Review Article
Algae as a Nutritional and Functional Food Source
1,2Chaudhary Devilal University, Sirsa, India
3Jamia Millia Islamiya University, New Delhi, India
*Corresponding author: Manju Nehra, Associate Professor, Department of Food Science and Technology, Chaudhary Devilal University, Sirsa, India, Tel: +91 9996604977, E-mail: manju.vnehra@gmail.com
Received: February 19, 2022 Accepted: March 16, 2022 Published: March 23, 2022
Citation: Jyoti A, Nehra M, Khan M. Algae as a Nutritional and Functional Food Source. Madridge J Food Technol. 2022; 7(1): 189-199. doi: 10.18689/mjft-1000129
Copyright: © 2022 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Algae are organisms that can survive in a wide range of environments, usually in aquatic situations. They have the ability to photosynthesize and reproduce rapidly. The plant and algae have similar in physiological properties but different in cellular structure and reproduction. Algae are photosynthetic organisms with great diversity in size and form, ranging from unicellular microscopic organisms (microalgae) to large multicellular forms (macroalgae). Algae can be a very interesting natural source of new compounds with biological activity that could be used as functional ingredients [1]. Algae are biological compound which can be applied as functional ingredients.
Keywords: Proteins; Amino Acids; Nutrients; Lipids; Carotenoids.
Introduction
Besides the fact that algae are found in natural resource, also have other important aspects. For example, it is easy to cultivate them; grow rapidly and it is possible to control the production of some bioactive compounds. Compounds by manipulating cultivation conditions. The process of algal cultivation in ponds can be divided into three stages: harvesting, culturing and harvesting after treatment. Foods made from algae are a promising source of ingredients for the development of novel food products. Algal metabolites are good source of proteins, minerals, vitamins, amino acids, lipids, fatty acids, polysaccharides, nucleic acid and carotenoids. Algal metabolites are great nutritional supplements [2,3]. Algae play an important role in the food chain as they produce oxygen and biomass.
Algae are eukaryotic microorganisms that contain chlorophyll and store carbohydrates as starch, glycogen or lignin. Many are cultivated for their nutritional value because they provide many vitamins. These include A, B1, B2, B6, niacin and vitamin C [4].
Edible seaweeds are rich in nutrients that are beneficial its human health. It contains bioactive antioxidants, soluble dietary fibers, minerals, phytochemicals and polyunsaturated fatty acid, which are low in calories. Nutrients content can effect by environmental factors such as location, environmental condition and season [5].
Microalgae have been studied for decades, but recently scientists have become interested in them again as a renewable energy source. Algae can be cultivated using commercially available growth components and inexpensive growth media, so this could become a commercial technology in large-scale open pond cultivation. Microalgae are advantageous to the economy and environment. They are photoautotrophs, meaning they do not need organic substances for energy. Because of this, the large-scale culture of microalgae is theoretically simpler and cheaper than that of other organisms. Solar radiation, water, CO2, and inorganic nutrients are the basic requirement for algal growth. There are many other types of algae that can be grown in saline to hypersaline waters, so they do not compete with conventional agriculture for limited resources [6].
Microalgae have high nutritional values. This is due to their ability to produce (I) Long-Chain Polyunsaturated Fatty Acids; (II) Phenolic Compounds; (III) Volatile Compounds; (IV) Sterols; (V) Proteins, Amino Acids, Peptides; (VI) Vitamins; (VII) Polysaccharides; (VIII) Pigments and (IX) Food.
The Omega-3 family can be found in many foods, including fish oil and flaxseed oil. And the other omega-6 fatty acids are produced by microalgae. Omega-3s have been shown to reduce triglyceride concentrations; support healthy cardiovascular health; promote infant brain development; inhibit cancer growth; improve mood, cognition, and behavior; reduce inflammation etc.
Long-Chain Polyunsaturated Fatty Acids
Long-Chain Polyunsaturated Fatty Acids (LCPUFA) produced by microalgae can be used as food supplements by the food industry, because microalgae synthesize ω6 fatty acids (γ-linolenic acid and arachidonic acid) and ω3 fatty acids (eicosapentaenoic acid and docosahexaenoic acid) [7,8]. Several microalgae strains have been reported to be good producers of various PUFAs. Microalgae can be used as substitutes for fish oil, which will reduce the pressure on fish stocks that are already in danger of being depleted.
Essential fatty acids (EFAs) are linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). DHA and EPA are essential fatty acids that are found in the microalgae that are consumed by zooplankton, fish, and other multicellular organisms. The EFAs become increasingly concentrated up the food web [9]. Fish oils are rich in docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), because they represent the trophic integration of DHA-rich flagellates and EPA-rich diatoms in the food web [10]. There is evidence that the acidification of oceans, caused by changes in coastal processes and increased levels of CO2 in the atmosphere, can affect the supply of essential fatty acids to higher trophic levels [11]. Several factors affect the production of EFA in algal assemblages [12,13].
Several recent studies analyzed the constituent fatty acids of large numbers of red, brown, and green macroalgae from polar [14] (20 species), temperate [15] (16 species) [16] (10 species), and tropical [17] (27 species); [18] (22 species) habitats, and, despite some species variability, red (Rhodophyta) and brown (Phaeophyceae) macroalgae had a high proportion of total FAs in EPA and arachidonic acid across latitudes, whereas the green (Chlorophyta) algae had low EPA (as % of total FA) but some DHA, and, were enriched in C18 LC PUFA. Phytoplankton contains more PUFA when grown at lower temperatures, as expected [e.g., DHA in Crypthecodinium, 19]. Lower temperatures can be good for maximal biomass production, and 12 hours of lowering the temperature can induce maximal EPA content in diatoms [20]. The content of omega-3 FA can be manipulated by the timing of wild harvest or grow-out of sea vegetable crops in winter to increase EFA in whole foods.
Phaeodactylum tricornutum contains 30%–45% of the PUFAs of which EPA accounts for 20%–40% of their total fatty acids [21]. It can be found in Monodus subterraneus, Porphyridium cruentum, Chaetoceros calcitrans, and Nannochloropsis spp. [22,23].
