Research Article
Stabilization of Bioactive Betalain Pigment from Fruits of Basella rubra L. through Maltodextrin Encapsulation
Department of Plant Cell Biotechnology, Council of Scientific and Industrial Research- Central Food Technological Research Institute, Karnataka, India
*Corresponding author: Parvatam Giridhar, Principal Scientist, Department of Plant Cell Biotechnology, CSIR-Central Food Technological Research Institute (CFTRI), Karnataka, India, E-mail: parvatamg@yahoo.com
Received: November 29, 2016 Accepted: December 6, 2016 Published: March 21, 2017
Citation: Kumar SS, Giridhar P. Stabilization of Bioactive Betalain Pigment from Fruits of Basella rubra L. through Maltodextrin Encapsulation. Madridge J Food Technol. 2017; 2(1): 73-77. doi: 10.18689/mjft-1000111
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The ripened fruits of Basella rubra are rich in betalains that can be used as food grade natural colorants. Betalains were extracted from de-seeded fruits and encapsulated with maltodextrin and spray dried. The total betalains content of the fruit extracts was 348.2 with 296.6 betacyanins and 51.6 mg/100 g of betaxanthins. Further, betacyanins and betaxanthins were confirmed by HPLC. The optimization of B. rubra fruit juice powder was devised using the spray drying parameters of inlet air temperature (IAT) (150°C), maltodextrin (MD) addition rate 50%) and feed flow rate (FFR) (2.5 mL/h). Encapsulated betalains with maltodextrin showed about 10 fold increase in betalains content compared to normal fruit juice. Encapsulated betalain powder from fruits of B. rubra preserved at 4°C for two years and subjected to microbial analysis at six month interval and the quality was found good without any significant microbial count.
Keywords: Betalains; Gomphrenin I; Encapsulation; Stability studies.
Introduction
Betalains are the water soluble natural red and yellow colored indole derived pigments found in the vacuoles of plant cells and are having great potential as a natural dye in food processing industry and in pharma as antioxidant, anti-inflammatory, and detoxifying agents [1]. These pigments are well documented from plants of Caryophyllales and other plant species [2,3].
Recent reports on Malabar spinach (Basella rubra L.) belongs to Basellaceae indicates the potential benefits of pigment rich fruit extracts in food applications [4,5]. Moreover, the pink colored tender twines and leaves of B. rubra are known as a leafy vegetable [6]. Similarly, the phytonutrient composition and stability of betalain extracts using stabilizing agents have been reported [7]. During the last two decades significant contributions have been made towards understanding the betalains biosynthesis, chemistry, stability and physiological aspects, apart from various applications [8]. At pH range of 3-7, extracted form of betalains are fairly stable [9]. However, the stability of the pigment extract in food matrix is a concern while making appropriate formulations as it varies with different physical and physiological aspects, such as water activity, temperatures, exposure to oxygen, and light. In addition, upon application of colors to food, it should impart uniformity of color across batches of a product [10]. In this regard, encapsulation based formulations offer good stability to the pigment and also advantageous in view of their low water activity and easier transport and storage [11,12]. Moreover, encapsulated pigments serve as alternative for the substitution of artificial colorants for natural colorants [13]. Maltodextrin is one of the most suitable matrix for the betalains encapsulation when compared to chitosan and inulin etc. which was reported earlier for application in food industry [14]. In the present communication, we report the maltodextrin encapsulation of betalains pigment extract from the ripened fruits of B. rubra, along with stability studies of powder over a period of time.
Materials and Methods
Materials
Maltodextrin and HPLC grade methanol were procured from Himedia Chemicals (Mumbai, India). Trifluoroacetic acid (TFA) was purchased from Sigma-Aldrich Co (St. Louis, MO, USA). All other chemicals used were of analytical grade. For HPLC analysis, de-gassed and 0.22 mm membrane filtered Milli-Q water was used.
Source of fruits
Basella rubra L. ripened red-violet fruits were collected from 3-month-old twine of greenhouse maintained potted plants (Figure 1) at CFTRI, Mysore during March-April. The Herbarium specimen was deposited at the Herbarium Collection Centre, University of Mysore (Reference No. 02/08/05/13) upon confirmation of its taxonomical features. The fresh fruits were cleaned under running tap water, followed by ethanol disinfection and blotting on hand made filter paper to remove water and then subjected to extractions. For experiment purpose, 1.5-2 Kg fresh fruits were collected from the single plant and from this lot pigment extractions, spray drying and encapsulated betalain powder analyses were performed.
