Research Article
Analysis of the Antioxidative Properties and Maillard Reaction Products in Ginger Cakes Enriched with Rutin
1Division of Food Science, Institute of Animal Reproduction and Food Research, Polish Academy of Sciences, Tuwima 10, Poland
2National Agriculture and Food Centre– The Food Research Institute, Priemyselná 4, Slovakia
*Corresponding author: Henryk Zieliński, Professor, Division of Food Science, Institute of Animal Reproduction and Food Research, Polish Academy of Sciences, Tuwima 10, Poland, Tel: +48 89 5234682, Fax: +48 89 5240124, E-mail: h.zielinski@pan.olsztyn.pl
Received: September 7, 2016 Accepted: September 19, 2016 Published: February 15, 2017
Citation: Starowicz M, Ciesarová Z, Zieliński H. Analysis of the Antioxidative Properties and Maillard Reaction Products in Ginger Cakes Enriched with Rutin. Madridge J Food Technol. 2017; 2(1): 44-51. doi: 10.18689/mjft-1000107
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Study of antioxidative properties and Maillard reaction (MR) products in ginger cakes enriched with rutin was conducted. The cakes were formulated on rye flour substituted by 30% (w/w) of flour from husked buckwheat or flour from roasted buckwheat groats. Enrichment of rye-buckwheat ginger cakes with rutin improved their antioxidative properties, showed protective effect on lysine blockage and stimulated the MR progress to the melanoidin formation, thus supporting their contribution to the antioxidative capacity. In contrast, the loss of nutritional quality of rye-buckwheat ginger cakes enriched with rutin was noted due to carboxymethyllysine and fluorescent compounds linked-to-protein formed at the advanced stage of MR. Acrylamide was formed in control rye and rye-buckwheat ginger cakes in moderate level within the range of 72.2 – 149.2 µg/kg DM.
Keywords: Rye-buckwheat ginger cakes; Rutin; Maillard reaction products; Antioxidative capacity; Acrylamide.
Introduction
The ginger cake products vary across Europe, depending on the regional formulation, quality of ingredients and manufacturing process. Traditional ginger cake composition consists of rye flour, sugar, honey, fat and spices [1]. Rye flour is commonly used for bread baking in Eastern Europe (Secale cereale L.) showing beneficial effects when it substitutes typical wheat flours [2,3]. More recently, buckwheat has been used as an important raw material for functional food development, because of its functionalities and compounds content, such as proteins, flavonoids, phytosterols and other [4]. It was reported that buckwheat contains balanced amino acid composition and it is rich of essential amino acids such as lysine, tryptophan, threonine, leucine and isoleucine [5]. It was already proven that buckwheat is a rich source of flavonoids, which are linked to the functional properties of the buckwheat-based products [6]. The presence of rutin (3ʼ,4ʼ,5,7-tetrahydroxyflavone-3-rutinoside) in buckwheat groats, was recognized as an important flavonoid due to its wide spectrum of pharmacological activities, including anti-inflammatory, anticancer, antiatherogenic, and antioxidant activity [5,7]. Most of these pharmacological properties are based on the antioxidant property of rutin which was measured in numerous studies [1,8].
Nowadays, a wide spectrum of functional products with buckwheat, such as bread, biscuits, snacks, tea or gluten-free noodles were developed due to the expected potential for preventing or delaying a number of age-related diseases [3,9,10]. Buckwheat enriched biscuits and snacks are new buckwheat derived products [9,11]. Wójtowicz et al. [12] showed a good acceptability of buckwheat enriched snacks at a level no higher than 30%, proposing corn-buckwheat snacks as an attractive type of appetizer with increased nutritional properties. Baljeet et al. [11] successfully incorporated buckwheat flour into refined wheat flour up to a level of 20% to yield biscuits of enhanced nutritional quality. In a study carried out by Filipčev et al. [9], the sensory analysis of ginger nut biscuits, popular traditional biscuits containing honey, indicated that addition of 40% buckwheat flour was the best scored, but 50% provided a sensory acceptable product with enhanced bio-functional properties. Therefore, obtaining different partially buckwheatbased bakery products with health-promoting components, the optimization of recipes and technological process parameters, and the characterization of final products in terms of potential functional properties, have acquired a considerable interest in the past few years [13].
The impact of heat treatment on proteins in the final products is positive by increasing the digestibility of proteins, but at the same time it decreases their nutritional value due to the Maillard reaction. The progress of the reaction can be considered in the context of early, advanced and final Maillard reaction products formation such as furosine (ε-N-2-furoylmethyl-L-lysine) [14], fluorescence of intermediatory compounds (FIC) formed at the advanced stage [15], carboxymethyllysine (CML) [16] and melanoidins [17]. The latest are responsible for productʼs colour formation, nevertheless possessing the ability to scavenge free radicals. The degradation of proteins is usually expressed as FAST index [18], based on the measurement of the fluorescence of tryptophan and formation of intermediatory compounds. However, lysine is one of the limiting factor of Maillard reaction products formation, because of its highly reactive two amino groups.
Gathering all latest research areas of functional buckwheat-based food, the present study has been focused on elaboration of rye-buckwheat ginger cakes which are a traditional pastry in East Europe. The novelty is related to the cakes formula based on the application of rye flour substituted by flour from husked buckwheat or flour from roasted buckwheat groats at 30 % level, buckwheat honey [19], spice mix for ginger cakes (Przygodzka et al., 2014) and fortification of the cakes with specified quantity of rutin where 100 g of cakes corresponds to the rutin content up to 50 mg per one tablet per day in popular pharmaceutical drugs offered in drugstores. This idea may differentiate peopleʼs diet with functional cookies enhanced with rutin [20]. Characterization of the changes occurring during processing and effect of rutin supplementation should be valuable for the understanding of quality and safety of rye-buckwheat ginger cakes.