Producers of DHA include Crypthecodinium cohnii (up to 39% of total fatty acids; 22), Isochrysis galbana, and Pavlova salina [24,25].
Dunaliella salina and Spirulina platensis have been reported to be good sources of linolenic, oleic acids, and DHA [26,27]. While Porphyridium cruentum contains only a small amount of lipids in its biomass (6.5%), the majority are palmitic acid, arachidonic acid, EPA, and linoleic acid [28]. Nostoc commune is a species of cyanobacteria that are found in acidic ponds, lakes, and ditches. They are used in the treatment of cancer, viruses, burns, and chronic fatigue. However, the compounds responsible for these effects have not yet been identified [29].
Phenolic compounds
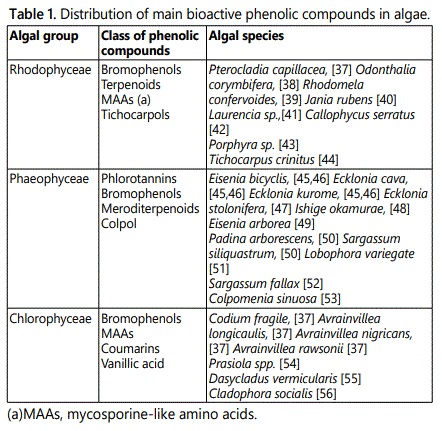
Phenolic compounds are one of the most important classes of antioxidants, which can be used as dietary supplements. Phenolic compounds are produced as secondary metabolites in response to stress. They play a role in the protective mechanism of cells against biotic factors such as grazing and UV radiation, bacteria settlement, and other fouling organisms or metal contamination [28]. Phenolic compounds produced by microalgae include caffeic acid, ferulic acid, p-coumaric acid that can be extracted with subcritical water technology [30,31].
There are a number of different types of microalgae to choose from for use as a dietary supplement. Some of the most commonly studied species include Nostoc ellipsosporum, Nostoc piscinale, Chlorella protothecoides, Chlorella vulgaris, Chlamydomonas nivalis and several others [32,33]. They have similar or higher total phenolic contents than some fruits and vegetables such as kale, shallots, oriental plum, cashews, apple and strawberries [34-36].
Volatile compounds
Certain volatile compounds, like carbonyls, alcohols, aldehydes, esters and terpenes are naturally produced by algae. These volatile compounds can be used as flavoring agents. Algae are attractive for producing flavors because they produce these compounds under mild conditions, high region-and enantio-selectivity and no toxic waste generate [57]. Spirulina is good source of heptadecane and tetradecane, which are volatile compounds known to have antibacterial capacity. D. salina is reported to contain volatile compounds such as β-cyclocitral, α- and β-ionone, neophytadiene, and phytol that have antimicrobial activity [58,59].
Sterol
Sterols in microalgae play a major role in the algaeʼs physiology, including membrane integrity. Phytosterol is one of the most promising sterols because it is used in healthy diets and can be used to reduce coronary heart disease [60]. There are different types of algae that have varying amounts of sterols. While some may have more cholesterol, others may have more other types of sterols. Older analytical techniques may have misidentified algal sterols as cholesterol because their structures are similar. Fucosterol is a compound that occurs in many algae. It can be found in red and brown macroalgae [61]. This compound has potential to treat complications of diabetes and hypertension, as well as other major health concerns [62].
Protein
Proteins, amino acids and peptides are major components of microalgae. Microalgae have a high content of essential amino acids, which is useful for humans and animals. These amino acids are lysine, leucine, isoleucine, and valine. These four essential amino acids constitute 35 percent of the essential amino acids in human muscle protein [63].
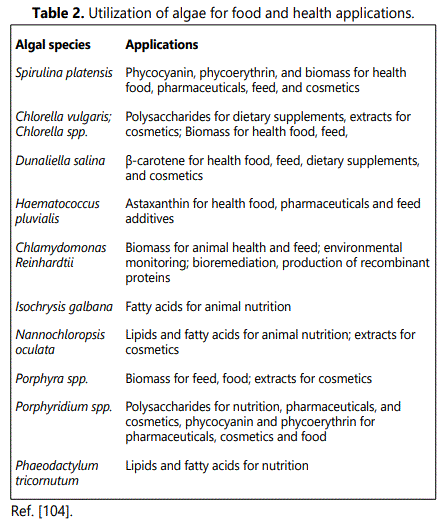
Chlorella sp. and Spirulina sp. have the highest protein content (more than 50% dry weight) [64]. Red and green algae contain a high level of protein in their bodies, but brown algae have lower levels [65-69]. For example Porphyra spp. (Blaver^), Pyropia spp. (Bnori^), Palmaria palmata (Bdulse^), Ulva spp. (Bsea lettuce^) often contain high levels of protein (as % dry wt). A. platensis is used as a dietary supplement due to its high protein content, which is useful for young children and the elderly (Cyanotech Corporation, Hawaii, USA) [70]. S. latissima has higher protein content than other seaweed species due to the environmental conditions in which it grows [71]. The seaweed Chlorella vulgaris (Chlorophyta) is one of the most popularly consumed algae in the world because of its high protein content. It contains 51–58% protein by dry weight [72]. This species of alga is commonly used for human food, animal feed, medical applications etc. [73].
Ulva species have the nine essential amino acids that are needed for human beings, and contain them in high levels and equivalent to soybean proteins [74].