Pigment extraction
Manually de-seeded fruit pulp (1.5-2 Kg) was extracted using distilled water (1 L) with a mortar and pestle until the macerate becomes colourless. Then, the macerate was centrifuged at 12,000 rpm for 10 min. All clear supernatants were pooled and stored at -20°C until use. The pigment extracts have been subjected to fermentation with Saccharomyces cerevisiae to reduce the fermented sugar constituents in the fruit extracts as reported earlier [15]. The clear juice obtained after fermentation was subjected to centrifugation at 12,000 rpm for 20 min and used for further experiments.
Encapsulation of betalains
The above prepared betalains rich extract was mixed with 50% maltodextrin (10 DE) as a carrier agent under vigorous vortexing. Particles were prepared in a Buchi B-290 mini spray dryer (Buchi Labortechnik AG, Flawil, Switzerland). The input air temperature was 150°C and the outlet air temperature was kept at 68°C. The flow rate was maintained at 2.5 mL/min with an atomization air flow 246 L/h, and the drying air flow was 36 m3/h. The particles were separated from the drying air by a cyclone. The powders were stored in a desiccator over silica gel at 4°C until analysis.
Encapsulated powder analysis
Estimation of moisture content
The moisture content in the powder sample was performed according to AOCS method [16].
Total betalains content
The total betalain content was analysed in triplicates with spray dried betalain powder with the above said procedure. Quantification of total betalain content was performed using a UV-visible spectrophotometer (UV 1800, Shimadzu, Kyoto, Japan). Total betalain concentration was determined with betacyanins and betaxanthins expressed in terms of betanin and vulgaxanthin I with molar extinction coefficients of 60,000 L mol-1 cm-1 at 535 nm and 48,000 L mol-1cm-1 at 477 nm, respectively. While calculating total betalains, a correction factor was integrated, resulting from the absorbance of light by impurities present in the sample, on the basis of measurement of extinction at 600 nm. Total pigment content was expressed as the sum of betacyanins and betaxanthins [17].
HPLC analysis
HPLC analysis of betalains was conducted using an HPLC pump equipped with a C18 column (Sunfire, Waters Corporation, Milford, MA, USA) of 250 × 4.6 mm i.d. The UV detector was set at 477 and 535 nm [18].
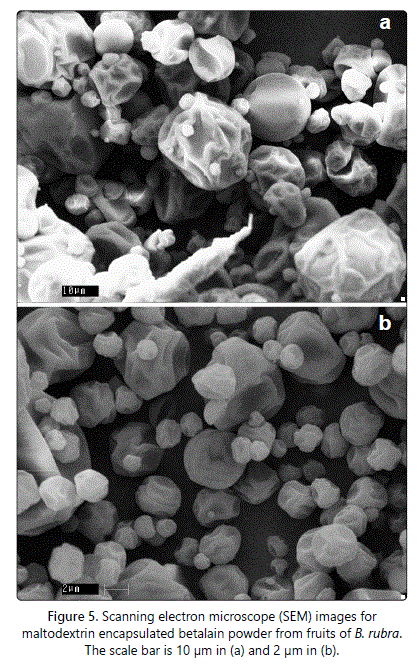
Scanning electron microscopy (SEM)
The betalain powders were analysed for the outer structure of the micro encapsules through SEM. The powders were coated with gold on a double sided adhesive tape mounted on SEM stubs under vacuum using an SEM coating system and examined in a LEO Scanning Electron Microscopy 435 VP (Leo Electron Microscopy Ltd., Cambridge, UK).
Stability of encapsulated betalains assessment
The encapsulated betalain powder was stored at 4°C in subdued light condition. A portion of the sample was withdrawn at six month interval up to two years and analysed for total betalain content analysis in triplicates with the above mentioned procedure.
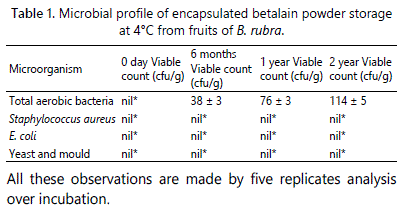
Microbial analysis
During the course of betalain powder storage at 4°C, the microbial assay was also performed to analyse the microbial contamination in the powder. One gram of the sample was dissolved in 100 mL of 0.9% saline. This solution was serially diluted up to 10-4 g/mL. Later, 0.1 mL of the diluted sample was aseptically spread onto prepared media plated containing plate count agar (for aerobic bacterial count), rose Bengal agar (for yeast and mould count) and Violet Red Bile Agar (VRBA) (for E. coli estimation) and Baird Parker Agar (BPA) supplemented with 5 mL egg yolk tellurite emulsion/100 mL (for Staphyllococcus aureus). Triplicate plates were used for each analysis with different dilutions (10-1, 10-2, 10-3 and 10-4). The inoculated plates were incubated at 37°C for the total bacterial count, 28°C for S. aureus and colonies were observed after 24 and 48 h. However, the fungal plates were incubated for 5 days at 31°C and observed for colony growth.