Therefore, evaluation of the total flavonoids, rutin, available lysine, products of early, advanced and final Maillard reaction stage, acrylamide contents and antioxidative capacity of rye-buckwheat ginger cakes enriched with rutin was addressed in this study.
Materials and Methods
Chemicals
2,2ʼ-Azinobis(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), rutin (quercetin-3-rutinoside), lysine (Nα-acetyl-L-lysine), pentadecafluorooctanoic acid were purchased from Sigma (Sigma Chemical Co., St. Louis, MO, USA). PCL ACW (Antioxidant Capacity of Water-soluble substances) kit for PCL assay was purchased from Analytik Jena AG (Jena, Germany). O-phtaldialdehyde for fluorescence (OPA) and sodium dodecylsulfonate (SDS) were supplied by Fluka (Buchs, Switzerland). Furosine (2-furoylmethyl-lysine) and Nα-carboxymethyl-L-lysine (CML) were purchased from PolPeptide (Strasbourg, France). Acrylamide-d3 (98%) was purchased from Cambridge Isotope Laboratories (USA). Acetonitrile and methanol (HPLC purity) were provided by POCh (Gliwice, Poland). Trifluoro acetic acid, β-mercaptoethanol, zinc sulphate and potassium ferrocyanide were provided by Merck (Darmstadt, Germany). Water was purified with Mili-Q-system (Milipore, Bedford, USA).
Ginger cakes preparation formula
The light common buckwheat flour (Eko, Poland) and rye flour type 720 were provided by food store in Olsztyn, Poland. The roasted buckwheat groats (common buckwheat variety Kora, Melvit S.A. Poland) was provided by local company located in the northeast of Poland. Then groats were milled using laboratory grinder obtaining flour. The dry matter (DM) in light buckwheat flour, flour from roasted buckwheat groats and rye flour were 87.6, 89.7 and 86.2% while their corresponding protein contents were 14.3, 10.6 and 8.7%. A commercial spice mix for ginger cakes was obtained from Mäspoma (Zvolen, Slovakia) and buckwheat honey from aspiary “LeśnyDwór” (Szczytno, Poland).
The light buckwheat flour and flour obtained from roasted groats was used to substitute rye flour at level of 30% w/w on total flour basis. The cookies making process involved dough preparation by mixing rye flour with light buckwheat flour or flour from buckwheat groats (30:70, w/w), addition of buckwheat honey, spice mix for ginger cakes and other typical bakery ingredients. According to the producer‘s declaration, spice mix contained cinnamon, pepper, clove, anise, coriander, fennel and nutmeg. The recipe of rye-buckwheat ginger cakes was modified by adding low and high amounts of rutin to the mixture of flours. The addition of rutin to rye-buckwheat ginger cake formula corresponded to the rutin content in one tablet of pharmaceutical drug available in drugstore (25 mg of rutin per tablet). The dough was cut into 0.5-cm thick discs of 5.5 cm diameter and baked at 180°C for 18 min in a DC-32E electric oven (Sveba-Dahlen, Fristad, Sweden). Finally, the cakes were freeze-dried and grounded into powder after cooling. The powdered samples were sieved through a 60-mesh screen and then stored at –20°C until analyzed. The formulation of rye-buckwheat ginger cakes enriched with rutin is shown in table 1.
Preparation of ginger cakes extracts
100 mg of powdered ginger cakes samples were extracted with 1 mL of 80% (v/v) methanol. Next, the mixture was sonificated, vortexed each for 30 s, again sonicated and vortexed and centrifuged for 5 min (5000 x g at 4°C). That step was repeated 5 times and the supernatants were collected into 5mL flask. Final extracts concentration was 20 mg/mL. Extraction of each sample was performed in triplicate. Prepared extracts were used to determine total flavonoids (TF), rutin contents and antioxidative capacity (AC) against ABTS•+ and O2-• radicals.
Determination of total flavonoids (TF), rutin contents and antioxidant capacity (AC)
TF was determined according to Jia et al. [21]. The method described by Re et al. [22] was used to determine the antioxidant capacity of 80% methanol extracts of rye-buckwheat ginger cakes as determined against ABTS•+ radicals. The Trolox solutions within the range of 0.1-2.5 mM was used for calibration curve construction (y = 36.03x + 1.61; R2=0.99). The measurements were carried out using spectrophotometer UV-160 1PC (Shimadzu, Japan). Photo chemiluminescence (PCL ACW) assay was used to measure the antioxidant activity of cakes extracts against superoxide anion radicals (O2-•) generated from luminol, a photo sensitizer when exposed to UV light [23]. The analysis was conducted by Photochem® apparatus provided by Analytical Jena (Germany). The Trolox was used as a standard according to the ACW protocol for calibration curve construction (y = -1.59x2 + 56.13x + 5.22; R2 =0.99). The details of each assay have been recently reported by Przygodzka et al. [24]. The content of rutin in ginger cakes was determined with HPLC (LC-10, Shimadzu, Japan) with a UV detector (SPD-10A, Shimadzu, Japan) set up 330 nm.
Determination of available lysine content (OPA assay)
The available lysine content was measured according to Ramírez-Jiménez et al. [25] with application to the micro plate reader (Infinite® M1000 PRO, Tecan, Switzerland). The OPA reagent was prepared by mixing 1.25 mL OPA solution (18.9 mg OPA + 2.88 mL 95% methanol) with 2.50 (w/v) SDS solution (20%), 12.50 mL of 0.1 M borate buffer (pH 9.5) and 200 µL of 10% (v/v) β-mercaptoethanol and filled up to 50 mL with distilled water. Next, 50 µL of sample, 100 µL of OPA reagent and 100 µL of water were added to well and incubated for 3 min (96-well micro plate, Porvair Sciences). After that, the fluorescence was reading at extinction wavelength 340 nm and emission wavelength 455 nm. For quantitative analysis a calibration curve of Nα-acetyl-L-lysine ranged from 10 to 250 µM was constructed. Results are reported as a mean of three independent extraction.