Cultivation is necessary to increase the protein content of plant species used for oil and biofuels production [75]. According to a review, the crude protein content of microalgae biomass ranges from 6 to 63%. Most species have over 40% crude protein on the basis of dry mass. The protein content of 16 microalgae ranged from 12% to 35%. The diatom Chaetoceros gracilis had the lowest percentage, while Nannochloropsis oculata had the highest [76]. A 2007 report by Becker provided an overview on the major constituents of 13 species of microalgae. The protein content in each varied between 6% and 71% of dry matter, with at least 50% in most species [75]. A majority of species in the genus Spirogyra had a protein content ranging from 6.0% to 20.0% of dry matter, which is lower than that reported by Tipnee et al., (2015) and Saragih et al., (2019) [77,78]. Acquah et al. reported a range of protein content of 6–58% in 17 microalgal species in 2020 [79]. A similar range (10–71%) of protein content was reported by Becker in 33 microalgal species [80]. Another review of 22 algal species showed the protein content in dry biomass to be 6–20% for Spirogyra sp. and 8–18% for Scenedesmus dimorphus. The majority of species had over 50% protein [81]. Most microalgae species contain a high content of protein and some have been used for food proteins or dietary supplements. For example, Chlorella sp. and Scenedesmus obliquus are available as food proteins, while Arthrospira sp. is a dietary supplement. It appears that Arthrospira sp. and Chlorella vulgaris are the most common species of cyanobacteria to be used in industrial processes due to their high protein content (51–58% of dry matter) and favorable essential amino acid profiles [72,82].
Vitamin
Some microalgae contain both water and lipid soluble vitamins in higher amounts than conventional vitamin rich foods. This is because these microalgae have a high nutritional value and can provide certain vitamins to humans and animals. Vitamin contains β-carotene (provitamin A), tocopherol (vitamin E), thiamin (vitamin B1) and folic acid, was higher in many microalgae than in conventional foods. Some cyanobacteria, including Arthrospira (Spirulina), Aphanizomenon and Nostoc are produced for the food industry. They produce up to 3,000 tons of cyanobacteria per year [83]. Since the 1990s, spirulina has been known to be a useful and superior source of vitamins. Seaweed has many nutrients that can be used as a food source for humans, including vitamins and minerals. Arthrospira contains other vitamins as well. It is a source of vitamin B12, which can be found in commercially available Arthrospira. The amount of vitamin B12 per gram is 244 µg [83,84]. Despite the fact that most of this vitamin is believed to be pseudovitamin B12, which might barely be absorbed by the mammalian intestine, further investigation is needed for a certain prediction. The main form of vitamin B12 in Aphanothece sacrum is also pseudovitamin B12 and its availability must be considered doubtful. Belay et al. reported that Spirulina powder contains provitamin A (2.330_103 IU/kg), β-carotene (140 mg 100/g), vitamin E (100 mg 100/g), thiamin B1 (3.5 mg 100/g), riboflavin B2 (4.0 mg 100/g), niacin B3 (14.0 mg 100/g), vitamin B6 (0.8 mg 100/g), inositol (64 mg 100/g), vitamin B12 (0.32 mg 100/g), biotin (0.005 mg 100/g), folic acid (0.01 mg 100/g), pantothenic acid (0.1 mg 100/g), and vitamin K (2.2 mg 100/g) [85]. It is unclear whether this form of vitamin B12 can be absorbed by the human body or not. More studies have to be conducted in order to evaluate its effectiveness.
Tetraselmis suecica was found to be a rich source of thiamin (vitamin B1), pyridoxine (vitamin B6), nicotinic acid (vitamin B3), pantothenic acid (vitamin B5), and ascorbic acid (vitamin C). On the other hand, Dunaliella tertiolecta contents a good source of β-carotene (provitamin A) riboflavin (vitamin B2), cobalamin (vitamin B12), and tocopherol (vitamin E). Moreover, biotin (vitamin B7) was found in high concentrations in Chlorella. In brown algae, α-tocopherol is the most common tocopherol. It has high stability in heat and acid but low stability in alkali, UV radiation and oxygen. It fixes free radicals due to the presence of a phenol group in its structure.
Carotenoids are a family of compounds, which are also important due to their high antioxidant activity. Studies have shown that the antioxidant activity of different algae is directly linked to the amount of carotenoids present in them. Research has suggested that xantophylls derived from U. pinnatifida may be useful in the treatment of cerebrovascular diseases [86].
Sea vegetables are a good source of vitamins B1, B12, and A. The food-grade sea vegetable Nori is particularly rich in vitamin K. This nutrient is important for blood clotting and bone building.
Kelp (Macrocystis pyrifera) can contain levels of α-tocopherol comparable to those found in palm, sunflower seed and soybean oils [87,88]. In addition, the values of β-carotene (pro-vitamin A) found in seaweeds Codium fragile and Gracilaria chilensis can exceed those measured in carrots. The composition of vitamins in microalgae varies greatly. Tetraselmis suecica, Isochrysis galbana, Dunaliella tertiolecta, and Chlorella stigmatophora are particularly high in lipid-soluble (A and E) and B-group vitamins including vitamins B1, B2 (riboflavin), B6 (pyridoxal), and B12.
Polysaccharide
A polysaccharide is a polymeric chain of monosaccharide bonded together with glycosidic bonds. Algal polysaccharides are obtained from algae. There are many different types of polysaccharides found in algae that break down into oligosaccharides and monosaccharideʼs when hydrolyzed. Among the polysaccharides found in marine algae are mucopolysaccharides, cell wall-structured polysaccharides, and storage polysaccharide [50,89]. In addition to thickeners, emulsifiers, feed, stabilizers, food, and beverages [90], polysaccharides are found in more than 4,000 species of seaweeds, ranging from 4.6% to 76.6% by weight.
Polysaccharide levels are high in the polysaccharides of Ascophyllum, Palmaria, and Porphyra. Ulva, a green seaweed species, has a polysaccharide content of 65% by dry weight. In terms of nutrition, seaweeds are low in calorie and lipid content. While they are rich in carbohydrates, most of this carbohydrate content consists of dietary fibers that the human body cannot digest.
As dietary fibers, they are beneficial for human health because they ensure a healthy digestive environment in the intestines [66].
Cell walled species have polysaccharides mainly composed of cellulose, hemicellulose, and neutral polysaccharides. These polysaccharides play a crucial role in physically supporting the thallus. Seaweed species contain up to 2% cellulose and 9% hemicellulose of their dry weight. Only green seaweed species such as Ulva have 3% lignin of their dry weight. Brown algae are a group of seaweeds, and contain laminarin, alginic acid and sargassan. Red algae contain carrageenans, agars and xylans as water-soluble polysaccharides. Green algae contain sulfated galactans, xylans and sulfuric acid polysaccharides [50,89].