Statistical Analysis
All values presented are mean ± SD of three analyses. The data was subjected to one-way ANOVA followed by post hoc Duncanʼs Multiple Range Test (DMRT) using SPSS 17 (SPSS Inc., Chicago, IL, USA) for determining significant differences. A difference was considered significant when p<0.05.
Results and Discussion
Pigment quantification
The photometric quantification of betalains from fresh de-seeded B. rubra ripened fruits extract results total betalain content (348.2 mg/100 g) with 296.6 betacyanins and 51.6 mg/100 g of betaxanthins. The obtained values were in concomitant with the previous reports [4].
Encapsulation of betalains
Encapsulated Spray drying is a feasible downstream processing technique for betalain production. The spray dried samples of the fermented betalains extract was shown in figure 2. Betalains are highly stable at low water activities. This phenomenon has been proved by a water-glucose system where degradation was reduced by decreasing water activity [19]. In view of this, B. rubra fruit pigments are a novel source of natural colourant used in minimally processed foods, such as desserts, drinks, yoghurts, ice creams, etc [5].
Encapsulated powder analysis
The moisture content of the encapsulated spray dried betalain powder was about 3.2 ± 0.02%. Similar observations with the bioactive compound encapsulation by maltodextrin and inulin from cactus pear fruit was reported [12].
Total betalain estimation
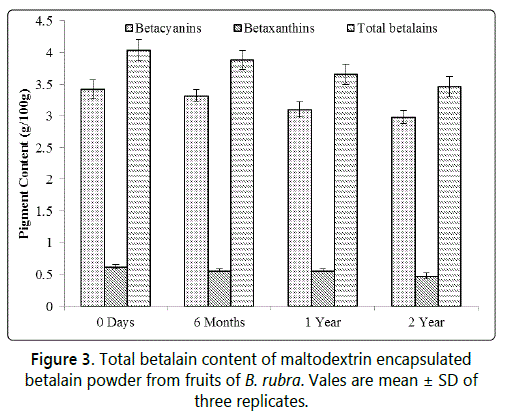
The total betalain retention in the encapsulated betalain powder showed that there was 96.81% pigment retention after 2 years of storage at 4°C (Figure 3). Hence, the spray dried betalain powder can be further explored for its use in food industry as a natural colourant.
HPLC analysis
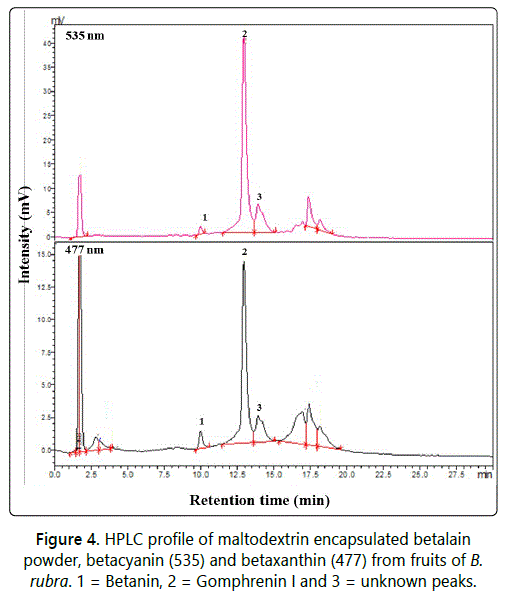
According to earlier reports [18] the major betalain pigment characterized in B. alba fruits were gomphrenin I the concentration of which increases on fruit maturity. The identification and characterization during ontogeny of B. rubra fruit extracts by HPLC and MS was reported recently [4] [5]. Lin et al. [18]. had reported 36 mg/100 g of gomphrenin I pigment from ripened B. alba de-seeded fruits on fresh weight basis. In the present study, the gomphrenin I pigment content quantified by HPLC on the spray dried betalain powder was 0.64 g/100 g (Figure 4). This study shows that spray dried betalain powder showed 25 folds more intense colour (in terms of gomphronein I) than the gomphrenin I pigment content in ripened fruits extract as reported earlier in fresh deseeded fruits [5].
Scanning electron microscopy (SEM)
Figure 5 presents the SEM photograph of encapsulated betalain powder. The morphology of the spray dried powder was regularly spherical in shape. Similar morphology was reported for betalains in Lampranthus productus plant flowers [14].
Microbial analysis
The prepared spray dried powders of betalains were free of all types of aerobic bacteria, coliforms, Staphylococcus aureus, yeast and moulds, etc. after two years of storage at 4°C. The total aerobic bacterial count observed was within the limit as shown in table 1. There was no growth of any other microbes or moulds identified. This may be due to the presence of high dextrose content in the powder. The overall microbial load data showed indicates that the powder is safe for consumption and has a very good shelf life.