Furosine assay
Furosine content was determined as described by Delgado-Andrade et al. [26]. An accurately weighted 30 mg of powdered ginger cake sample was hydrolysed with 4mL of 4.9 M HCl at 110°C for 23 h in a Pyrex screw-capped vial with PTFE-faced septa. Hydrolysis tubes were sealed under nitrogen. The hydrolysate was centrifuged for 10 min. A 0.5 mL portion of the supernatant was applied to a Sep-pak C18 cartrigde (Millipore) conditioned with 5 mL of methanol and 10 mL of distilled water, then eluted with 3 mL of 3M HCl and evaporated under vacuum. The dried sample was dissolved in 1 mL of a mixture of water, acetonitrile and formic acid (95:5:0.2) before HPLC analysis. The furosine was quantified by HPLC system (LC-20, Shimadzu, Japan) comprised of a controller (SCL-10AVP, Shimadzu, Japan), a PDA detector (SPD-M10AVP, Shimadzu, Japan). A Cadenza CD-C18 column (250 x 2 mm, 3 µm, Imtakt, Japan) at 35°C was used. The mobile phase consisted of a solution of 5 mM sodium heptanases sulfonate containing 20% of acetonitrile and 0.2% of formic acid. The elution was isocratic and the flow rate was 0.2 mL/min. The UV detector was set at 280 nm. The linear response of external standard of furosine solution within the concentration of 0.2-9.0 µg/mL was used for standard curve construction (y = 0.49x – 0.39; R2 = 0.99) and it was applied to quantify furosine in the samples.
Carboxymethylolysine (CML)
The extraction of CML from the samples was carried out according to the procedure described by Peng et al. [27]. In this procedure the 0.5 g of powdered ginger cake sample was defatted with 3mL of chloroform/methanol (2:1, v/v) in triplicate, then the solvent was removed and the samples were dried at 50°C in the oven. To 50-100 mg of defatted sample 1mL of sodium borate buffer (0.2 M, pH 9.4) and 0.5 mL sodium tetrahydridoborate were added. Then the samples were incubated for 4 h at 25°C. In the next step, 0.75 mL of hydrochloric acid (40%) was added and under nitrogen, and hydrolyzed at 110°C for 20 h. The extracts were concentrated and were kept in refrigerator until the analysis. The OPA reagent was prepared by dissolving 10 mg OPA in 2 mL of methanol, then to 1mL of OPA solution 8 uL of β-mercaptoethanol and 0.2 M sodium borate buffer (pH 9.9) were added and the reagent was ready to use. Before the analysis the samples were dissolved in 0.1 mL water with 0.05% orthophosphoric acid. Then to 50 µL of extract 50 µL of OPA solution was added and the mixture was incubated for 5-20 min and injected on to the column. The determination was conducted by HPLC (LPG-3400M WPS-3000TSL, Dionex, USA) with fluorescence detector (SFLD-3400RS, Dionex, USA) set up the emission wavelength 340 and excitation 455 nm. Chromatographic determinations were performed at 35°C with flow rate of 0.2 mL/min on Luna C18 column (2 x 150 mm, 3 µm) (Phenomenex, USA). CML was eluted using a gradient composition of water with 0.05% of orthophosphoric acid (solvent A) and acetonitrile with 0.05% of orthophosphoric acid (solvent B). The gradient elution programme was as follows: 5-5-70-5-5% B at gradient time tG = 0-9-30-35-40 min. For quantitative analysis, calibration standard was prepared in triplicate within the range of 2.5-20 µM. The results were expressed in µg per g of dry matter.
Measurement of MRP fluorescence and calculation of the FAST index
The fluorescence of free and total intermediary compounds (FIC) was determined after sample extraction and further enzymatic hydrolysis using pronase E according to Delgado-Andrade et al. [15]. Readings were recorded in a luminescent spectro fluorimeter (LS 50B, Perkin Elmer, USA) setting at λext.=347 nm and λem.=415 nm. Tryptophan fluorescence was measured at λext.=290 nm and λem.=340 nm. Results are expressed in fluorescence intensity (FI) per mg of sample DM. The FAST index was calculated as recently reported by Zieliński et al. [28] with a one novelty modification based on the use of fluorescent compounds linked-to-proteins for index calculation. The samples were analysed in triplicate and FAST index data were expressed as a percentage (%).
Brown pigments assay
Formation of brown pigments was estimated as reported in details by Zieliński et al. [28]. Readings were recorded in a spectrometer (UV-1601PC, Shimadzu, Japan) setting at 420 nm. All measurements were performed in triplicate. Results were expressed as arbitrary absorbance units.
Determination of acrylamide content
The LC/ESI-MS-MS analyses of acrylamide content were performed by an HPLC system 1200 series (Agilent Technologies, USA) coupled to an Agilent 6410 Triple Quad detector equipped with ESI interface. The method was described in details by Markova et al. [29].
Statistical analysis
The results of the chemical analyses are given as the means and the standard deviation of three independent measurements. Statistical one-way analysis of variance (ANOVA) using Fischer test was performed. The significance level was set at p<0.05. The correlation test between rutin content, MRPs formation and antioxidant capacity was performed and the Pearson correlation coefficients were calculated. Statistical analyses were performed using software package (StatSoft Inc., v. 7.1, Tulsa, OK, USA).