Alginates are available in both salt and acid forms. The salt form is an important component of the cell wall of all brown seaweed species, consisting of up to 40%-47% of their total dry algal biomass weight, whereas acid form consists of linear polychronic acid known as alginic acid [91,92]. Alginates are complex polysaccharides that have a chemical composition of linear blocks of covalently linked (b 1-4) C-5 epimer alguluronate with D-man urinate. These blocks could contain one or both of these monomers. The ratio and number of these monomers vary from species to species. Alginates are used in a number of industries. They are used in the manufacturing of processed food, cosmetic creams, paper and cardboard, and pharmaceuticals [93]. In Korea and Japan, Laminaria japonica is in high demand. There has been an increase in price due to the popularity of the product, as well as imports of alginates. The annual growth rate for alginates is approximately 2%. Textile printing accounts for nearly half of the global market. Medical and pharmaceutical applications make up about 20% of the global market with 2-4% annual growth based on the use of alginate in wound healing applications and continuous developments in controlled release technologies. The paper industry is 5% of the global market [94].
Red algal polysaccharides contain the nutritionally important floridean starch, and their sulfated galactans initiate or modulate a large number of biological activities of importance to human health [95]. The most studied seaweed polysaccharides are the sulfated agarocolloids and carrageenans. These two were derived from macroalgae in the orders Gelidiales, Gigartinales, and Gracilariales. Anti-viral activities include those against herpes simplex, herpes zoster, dengue-2, vaccinia, rabies, and vesicular stomatitis virus. Patents exist for these anti-virals and there are some commercial projects resulting from this research [96-103]. Consumption of red algae or their extracts in foods may be protective against viruses. The extract from the seaweed known as carrageen an has been used in many medicinal applications for millennia. Today, compounds within this natural extract have a wide range of uses, including anti-cancer and immune system stimulation.
Bioactivities
Antioxidant properties
The body uses several different types of antioxidants to fight the damage caused by free radicals. The body produces reactive oxygen species (ROS) in normal metabolism [105]. ROS reacts with molecules in living cells, such as lipids, sugars, amino acids, and nucleotides [106].
The antioxidant system in the body is composed of endogenous and non-enzymatic antioxidants. The endogenous antioxidants include superoxide dismutase, catalase and glutathione peroxidase, which protect cells from damage caused by free radicals. Non-enzymatic antioxidants such as vitamin C, α-tocopherol and selenium. Inorganic compounds may react with oxygen to form toxic free radicals. Antioxidants can reduce this reaction [107] and prevent damage to cells and tissues [105]. The balance between antioxidants and oxidants is vital to maintain good health. Antioxidants are compounds that prevent oxidation in the body, while oxidants are compounds that produce free radicals. The increased production of oxidants can lead to many health issues such as cancer, neurodegenerative diseases, autoimmune conditions, cardiovascular disease, hypertension diabetes mellitus, inflammatory disease and aging [108,94,109,110].
The consumption of seaweed generally increases the activity of endogenous antioxidant enzymes such as superoxide dismutase, glutathione peroxidise and sometimes catalase [111,112,123].
Sulphated polysaccharides from algae have the potential to be used as antioxidants. Fucoidan, in particular, has been shown to have potent antioxidant activity [113]. A study published in 2013 has shown that SP from Porphyra haitanensis has antioxidant activity in vivo. Another study done in 2000 showed that Fucoidan from Fucus vesiculosus prevented the formation of superoxide radicals, hydroxyl radicals, and lipid peroxidation [114,115]. There has been some evidence that SP from Porphyra haitanensis has antioxidant activity in mice [114]. Fucosecan from Fucus vesiculosus decreased the formation of superoxide radicals, hydroxyl radicals and lipid peroxidation [115]. Hipochlorous acid is a powerful oxidant that contributes to the microbial killing which leads to the injury of host tissue and triggers severe inflammation disorders [116].
Furthermore, in an in vivo experiment on diabetic mice, fucoidans from this species prevented the increase of lipid peroxidation in serum, liver and spleen [117]. These results on fucoidans indicate their potential for preventing free radical-mediated diseases [118].
Algae are aquatic organisms that are part of the plant kingdom. Marine brown algae are known to contain polyphenols, also called phenolic compounds, which can help prevent cancer and age-related disorders. The phloroglucinol-based polyphenols in marine brown algae are phlorotannins [119].
In recent years, researchers have been studying the antioxidant properties of protein hydrolysates and peptides. In one study, an alkali-soluble protease enzyme extract from edible brown seaweeds was shown to have antioxidative effects. These included Ecklonia cava, Scytosiphon lomentari, Ishige okamurae, Sargassum fullvelum, Sargasum horneri and Sargassum thunbergii. The extract of E. cava was able to scavenge DPPH free radicals more effectively than the extract of S. lomentaria in one study [120]. Another study found that the enzyme extract of S. lomentaria showed stronger lipid peroxidation inhibition in linoleic acid than the extracts of E. cava and P. vulgaris [121].
A recent study [122] used the coastal diatom Skeletonema marinoi to investigate the modulation of lipophilic antioxidant compounds and the hydrophilic vitamin C by light manipulation. The study was done in order to examine the effects of light on carotenoid production and lipid peroxidation. The results showed that certain carotenoids were able to act as antioxidants by preventing the creation of radicals. In addition, they found a link between the carotenoid operating photoprotection and the antioxidant molecules and activity modulation. This study confirms the role of light manipulation as a powerful tool for manipulating antioxidant compounds in microalgae [122].
By the 20th century, scientists began to research carotenoids, which are a major component of microalgae. These compounds are known for their bioactivity and human wellness benefits as well as their versatility. Scientists have been able to enhance carotenoids through light modulation in microalgae [123,124].
