant losses in quality as well as quantity of the persimmon fruits.
Conflicts of Interest
The authors confirm that there is no conflicts of interest regarding this manuscript.
References
- del Mar NM, Zuriaga E, Pecchioli S, Llácer G, Giordani E, Badenes ML. Analysis of genetic diversity among persimmon cultivars using microsatellite markers. Tree Genet Genomes. 2010; 6(5): 677-87. doi: 10.1007/s11295-010-0283-0
- Jing Z, Ruan X, Wang R, Yang Y. Genetic diversity and relationships between and within persimmon (Diospyros L.) wild species and cultivated varieties by SRAP markers. Plant Syst Evol. 2013; 299(8): 1485-92. doi: 10.1007/s00606-013-0810-1
- Leng P, Yamamura H. Fruit set and embryo rescue in crosses using parthenocarpic ‘Mopanshiʼpersimmon. Sci Hortic. 2006; 107(4): 332-36. doi: 10.1016/j.scienta.2005.10.005
- Ogata T, Takeichi T, Matsunaga K, Hasegawa K, Yamane S, Sugiyama K. Seed abortion of ‘Tosa-Buntanʼpummelo pollinated with soft-X-irradiated pollens. Sci Hortic. 2008; 116(2): 180-85. doi: 10.1016/j.scienta.2007.12.006
- Vázquez-Gutiérrez J, Quiles A, Hernando I, Pérez-Munuera I. Changes in the microstructure and location of some bioactive compounds in persimmons treated by high hydrostatic pressure. Postharvest Biol Technol. 2011; 61(2-3): 137-44. doi: 10.1016/j.postharvbio.2011.03.008
- Novillo P, Besada C, Tian L, Bermejo A, Salvador A. Nutritional Composition of Ten Persimmon Cultivars in the” Ready-to-Eat Crisp”Stage. Effect of Deastringency Treatment. Food Nutr Sci. 2015; 6(14): 1296-1306. doi: 10.4236/fns.2015.614135
- Salvador A, Arnal L, Besada C, Larrea V, Quiles A, Pérez-Munuera I. Physiological and structural changes during ripening and deastringency treatment of persimmon fruit cv.‘Rojo Brillante’. Postharvest Biol Technol. 2007; 46(2): 181-88. doi: 10.1016/j.postharvbio.2007.05.003
- Ziosi V, Noferini M, Fiori G, et al. A new index based on vis spectroscopy to characterize the progression of ripening in peach fruit. Postharvest Biol Technol. 2008; 49(3): 319-29. doi: 10.1016/j.postharvbio.2008.01.017
- Zhou R, Li Y, Yan L, Xie J. Effect of edible coatings on enzymes, cellmembrane integrity, and cell-wall constituents in relation to brittleness and firmness of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua) during storage. Food Chem. 2011; 124(2): 569-75. doi: 10.1016/j.foodchem.2010.06.075
- Workneh TS, Osthoff G, Steyn M. Effects of preharvest treatment, disinfections, packaging and storage environment on quality of tomato. J Food Sci Technol. 2012; 49(6): 685-94. doi: 10.1007/s13197-011-0391-3
- Jung SK, Watkins CB. Superficial scald control after delayed treatment of apple fruit with diphenylamine (DPA) and 1-methylcyclopropene (1-MCP). Postharvest Biol Technol. 2008; 50(1): 45-52. doi: 10.1016/j. postharvbio.2008.05.006
- Alia-Tejacal I, Villanueva-Arce R, Pelayo-Zaldívar C, Colinas-León M, López-Martínez V, Bautista-Baños S. Postharvest physiology and technology of sapote mamey fruit (Pouteria sapota (Jacq.) HE Moore & Stearn). Postharvest Biol Technol. 2007; 45(3): 285-97. doi: 10.1016/j.postharvbio.2006.12.024