Results and Discussion
Total flavonoids and rutin contens in rye-buckwheat ginger cakes
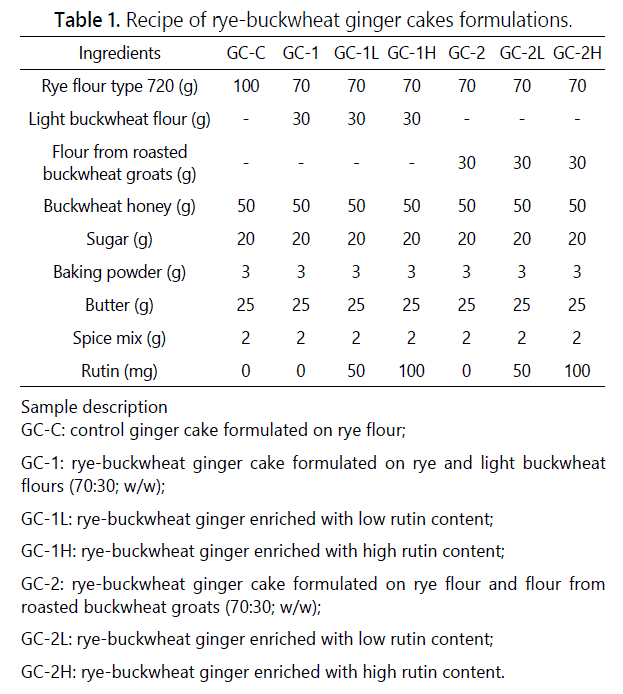
Table 2 shows total flavonoids and rutin contents in rye-buckwheat ginger cakes. In the rye-buckwheat ginger cakes GC-1 and GC-2, the replacement of rye flour by light buckwheat flour or flour obtained after whole milling of roasted buckwheat groats at level of 30% w/w, led to obtain significantly higher TF by 49 and 42% as compared to control rye ginger cake GC-C (p<0.05). The source of TF in GC-C was rye flour, buckwheat honey [19,30], and spice mix [24] while buckwheat flours at substitution level of 30% were additional source of flavonoids [1]. Then, adding high dose of rutin increased TF about 24% in GC-1H and 6% in GC-2H in comparison to GC-1 and GC-2. GC-1 and GC-2 ginger cakes showed also statistically significant higher content of rutin by 81 and 22% as compared to GC-C control cake. The lower content of TF and rutin in GC-2 cakes in comparison to that noted in GC-1 was due to the thermal degradation of these compounds as it was previously described by Zieliński et al. (2009). The supplementation of rye-buckwheat ginger cake formula by low and high amounts of rutin (Table 2) resulted in progressive increase of TF and rutin contents in cakes (GC-1L, GC-2L and GC-1H and GC-2H). A positive correlation between TF and rutin contents was noted in rye-buckwheat ginger cakes (r=0.73).
The rutin content in 100 g of ginger cakes enriched with low dose of rutin (GC-1L and GC-2L) was 34.6 and 38.2 mg whereas those enriched with high dose (GC-2H and GC-1H) showed 47.6 and 72.3 mg, respectively. It means that consumption of 250 g of GC-2H and GC-1H will provide to the organism enough amount of rutin (120-180 mg) to observe the health effect. It has been already reported that the dose of rutin which demonstrated therapeutically effects is within the range of 180 - 350 mg [7]. In contrast to the doses applied in theraphy, the rutin content ranged between 35 – 72 mg/100 g of cakes reached in our study may be used in the prophylactic treatment as it corresponds to 1-2 tablets of typical pharmacological drugs with rutin as the bioactive component.
Antioxidant properties
The substitution of rye flour by the light buckwheat flour and flour obtained from roasted groats at the level of 30% in the formulation of ginger cakes resulted in significant differences (p<0.05) in the antioxidant capacity (AC) determined against 2,2ʼ-azinobis-(3-ethylbenzothiazoline-6-sulphonate) radical cation (ABTS•+) and superoxide anion radical (O2-•) as presented in table 2. The GC-1 and GC-2 cakes showed significantly higher AC by 75 and 31% than control rye ginger cake GC-C (p<0.05). This finding indicates a clear relationship between considerably higher polyphenols in two types of buckwheat flours in comparison with rye flour type 720 [9,11,28,31]. The supplementation of rye-buckwheat ginger cake formula by low and high amounts of rutin showed no effect on AC. No statistical differences were noted for GC-1H and GC-2H as compared to GC-1 and GC-2 cakes (p<0.05). Therefore, no clear correlation was observed between ABTS data and TF and rutin contents (r = 0.52). This finding may be due to the reasonable low doses of rutin applied in ginger cakes formulation (0.25 mg and 0.5 mg per g of formulation) which consisted only up to 8 and 15% of TF content (Table 2). However, a better picture was found when AC was determined against O2-•. The about twice and almost three-fold increase of AC in GC-1 and GC-2 in comparison to control cake was noted. Moreover almost three-fold increase of AC in GC-1H and GC-2H as compared to GC-C was noted. This finding may be explained by higher antioxidant activity of rutin against O2-• determined with PCL ACW method (1.38 mmol of Trolox) than activity evaluated against ABTS•+ (1.16 mmol of Trolox) as it was described by Zielińska et al. (2010). Our findings confirmed the ability of rutin to scavenge superoxide radicals as reported by Yang et al. [8], thus supporting positive correlation between TF content and PCL ACW results (r=0.95).