Dunaliella salina is a chlorophyte that is used mostly for biotechnological investigations and applications, mainly relying on the production of β-carotene. This carotenoid has been used as a model to study the modulation of carotenoids with respect to light intensity and wavelength [125,126]. In this study, the researchers use monochromatic red light to examine how it affects carotenoids. They found that red light increased the concentration of β-carotene in tomatoes and also changed the ratio of different forms of β-carotene to 9-cis β-carotene. This study confirms the role of light in carotenoid production in microalgae. It shows that the modulation of light is important for biotechnological production of these bioactive compounds. Enhancing carotenoid production in algae can be done by genetic engineering and biomanipulation. To reach this goal, it is necessary to increase the knowledge about the biosynthetic pathways of these compounds as well as the modulation factors affecting gene expression involved. Two brown algae, Saccharina japonica and Cladosiphon okamuranus, were investigated thanks to the analysis of Genome–Scale Metabolic Networks (GSMNs) [127]. The authors of the study were able to reconstruct the biosynthetic pathways of the main carotenoids in these two algae, highlighting how interesting and scientifically rich such an approach is for studying targeted biochemical pathways.
Chlorophyll molecules are found in most plants and algae. In addition to their well-known role in photosynthesis, chlorophylls function as antioxidants. Their structure is similar to that of hemoglobin. Therefore, chlorophylls can be used for the oxygenation of blood substitutes [128].
Chlorophyll molecules are found in most plants and algae. These molecules also function as antioxidants, due to their similar structure to hemoglobins. Chlorophylls can be used for the oxygenation of blood substitutes. The study by Maroneze and collaborators [128] reported that the presence of light influenced the chlorophyll and carotenoid content of Scenedesmus obliquus. It was also found that the increase in sunlight led to an increase in chlorophyll (Chl) levels, while it decreased when there was no sunlight. The authors showed that the concentrations and chemical forms of compounds vary with different conditions. This lays the foundation for large-scale production of these compounds, which are useful to industry.
Fucoxanthin, a carotenoid present in brown algae, has been tested for its potential health benefits. Fucoxanthin, a carotenoid compound, might be extracted from numerous classes of microalgae. The anti-inflammatory, antioxidant, and antiproliferative effects of fucoxanthin were investigated on blood mononuclear cells and different cell lines. The study showed that the clearly displayed the antiproliferative and antioxidant activities of fucoxanthin in vitro, highlighting the great interest in its potential use in nutraceuticals [129].
Anticancer activity
Marine flora is made up of brown, blue, green, blue-green, and red algae. Of these, brown algae were the most common source of secondary metabolites with multiple biological activities. Phytoplankton are important constituents in the marine food chain [130,131]. The use of marine organisms as a source for new drugs has been attracting enormous attention. Few molecules from marine organisms are used today in the field of medicine, but they have great potential to provide new medicines with diverse biological efficacies. Biomolecules are molecules that are active against lung cancer. They can be derived from algae such as microalgae, macroalgae and cyanobacteria [132].
Marine macroalgae, also known as seaweeds, are the most common plants in the marine ecosystems. They account for 90% of all marine plants and 50% of the earthʼs photosynthesis [133]. Seaweeds have been used in traditional Chinese medicine for more than 2000 years [134]. As more scientists have examined seaweeds, their health benefits have been discovered. Seaweeds can fight numerous diseases such as gall stones, stomach ailments, eczema, cancer, renal disorders, scabies, psoriasis, asthma and ulcers. The pharmaceutical potential of those molecules was amply investigated for drug discovery. As a result of these findings, several high-value compounds were identified. Carotenoid, polysaccharides, fatty acids, glycoproteins, haloforms, halogenated alkanes and alkenes were found. Alcohols, aldehydes, hydroquinones and ketones were also discovered. Phlorotannins, pigments, lectins, alkaloids, terpenoids, sterols, and some heterocyclic and phenolic compounds. Many of these HVC are in clinical or preclinical trials.
Microalgae are autotrophic microorganisms that consume CO2, light, and inorganic nutrients to produce biomass. Microalgae have been used for centuries as a food source; however, the discovery of their health benefits has created more opportunities for this group of organisms in the medical field. In addition, microalgae can produce HVC such as polysaccharides, PUFAs, carotenoids (lutein, zeaxanthin and astaxanthin), and vitamins with very high nutraceutical and pharmaceutical potentials [135]. A type of marine green microalgae called Tetraselmis suecica is a good source of polyunsaturated fatty acids (PUFAs). The amount of PUFAs in this microalga is 74 grams per kilogram of algae harvested [136]. In a 2009 study, researchers tested the antioxidant and cell repair properties of an extract from the root of “Euphorbia hirta” in A549 lung adenocarcinoma cells. The extract showed strong antioxidant and cell repair activity. A study demonstrated that a heterogeneous mixture of microalgae was able to inhibit the colony forming ability of A549 and H460 lung cancer cells at a dose of 5 µg µL−1 [137]. In addition, Chlorella vulgaris extract inhibited the proliferation of H1299, A549, and H1437 lung cancer cells by 50% [138]. By using different polarities of solvents, the extraction will be able to dissolve and extract more biomolecules that are selectively active against specific types of cancer cells.
H. musciformis, P. gymnospora, and D. dichotoma were shown to be selectively active against NCI-H292 cells (human lung mucoepidermoid carcinoma) in ethanolic extracts at 22.0 ± 3.5 µg mL−1. The dichloromethane extract, chloroform and methanolic extracts of D. dichotoma were active against HEp-2 (human larynx epidermoid carcinoma) cells [139].
Blue green algae, which are members of the cyanophyta, have many different ways of producing cyclic nitrogenous compounds. These compounds have a variety of biological activities. Lyngbya majuscula produces molecules such as obyanamide, hectochlorin, lyngbyastatin 3, and apratoxin with proven cytotoxic activities [132]. Some cyanobacteria species also produce compounds like cryptophycin, which has an analog that is effective against cancer (Hela cells) [140]. Deniz et al. (2016) has emphasized the use of phycocyanins from Spirulina platensis against A549 lung cancer cell line with an IC50 value of 29.41 µg mL−1 after 24 h of incubation [141,142].
The likelihood of developing cancer increases as a person gets older. Only a small portion of cancers are hereditary [143]. Seaweed consumption has been associated with a lower risk of rectal cancer [144] and neoplasis cancer risk [145,146]; as well as human breast cancers [147] with suppressive effects on the development of benign and cancer neoplasis [148].