All these observations are made by five replicates analysis over incubation.
Conclusion
Fruits of B. rubra were investigated for its encapsulation using maltodextrin to produce spray dried betalain powder. Encapsulated betalains with maltodextrin bettered by 10 folds for betalains compared to normal fruit juice. Even two years storage at 4°C did not hamper the quality of this spray dried powder. These findings provide value addition to B. rubra fruit betalain powder as a potential source of natural colour and as a functional food to the food industry.
Acknowledgement
The authors are thankful to Department of Biotechnology, Government of India, New Delhi for financial assistance (BT/PR1238/FNS/20/524/2011). We greatly acknowledge the Director, CSIR-CFTRI for his kind support.
Conflict of Interest: The authors declare that there is no conflict of interest.
References
- Kanner J, Harel, S, Granit R. Betalains - a new class of dietary cationized antioxidants. J Agric Food Chem. 2001; 49(11): 5178-5185. doi: 10.1021/jf010456f
- Khan Md I, Sri Harsha PSC, Chauhan AS, Vijayendra SVN, Asha MR, Giridhar P. Betalains rich Rivina humilis L. berry extract as natural colorant in product (fruit spread and RTS beverage) development. J Food Sci Technol. 2015; 52: 1808-1813. doi: 10.1007/s13197-013-1175-8
- Khan Md I, Avinash K, Giridhar P. Betalains and expression of antioxidant enzymes during development and abiotic stress in Rivina humilis L. berries. Turkish J Bot. 2016; 40(1): 28-36. doi: 10.3906/bot-1405-32
- Kumar SS, Manoj P, Giridhar P. Nutrition facts and functional attributes of foliage of Basella spp. LWT-Food Sci Technol. 2015a; 64(1): 468-474. doi: 10.1016/j.lwt.2015.05.017
- Kumar SS, Manoj P, Giridhar P, Shrivastava R, Bharadwaj M. Fruit extracts of Basella rubra that are rich in bioactives and betalains exhibit antioxidant activity and cytotoxicity against human cervical carcinoma cells. J Funct Foods. 2015b; 15: 509-515. doi: 10.1016/j.jff.2015.03.052
- Kumar SS, Manoj P, Giridhar P. A method for red-violet pigments extraction from fruits of Malabar spinach (Basella rubra) with enhanced antioxidant potential under fermentation. J Food Sci Technol. 2015c; 52(5): 3037-3043. doi: 10.1007/s13197-014-1335-5
- Khan Md I, Giridhar P. Plant Betalains – Chemistry and Biochemistry. Phytochem. 2015; 117: 267-295. doi: 10.1016/j.phytochem.2015.06.008
- Shishir MRI, Taip FS, Aziz NA, Talib RA, Sarker RS. Optimization of spray drying parameters for pink guava powder using RSM. Food Sci Biotechnol. 2016; 25(2): 461–468. doi: 10.1007/s10068-016-0064-0
- Quek SY. The physicochemical properties of spray-dried watermelon powders. Chem Eng Process. 2007; 46(5): 386–392. doi: 10.1016/j.cep.2006.06.020
- Gandía-Herrero F, Cabanes J, Escribano J, García-Carmona F, Jiménez-Atiénzar M. Encapsulation of the most potent antioxidant betalains in edible matrixes as powders of different colors. J Agric Food Chem. 2013; 61(18):4294-4302. doi: 10.1021/jf400337g
- Kumar SS, Manoj P, Shetty NP, Prakash M, Giridhar P. Characterization of major betalain pigments-Gomphrenin, Betanin and Isobetanin from Basella rubra L. fruit and evaluation of efficacy as a natural colourant in product (ice cream) development. J Food Sci Technol. 2015d; 52:4994-5002. doi: 10.1007/s13197-014-1527-z
- Castellanos-Santiago E, Yahia EM. Identification and Quantification of Betalains from the Fruits of 10 Mexican Prickly Pear Cultivars by High-Performance Liquid Chromatography and Electrospray Ionization Mass Spectrometry. J Agric Food Chem. 2008; 56: 5758–5764. doi: 10.1021/jf800362t
- Lin SM, Lin BH, Hsieh NM, et al. Structural identification of Bioactivities of red-violet pigments present in Basella alba fruits. J Agric Food Chem. 2010; 58:10364-10372.
- Delgado-Varges F, Jiménez AR, Paredes-López O. Natural pigments, carotenoids, anthocyanins, and betalains. Characteristics, biosynthesis processing and stability. Critical Rev Food Sci Nutr. 2000; 40:173-289. doi: 10.1080/10408690091189257