It can be concluded that enrichment of rye-buckwheat ginger cakes with rutin offered final product with beneficial high antioxidant potential as well as flavonoids content which was confirmed in this study. Several authors reported an increase of antioxidant activity in cooked cereal foods and investigating a link between antioxidant activity and the formation of advanced Maillard products. For example Moore et al. [32] observed a raise of antioxidant activity in whole-wheat pizza crust after baking, and the effect was stronger with longer cooking time or higher temperature. Similarly Capuano et al. [33] showed the relationship of antioxidant activity in bread crisps as a function of toasting time. Likewise Gallegos-Infante et al. [34], was studying the effect of traditional Mexican processing of barley, reported higher antioxidant activity of the roasted or cooked germinated kernels than AC of the control. However, baking often lead to a loss of nutritional properties due to the Maillard reaction development [34]. Therefore the dose-depend effect of rutin on the available lysine content and formation of early, advanced and final MRPs in rye-buckwheat ginger cakes was further investigated.
Available lysine content (OPA assay)
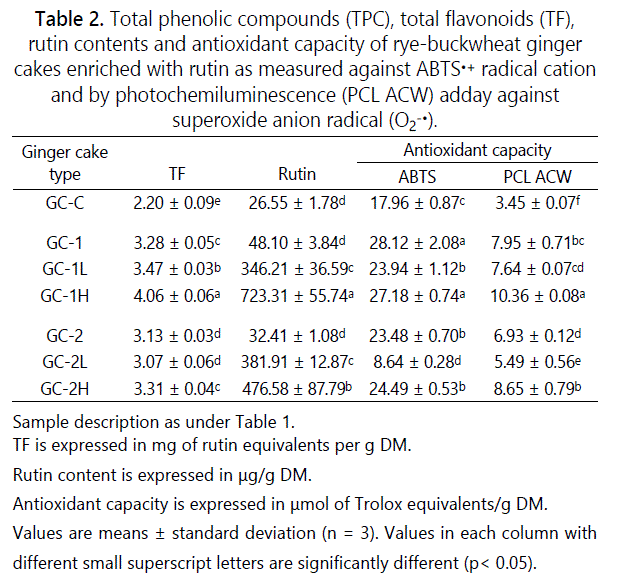
Results of OPA assay are shown in table 3. Available lysine values of 265, 516 and 453 mg/g DM were found in control rye ginger cake GC-C, and rye-buckwheat ginger cakes GC-1 and GC-2, respectively. Results suggested that substitution of rye flour by light buckwheat flour or flour obtained after whole milling of roasted buckwheat groats at level of 30% w/w in cake formula had a beneficial effect since available lysine content was almost twice higher as compared to GC-C control cake. This finding was not only with agreement to the higher protein contents in buckwheat flours but also to their balanced amino acid composition being a good source of essential amino acids such as lysine, tryptophan, threonine, leucine and isoleucine [5]. According to the OPA values found in rye-buckwheat ginger cake formula enriched with low and high amounts of rutin (0.25 mg and 0.5 mg per g of formulation) a protective effect on lysine blockage was found. The significantly higher available lysine content by 12 and 15% was noted in GC-1L and GC-1H as compared to GC-1 cake and by 6 and 19% in GC-2L and GC-2H as compared to GC-2 cake, respectively. The observed protective effect on lysine blockage in cakes was confirmed by positive correlation between OPA values and TF and rutin contents (r=0.95 and r=0.68, respectively). Results suggest that the protective effect of polyphenols may be extended on Maillard reaction development. In order to confirm this hypothesis early, advanced and end MRPs were determined.
Maillard reaction products
Table 3 shows early, advanced and final Maillard reaction products contents in rye-buckwheat ginger cakes enriched with rutin.
Furosine is a marker of the early stage of the Maillard reaction [34]. In this study furosine formation was observed in all types of cakes. Twice lower formation of furosine was noted in GC-1 and GC-2 rye-buckwheat ginger cakes. The protective effect of polyphenols on furosine formation was found in rye-buckwheat ginger cakes enriched with rutin and formulated on rye flour and flour from milled groats. It was confirmed by the high negative correlation between furosine and TF and rutin contents (r = -0.94 and r = - 0.84). In contrast, such effect was not found in cakes enriched with rutin when formulated on rye and light buckwheat flour. The content of furosine noted in this study was within the range previously reported for rye and wheat-rye ginger cakes [4]. The formation of furosine during ginger cakes preparation may be associated with the presence of buckwheat honey included in the recipe since its concentration depends on the total reducing sugar content and parameters of the process. However, these factors were excluded since all ginger cakes were prepared under the same baking conditions and the recipe included the same amounts of sugar and honey. For these reason, the only protective effect of polyphenols was discussed. It should be pointed out that furosine data negatively correlated with available lysine content (r= -0, 65) thus suggesting free amino groups degradation by Maillard reaction even when protective effect of polyphenols was observed. This relationship was previously described by Michalska et al. [35] in relation to CML formation in rye bread.
Table 3 also shows carboxymethyllysine (CML) content formed at the advanced Maillard reaction stage in ryebuckwheat ginger cakes enriched with rutin. CML, which has been described as unhealthy product, is a non-flourescent compound formed by the reaction between glyoxal and the epsilon-amino group of lysine. CML can be used as markers of MR, oxidative stress, and thermal damage in foods [36]. In this study, CML formation was detected in all ginger cake samples. The lowest CML content was found in control rye ginger cake (12.7 µg/g DM) however higher concentrations by 23% and 41% amounts were detected in GC-1 and GC-2 samples. Enrichment with rutin resulted in 24% lower formation of CML in GC-2H sample in comparison to GC-1 sample whereas no effect was found in GC-2L and GC-2H samples. CML content was negatively correlated with furosine data (r= -0.72) suggesting loss of nutritional quality due to Maillard reaction progress at the advanced stage.