The presence of free radicals in the body can induce cancer cells to form, making oxidative damage an important contributor in cancer formation. Thus, compounds that have radical scavenging activity, such as NP [149], SP [150] and phlorotannins [151], may be used to reduce cancer formation. Phlorotannins from E. cava demonstrated cytotoxic effects on human cancer cells including HT1080 (fibrosarcoma), AS49 (lung cancer) and HT-29 (human colon adenocarcinoma). However, they were less cytotoxic to normal lung fibroblasts [120]. In a study by Kong, C.S. et al. 2009, dioxinodehydroeckol, a derivative of phlorotannin from E. cava, was found to reduce the growth of human breast cancer cells (MCF-7) through induction of apoptosis [152].
Fucoidans have been shown to suppress the growth of cancer cells by inhibiting their proliferation, stimulating apoptosis of tumour cells, preventing metastasis and enhancing immune responses [153,154].
The anti-proliferative activity of Cladosiphon okamuranus fucoidans was observed in U 937 cell line. This was caused by inducing apoptosis via caspase-3 and 7 activation dependent pathways [155]. A study was conducted on the peripheral blood mononuclear cells of adult T-cell leukaemia patients and human T-cell leukaemia virus (HTLV) type 1-infected T-cell lines. The growth of both was inhibited, but the normal peripheral blood mononuclear cells were not. Apoptosis of HTLV-1 infected T cell was achieved by down regulation of cellular inhibitor of apoptosis protein 2 [156].
Furthermore, MDA-MB-231 breast carcinoma cells adhered less to platelets when treated with fucoidans from L. saccharina, L. digitata, Fucus serratus, Fucus distichus and Fucua vesiculosus. This might have critical implications in tumor [118]. Fucoidan from Fucus evanescens was also shown to have antimetastatic activity when 10mg/KG of it was administrated to C57Bl/6 mice with Lewis lung adenocarcinoma [157].
References
- Carlucci MJ, Scolaro LA, Damonte EB. Inhibitory action of natural carrageenans on Herpes simplex virus infection of mouse astrocytes. Chemotherapy. 1999; 45(6): 429-36. doi: 10.1159/000007236
- Tokuşoglu O, Una MK. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. Journal of Food Science. 2003; 68(4): 1144-1148. doi: 10.1111/j.1365-2621.2003.tb09615.x
- Rhodes CJ. Oil from Algae; Salvation from Peak Oil? Sci Prog. 2009; 92: 39-90. doi: 10.3184/003685009X440281
- Mohamed S, Hashim SN, Rahman A. Seaweeds: A Sustainable Functional Food for Complementary and Alternative Therapy. Trends in Food Science and Techonology. 2012; 23: 83-69. doi: 10.1016/j.tifs.2011.09.001
- Berquin IM, Edwards IJ, Kridel SJ, Chen YQ. Polyunsaturated fatty acid metabolism in prostate cancer. Cancer Metast Rev. 2011; 30(3-4): 295-309. doi: 10.1007/s10555-011-9299-7
- Golmakani MT, Rezaei K, Mazidi S, et al. γ-Linolenic acid production by Arthrospira platensis using different carbon sources. Eur J Lipid Sci Technol. 2012; 114(3): 306-314. doi:10.1002/ejlt.201100264
- Jubie S, Ramesh PN, Dhanabal P, et al. Synthesis, antidepressant and antimicrobial activities of some novel stearic acid analogues. Eur J Med Chem. 2012; 54: 931-935. doi: 10.1016/j.ejmech.2012.06.025
- Legezynska J, Kedra M, Walkusz W. Identifying trophic relationships within the high Arctic benthic community: how much can fatty acids tell? Mar Biol. 2014; 161: 821-836. doi: 10.1007/s00227-013-2380-8
- Viso AC, Marty JC. Fatty-acids from 28 marine microalgae. Phytochemistry. 1993; 34: 1521-1533. doi: 10.1016/S0031-9422(00)90839-2
- Rossoll D, Bermudez R, Hauss H, et al. Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS One. 2012; 7(4): 34737. doi: 10.1371/journal.pone.0034737
- Chrismadha T, Borowitzka MA. Effect of cell-density and irradiance on growth, proximate composition and eicosapentaenoic acid production of Phaeodactylum tricornutum grown in a tubular photo bioreactor. J Appl Phycol. 1994; 6: 67-74. doi: 10.1007/BF02185906
- Pasquet V, Ulmann L, Mimouni V, et al. Fatty acids profile and temperature in the cultured marine diatom Odontella aurita. J Appl Phycol. 2014; 26: 2265-2271. doi: 10.1007/s10811-014-0252-3
- Graeve M, Kattner G, Wiencke C, Karsten U. Fatty acid composition of Arctic and Antarctic macroalgae: indicator of phylogenetic and trophic relationships. Mar Ecol Prog Ser. 2002; 231: 67-74.