In this study, a special focus was put on the use of fluorescent compounds linked-to-protein, which are formed also on the advanced stage of MR, for the FAST index calculation. This parameter has been usually calculated as a ratio between total fluorescence of intermediary compounds (FIC)/tryptophan fluorescence and it was related to protein nutritional loss due to the formation of new molecular structures formed between reducing sugars and amino residues of proteins [18]. However, the total pool of fluorescent Maillard compounds is formed by those that are free in the media and those linked-to-proteins fractions. Then the fluorescent compounds linked-to-protein fraction can be calculated by subtraction free from total FIC and then taken for FAST index calculation. The calculated FAST index is shown in table 3 while total, free and linked-to-proteins FIC are presented in figure 1. The total, free and linked-to-proteins FIC found in all cakes were within the range of 132.8-208.2, 59.3-85.2 and 75.3-143.9 FI/mg sample DM, respectively. Generally, a slightly higher FIC was found in rye-buckwheat ginger cakes when compared to control rye ginger cake whereas no effect was found in rye-buckwheat cakes enriched rutin. The effect of rutin enrichment was only noted when the ratio of total to free FIC in these cakes was compared to that noted for rye ginger cakes. In this case, the average ratio noted in enriched GC-1 (2.8) and GC-2 cakes (2.6) was higher than in GC-C cake (2.2). The calculated ratio was at least five-fold lower as compared to those noted in corn-based, wheat-based and rice-based breakfast cereals [15]. Moreover, a correlation was found between the total and linked-to-protein FIC values (r= 0.93) in our study. This finding suggest that both total FIC and linked-to-protein FIC are representative to evaluate the presence of fluorescent Maillard compounds in rye-buckwheat ginger cakes but the latest may offer a direct information on the loss of nutritional quality of cakes as FAST index.
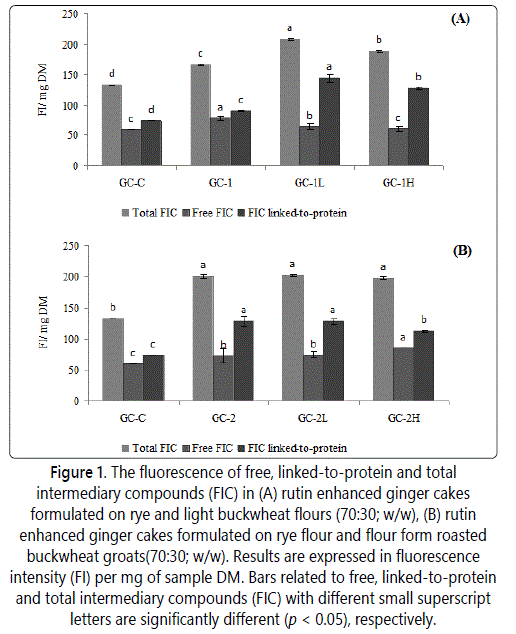
Table 3 shows the FAST index values. The values ranged from 461% to 1309% and they were at least twice lower as compared to those described for rye and rye-wheat ginger cakes [30]. The GC-1 sample showed FAST index value on the same level as it was noted in control GC-C cake while that found in GC-2 cake was above twice higher. It was clear since flour from roasted buckwheat groats used as ingredient in GC-2 formula showed significantly higher FAST values with comparison to raw buckwheat groats as it was reported by Zieliński et al. [31]. Therefore, the enrichment with rutin GC-2 cakes showed no effect on FAST values. In contrast, an increase in FAST values was observed in enriched GC-1 cakes due to use of non-thermally light buckwheat flour in the formula and then the better conditions for Maillard reaction development.
Melanoidin formations estimated by measuring browning values, which express formation of brown pigments, are shown in table 3. As it can be observed, brown polymers MRP were formed in control rye and experimental rye-buckwheat ginger cakes enriched with rutin. After substitution of rye flour by buckwheat flours in the formulas an increase by 88% and 5% in browning was noted in GC-1 and GC-2 type cakes, respectively. An additional increase in browning up to 21% (GC-1H) and 48% (GC-2H) was observed in cakes enriched with rutin as compared to GC-1 and GC-2 cakes, respectively. The browning values noted in our study were slightly higher than those previously found in rye ginger cakes formulated on dark and brown flours but they were twice as those in ryewheat ginger cakes [1].
Melanoidin formation, expressed in this study as arbitrary absorbance units, was positively correlated with antioxidant capacity measured by ABTS test (r= 0.61) and PCL ACW assay (r= 0.84) and with TF and rutin contents in the cakes (r= 0.85 and r=0.64). Manzocco et al. [37] reviewed the evidence available on the positive correlation between browning development and antioxidant properties and concluded that “colour can be considered an index of overall antioxidant properties of foods” if the pathways of antioxidant and colour formation are well known and similar. Therefore, it may suggest that TF and rutin stimulate the Maillard reaction progress to the melanoidin formation, thus supporting their contribution to the noted antioxidant capacity. Moreover, a weak but positive correlation between browning and total fluorescence of intermediary compounds (FIC) and fluorescent compounds linked-to-proteins was found (r=0.25 and r=0.32). In contrast, a weak negative correlation was noted between browning and furosine content and free fluorescent Maillard compounds (r= -0.20 and r= -0.12). It may be suggested that fluorophores linked-to-proteins may be precursors of the brown pigments and/or end products rather than early and free fluorescent Maillard compounds.
Acrylamide level
Since acrylamide is formed in foods during heat treatment at temperatures higher than 120°C, especially during the Maillard reaction, its content in rye-buckwheat ginger cakes is shown in table 3. Acrylamide was formed in control rye and rye-buckwheat ginger cakes within the range of 72.2 – 149.2 µg/kg DM. Its formation in rye-buckwheat ginger cakes has not exceeded twice higher the level noted in control rye ginger cakes which perfectly corresponded to its content in rye ginger cakes [4]. In contrast, the level of acrylamide found in rye-buckwheat ginger cakes was almost fourfold lower than noted in the same cakes without spice mix and honey in formula [29]. There was no relationship between TF and rutin contents as well as antioxidant capacity of rye-buckwheat ginger cakes enriched with rutin. Therefore, it may be suggested that other compounds originating from buckwheat honey or/and spice mix may a decrease acrylamide content. Moreover, a positive correlation between acrylamide formation and total FIC (r=0.62) and CML content (r=0.70) provides evidence supporting formation of acrylamide at the advanced stage of Maillard reaction. Previously Lasekan and Abbas (2010) reported presence of acrylamide in a wide range of common foods. The highest AA content was found in heated carbohydrate-rich foods: 150–4000 µg/kg, moderate levels of AA: 5–150 µg/kg in protein-rich foods and below 5µg/kg in unheated or boiled foods [38]. Therefore, it may be concluded that rye-buckwheat ginger cakes enriched with rutin may be included amongst food with moderate acrylamide level.
Conclusion
Rye-buckwheat ginger cakes enriched with rutin were developed and data on total flavoids, rutin, available lysine, early, advanced and final Maillard reaction products, acrylamide contents and antioxidative capacity of the developed cakes were provided. Enrichment of rye-buckwheat ginger cakes with rutin improved their antioxidant properties, showed protective effect on lysine blockage resulting in lower furosine formation whereas stimulated the Maillard reaction (MR) progress to the melanoidin formation. In contrast, the loss of nutritional quality of ryebuckwheat ginger cakes enriched with rutin was still noted due to formation of carboxymethyllysine and fluorescent compounds linked-to-protein at the advanced stage of MR. Formation of moderate level of acrylamide at the advanced stage of MR was confirmed but there was no relationship with total flavonoids and rutin contents as well as with antioxidant capacity of ryebuckwheat ginger cakes enriched with rutin. This study suggests that rye-buckwheat ginger cakes enriched with rutin may be recommended for wider nutrition since daily consumption of 250 g of cakes enriched with high dose of rutin may exert a prophylactic or therapeutically effect corresponding to 1-2 tablets of typical pharmacological drugs with rutin as the active component.
Conflict of Interest
The authors confirm that there is no conflicts of interest regarding this manuscript.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
References
- Zielińska D, Szawara-Nowak D, Zieliński H. Determination of the antioxidant activity of rutin and its contribution to the antioxidant capacity of diversified buckwheat origin material by updated analytical strategies. Polish Journal of Food and Nutrition Sciences. 2010; 60: 315-321.
- Zieliński H, Amigo BM, del Castillo MD, Horszwald A, Zielińska D. Formulation and baking process affect Maillard reaction development and antioxidant capacity of ginger cakes. Journal of Food and Nutrition Research. 2010; 49: 140-148.
- Szawara-Nowak D, Koutsidis G, Wiczkowski W, Zieliński H. Evaluation of the in vitro inhibitory effects of buckwheat enhanced wheat bread extracts on the formation of advanced glycation end-products (AGEs). LWT-Food Science and Technology. 2014; 58(2): 327-334. doi: 10.1016/j.lwt.2013.03.005
- Zieliński H, Ciesarová Z, Troszyńska A, et al. Antioxidant properties, acrylamide content and sensory quality of ginger cakes with different formulations. Polish Journal of Food and Nutrition Sciences. 2012; 62: 41-50.
- Zhang ZL, Zhou ML, Tang Y, et al. Bioactive compounds in functional buckwheat food. Food Res Int. 2012; 49(1): 389-395. doi: 10.1016/j.foodres.2012.07.035
- Jiang P, Burczynski F, Campbell C, Pierce G, Austria JA, Briggs CJ. Rutin and flavonoid contents in three buckwheat species Fagopyrutym esculentum, F. tataricum and F.homotropicum and their protective effects against lipid peroxidation. Food Res Int. 2007; 40: 356-364.
- Kreft I, Fabjan N, Yasumoto K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006; 98(3): 508-512. doi: 10.1016/j.foodchem.2005.05.081
- Yang J, Guo J, Yuan J. In vitro antioxidant properties of rutin. LWT-Food Science and Technology. 2008; 41(6): 1060-1066. doi: 10.1016/j.lwt.2007.06.010
- Filipčev B, Šimurina O, Sakač M, et al. Feasibility of use of buckwheat flour as an ingredient in ginger nut biscuit formulation. Food Chem. 2011; 125: 164-170. doi: 10.1016/j.foodchem.2010.08.055
- Giménez BJA, Piskuła MK, Zieliński H. Recent advances in processing and development of buckwheat derived bakery and non-bakery products-a review. Polish Journal of Food and Nutrition Science. 2015; 65(1): 9-20. doi: 10.1515/pjfns-2015-0005
- Baljeet SY, Yadav RB, Yadav R. Studies on functional properties and incorporation of buckwheat flour for biscuit making. Int Food Res J. 2010; 17: 1067-1076.
- Wójtowicz A, Kolasa A, Mościcki L. Influence of buckwheat addition on physical properties, texture and sensory characteristics of extruded corn snacks. Polish Journal of Food and Nutrition Sciences. 2013; 63(4): 239-244.
- Burluc RM, Vizireanu C, Dinica RM, Dima F. The use of pseudo-cereals flours in bakery. Chemistry & Chemical Engineering, Biotechnology, Food Industry. 2012; 13: 177-186.