- Schmid M, Guiheneuf F, Stengel DB. Fatty acid contents and profiles of 16 macroalgae collected from the Irish Coast at two seasons. J Appl Phycol. 2014; 26: 451-463. doi: 10.1007/s10811-013-0132-2
- McCauley JI, Meyer BJ, Winberg PC, Ranson M, Skropeta D. Selecting Australian marine macroalgae based on the fatty acid composition and anti-inflammatory activity. J Appl Phycol. 2015; 27: 2111-2121. doi: 10.1007/s10811-014-0465-5
- Kumari P, Kumar M, Gupta V, Reddy CRK, Jha B. Tropicalmarine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010; 120(3): 749-757. doi: 10.1016/j.foodchem.2009.11.006
- Kumar M, Kumari P, Trivedi N, et al. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J Appl Phycol. 2011; 23: 797-810. doi: 10.1007/s10811-010-9578-7
- Jiang HM, Chen F. Effects of temperature and temperature shift on docosahexanenoic acid production by the marine microalga Crypthecodinium cohnii. JAOCS. 2000; 77: 613-617. doi: 10.1007/s11746-000-0099-0
- Jiang HM, Gao KS. Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum (Bacillariophyceae). J Phycol. 2004; 40: 651-654. doi: 10.1111/j.1529-8817.2004.03112.x
- Ramírez-Fajardo A, Esteban-Cerdan L, Robles-Medina A, et al. Lipid extraction from the microalga Phaeodactylum tricornutum. European Journal of Lipid Science and Technology. 2007; 109: 120-126. doi: 10.1002/ejlt.200600216
- Borowitzka MA. High-value products from microalgae-their development and commercialisation. J Appl Phycol. 2013; 25: 443-756. doi: 10.1007/s10811-013-9983-9
- Guedes AC, Amaro HM, Barbosa CR, Pereira RD, Malcata FX. Fatty acid composition of several wild microalgae and cyanobacteria, with a focus on eicosapentaenoic, docosahexaenoic and α-linolenic acids for eventual dietary uses. Food Research International. 2011a; 44(9): 2721-2729. doi: 10.1016/j.foodres.2011.05.020
- Custódio L, Soares F, Pereira H, et al. Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: possible application in the pharmaceutical and functional food industries. J Appl Phycol. 2014; 26(1): 151-161. doi: 10.1007/s10811-013-0098-0
- Yukino T, Hayashi M, Inoue Y, Imamura J, Nagano N, Murata H. Preparation of docosahexaenoic acid fortified Spirulina platensis and its lipid and fatty acid compositions. Nippon Suisan Gakkaishi. 2005; 71: 74-79. doi: 10.2331/suisan.71.74
- Mendiola JA, Jaime L, Santoyo S, et al. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chemistry. 2007; 102(4): 1357-1367. doi: 10.1016/j.foodchem.2006.06.068
- Stengel DB, Connan S, Popper ZA. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol Adv. 2011;29(5): 483-501. doi: 10.1016/j.biotechadv.2011.05.016
- Rasmussen HE, Blobaum KR, Park YK, Ehlers S J, Lu F, Lee JY. Lipid extract of Nostoc commune var. sphaeroides Kützing, a blue-green alga, inhibits the activation of sterol regulatory element binding proteins in HepG2 cells. J Nutr. 2008; 138(3): 476-481.
- Cabrita MT, Vale C, Rauter AP. Halogenated compounds from marine algae. Mar Drugs. 2010; 8(8): 2301-2307. doi: 10.3390/md8082301
- La Barre S, Potin P, Leblanc C, et al. The halogenated metabolism of brown algae (Phaeophyta), its biological importance and its environmental significance. Mar Drugs. 2010; 8(4): 988-1010. doi: 10.3390/md8040988
- Li HB, Cheng KW, Wong CC, Fan KW, Chen F, Jiang, Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chemistry. 2007; 102(3): 771-776.
- Hajimahmoodi M, Faramarzi MA, Mohammadi N, Soltani N, Oveisi MR, Nafissi-Varcheh N. Evaluation of antioxidant properties and total phenolic contents of some strains of microalgae. J Appl Phycol. 2010; 22(1): 43-50. doi: 10.1007/s10811-009-9424-y
- Ismail A, Marjan Z M, Foong CW. Total antioxidant activity and phenolic content in selected vegetables. Food Chemistry. 2004; 87(4): 581-586. doi:10.1016/j.foodchem.2004.01.010
- Hassimotto NMA, Genovese MI, Lajolo FM. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J Agric Food Chem. 2005; 53(8): 2928-2935. doi: 10.1021/jf047894h
- Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chemistry. 2007; 101(1): 140-147. doi: 10.1016/j.foodchem.2006.01.014
- Whitfield FB, Helidoniotis F, Shaw KJ, Svoronos D. Distribution of bromophenols in species of marine algae from eastern Australia. J Agric Food Chem. 1999; 47(6): 2367-2373. doi: 10.1021/jf981080h
- Lee HS, Lee TH, Lee JH, et al. Inhibition of the pathogenicity of Magnaporthe grisea by bromophenols, isocitrate lyase inhibitors, from the red alga Odonthalia corymbifera. J Agric Food Chem. 2007; 55(17): 6923-6928. doi: 10.1021/jf071125r
- Zhao J, Fan X, Wang S, Li S, Shang S, Yang Y. Bromophenol derivatives from the red alga Rhodomela confervoides. J Nat Prod. 2004; 67: 1032-1035. doi: 10.1021/np030546+
- Awad NE. Bioactive brominated diterpenes from the marine red alga Jania rubens (L.) Lmx. Phytother Res. 2004; 18(4): 275-279. doi: 10.1002/ptr.1273
- Masuda M, Kawaguchi S, Abe T, Kawamoto T, Suzuki M. Additional analysis of chemical diversity of the red algal genus Laurencia (Rhodomelaceae) from Japan. Phycological Research. 2002; 50(2): 135-144. doi: 10.1046/j.1440-1835.2002.00267.x
- Lane AL, Stout EP, Lin AS, et al. Antimalarial bromophycolides J–Q from the Fijian red alga Callophycus serratus. J Org Chem. 2009; 74(7): 2736-2742. doi: 10.1021/jo900008w
- Carreto JI, Carignan MO. Mycosporine-like amino acids: relevant secondary metabolites. Chemical and ecological aspects. Marine Drugs. 2011; 9(3): 387-446. doi: 10.3390/md9030387
- Ishii T, Okino T, Suzuki M, Machiguchi Y. Tichocarpols A and B, two novel phenylpropanoids with feeding-deterrent activity from the red alga Tichocarpus crinitus. J Nat Prod. 2004; 67: 1764-1766. doi: 10.1021/np0498509