- Giannetti V, Boccacci MM, Mannino P. Furosine as a pasta quality marker: evaluation by an innovative and fast chromatographic approach. J Food Sci. 2013; 78(7): C994-C999.doi: 10.1111/1750-3841.12163
- Delgado-Andrade C, Rufián-Henares JA, Morales FJ. Study on fluorescence of Maillard reaction compounds in breakfast cereals. Mol Nutr Food Res. 2006; 50(9): 799-804. doi: 10.1002/mnfr.200500249
- Bosch L, Sanz ML, Montilla A, Alegría A, Farré R, del Castillo MD. Simultaneous analysis of lysine, Nε-carboxymethyllysine and lysinoalanine from proteins. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 860(1): 69-77. doi: 10.1016/j.jchromb.2007.10.011
- Wang HY, Qian H, Yao WR. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011; 128(3): 573-584. doi: 10.1016/j.foodchem.2011.03.075
- Damjanovic Desic S, Birlouez-Aragon I. The FAST index - a highly sensitive indicator of the heat impact on infant formula model. Food Chem. 2011; 124(3): 1043-1049. doi: 10.1016/j.foodchem.2010.07.071
- O'Sullivan AM, O'Callaghan YC, O'Connor TP, O'Brien NM. Comparison of the antioxidant activity of commercial honeys, before and after in-vitro digestion. Pol J Food Nutr Sci. 2013; 63(3): 167-171. doi: 10.2478/v10222-012-0080-6
- Potter R, Stojceska V, Plunkett A. The use of fruit powders in extruded snacks suitable for children's diet. Lebenson Wiss Technol. 2013; 51(2): 537-544. doi: 10.1016/j.lwt.2012.11.015
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxides radicals. Food Chem. 1998; 64(4): 555-559. doi: 10.1016/S0308-8146(98)00102-2
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26(9-10): 1291-1237.
- Popov I, Lewin G. Oxidants and Antioxidants Part B - Antioxidative homeostasis: characterization by means of chemiluminescent technique. Methods in Enzymology. 1999; 300: 437-456.
- Przygodzka M, Zielińska D, Ciesarová Z, Kukurová K, Zieliński H. Comparison of methods for evaluation of the antioxidant capacity and phenolic compounds in common spices. Lebenson Wiss Technol. 2014; 58(2): 321-326. doi: 10.1016/j.lwt.2013.09.019
- Ramírez-Jiménez A, García-Villanova B, Guerra-Hernández E. Loss of o-phthaldialdehyde reactivity during storage of infant cereals. Int J Food Sci Nutr. 2004; 55(2): 143-148. doi: 10.1080/09637480410001666513
- Delgado-Andrade C1, Seiquer I, Navarro MP, Morales FJ. Maillard reaction indicators in diets usually consumed by adolescent population. Mol Nutr Food Res. 2007; 51(3): 341-351. doi: 10.1002/mnfr.20060007
- Peng X, Ma J, Cheng KW, Jiang Y, Chen F, Wang M. The effects of grape seed extract fortification on the antioxidant and quality attributes of bread. Food Chem. 2010; 119(1): 49-53. doi: 10.1016/j.foodchem.2009.05.083
- Zieliński H, del Castillo MD, Przygodzka M, Ciesarová Z, Kukurová K, Zielińska D. Changes in chemical composition and antioxidative properties of rye ginger cakes during their shelf-life. Food Chem. 2012; 135(4): 2965-2973. doi: 10.1016/j.foodchem.2012.07.009
- Marková L, Ciesarová Z, Kukurová K, Zieliński H, Przygodzka M, Bednáriková A, Šimko P. Influence of various spices on acrylamide content in buckwheat ginger cakes. Chem Pap. 2012; 66(10): 949-954. doi: 10.2478/s11696-012-0218-3
- Sergiel I, Pohl P, Biesaga M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2014; 145: 404-408. doi: 10.1016/j.foodchem.2013.08.068
- Zieliński H, Michalska A, Amigo-Benavent M, del Castillo MD, Piskuła MK. Changes in protein quality and antioxidant properties of buckwheat seeds and groats induced by roasting. J Agric Food Chem. 2009; 57(11): 4771-4776. doi: 10.1021/jf900313e
- Moore J, Luther M, Cheng Z, Yu LL. Effects of baking conditions, dough fermentation, and bran particle size on antioxidant properties of whole-wheat pizza crusts. J Agric Food Chem. 2009; 57(3): 832-839.doi: 10.1021/jf802083x
- Capuano E, Ferrigno A, Acampa I, et al. Effect of flour type on Maillard reaction and acrylamide formation during toasting of bread crisp model systems and mitigation strategies. Food Res Int. 2009; 42(9): 295-1302. doi: 10.1016/j.foodres.2009.03.018
- Gallegos-Infante JA, Rocha-Guzman NE, Gonzalez-Laredo RF, Pulido-Alonso J. Effect of processing on the antioxidant properties of extracts from Mexican barley (Hordeum vulgare) cultivar. Food Chemistry. 2010; 119: 903-906. doi: 10.1016/j.foodchem.2009.07.044
- Michalska A, Amigo-Benavent M, Zieliński H, del Castillo MD. Effect of baking on the formation of MRPs contributing to the overall antioxidant activity of rye bread. J Cereal Sci. 2008; 48(1): 123-132. doi: 10.1016/j.jcs.2007.08.012
- He J, Zeng M, Zheng Z, He Z, Chen J. Simultaneous determination of Nε (carboxymethyl)lysine and Nε(carboxyethyl)lysine in cereal foods by LC-MS/MS. Eur Food Res Technol. 2014; 238(3): 367-374. doi: 10.1007/s00217-013-2085-8
- Manzocco L, Calligaris S, Mastrocola D, Nicoli MC, Lerici CR. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci Technol. 2000; 11(9): 340-346. doi: 10.1016/S0924-2244(01)00014-0
- Lasekan O, Abbas K. Investigation of the roasting conditions with minimal acrylamide generation in tropical almond (Terminalia catappa) nuts by response surface methodology. Food Chem. 2010; 125(2): 713-718. doi: 10.1016/j.foodchem.2010.09.073