- Shibata T, Nagayama K, Tanaka R, Yamaguchi K, Nakamura T. Inhibitory effects of brown algal phlorotannins on secretory phospholipase A2s, lipoxygenases and cyclooxygenases. J Appl Phycol. 2003; 15: 61-66. doi: 10.1023/A:1022972221002
- Shibata T, Ishimaru K, Kawaguchi S, Yoshikawa H, Hama Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J Appl Phycol. 2008; 20: 705-711. doi: 10.1007/s10811-007-9254-8
- Shim SY, Choi JS, Byun DS. Inhibitory effects of phloroglucinol derivatives isolated from Ecklonia stolonifera on FcεRI expression. Bioorg Med Chem. 2009; 17(13): 4734-4739. doi: 10.1016/j.bmc.2009.04.050
- Heo SJ, Jeon YJ. Radical scavenging capacity and cytoprotective effect of enzymatic digests of Ishige okamurae. J Appl Phycol. 2008; 20: 1087-1095. doi: 10.1007/s10811-008-9320-x
- Sugiura Y, Matsuda K, Yamada Y, et al. Isolation of a new anti-allergic phlorotannin, phlorofucofuroeckol-B, from an edible brown alga, Eisenia arborea. Biosci Biotechnol Biochem. 2006; 70(11): 2807-2811. doi: 10.1271/bbb.60417
- Chandini SK, Ganesan P, Bhaskar N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008; 107(2): 707-13. doi: 10.1016/j.foodchem.2007.08.081
- Chung H Y, Ma WCJ, Ang PO, Kim J S, Chen F. Seasonal variations of bromophenols in brown algae (Padina arborescens, Sargassum siliquastrum, and Lobophora variegata) collected in Hong Kong. Journal of Agricultural and Food Chemistry. 2003; 51(9): 2619-2624. doi: 10.1021/jf026082n
- Reddy P, Urban S. Meroditerpenoids from the southern Australian marine brown alga Sargassum fallax. Phytochemistry. 2009; 70: 250-255. doi: 10.1016/j.phytochem.2008.12.007
- Green D, Kashman, Y, Miroz A. Colpol, a new cytotoxic C6-C4-C6 metabolite from the alga Colpomenia sinuosa. J Nat Prod. 1993; 56(7): 1201-1202. doi: 10.1021/np50097a033
- Sinha RP, Singh SP, Hader DP. Database on mycosporines and mycosporine-like amino acids ¨ (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J Photochem Photobiol B. 2007; 89(1): 29-35. doi: 10.1016/j.jphotobiol.2007.07.006
- Perez-Rodriguez E, Aguilera J, Figueroa FL. Tissular localization of coumarins in the green alga Dasycladus vermicularis (Scopoli) Krasser: a photoprotective role? Journal of Experimental Botany. 2003; 54(384): 1093-1100. doi: 10.1093/jxb/erg111
- Feng Y, Carroll AR, Addepalli R, Fechner GA, Avery VM, Quinn RJ. Vanillic acid derivatives from the green algae Cladophora socialis as potent protein tyrosine phosphatase 1B inhibitors. J Nat Prod. 2007; 70(11): 1790-1792. doi: 10.1021/np070225o
- Fradique M, Batista AP, Nunes MC, Gouveia L, Bandarra NM, et al. Incorporation of Chlorella vulgaris and Spirulina maxima biomass in pasta products-Part 1: Preparation and evaluation. J Sci Food Agric. 2010; 90(10): 1656-1664. doi: 10.1002/jsfa.3999
- Ozdemir G, Ulku Karabay N, Dalay MC, Pazarbasi B. Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytother Res. 2004; 18(9): 754-757. doi: 10.1002/ptr.1541
- Herrero M, Ibáñez E, Cifuentes A, Reglero G, Santoyo S. Dunaliella salina microalga pressurized extracts as potential antimicrobials. J Food Prot. 2006; 69(10): 2471-2477. doi: 10.4315/0362-028x-69.10.2471
- Luo X, Su P, Zhang W. Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar Drugs. 2015; 13(7): 4231-4254. doi: 10.3390/md13074231
- Pereira CMP, Nunes CFP, Zambotti-Villela L, et al. Extraction of sterols in brown macroalgae from Antarctica and their identification by liquid chromatography coupled with tandem mass spectrometry. J Appl Phycol. 2016. doi: 10.1007/s10811-016-0905-5:1-7
- Abdul QA, Choi RJ, Jung HA, Choi JS. Health benefit of fucosterol from marine algae: a review. J Sci Food Agric. 2016; 96(6): 1856-1866. doi: 10.1002/jsfa.7489
- Dewapriya P, Kim SK. Marine microorganisms: an emerging avenue in modern nutraceuticals and functional foods. Food Res Int. 2014; 56: 115-125. doi: 10.1016/j.foodres.2013.12.022
- Mišurcová L, Buňka F, Vávra Ambrožová J, et al. Amino acid composition of algal products and its contribution to RDI. Food Chem. 2014; 151: 120-125. doi: 10.1016/j.foodchem.2013.11.040
- Dawczynski C, Schaefer U, Leiterer M, Jahreis G. Nutritional and toxicological importance of macro, trace, and ultra-trace elements in algae food products. J Agric Food Chem. 2007; 55(25): 10470-10475. doi: 10.1021/jf0721500
- Holdt SL, Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol. 2011; 23: 543-597. doi: 10.1007/s10811-010-9632-5
- Taboada MC, Millán R, Miguez MI. Nutritional value of the marine algae wakame (Undaria pinnatifida) and nori (Porphyra purpurea) as food supplements. J Appl Phycol. 2013; 25: 1271-1276. doi: 10.1007/s10811-012-9951-9
- Fleurence J. Seaweed proteins: biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol.1999; 10: 25-28. doi: 10.1016/S0924-2244(99)00015-1
- Bleakley S, Hayes M. Algal proteins: extraction, application, and challenges concerning production. Foods. 2017; 6(5): 33. doi: 10.3390/foods6050033
- Sánchez-Machado D I, López-Cervantes J, Lopez-Hernandez J, PaseiroLosada P. Fatty acids, total lipid, protein and ash contents of processe edible seaweeds. Food Chem. 2004; 85(3): 439-444. doi: 10.1016/j.foodchem.2003.08.001
- Aguilera-Morales M, Casas-Valdez M, Carrillo-Domınguez S, GonzálezAcosta, B, Pérez-Gil F. Chemical composition and microbiological assays of marine algae Enteromorpha spp. as a potential food source. J Food Compost Anal. 2005; 18(1): 79-88. doi: 10.1016/j.jfca.2003.12.012
- Becker E. Micro-algae as a source of protein. Biotechnol Adv. 2007; 25(2): 207-210. doi: 10.1016/j.biotechadv.2006.11.002
- Brown MR. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J Exp Mar Biol Ecol. 1991; 145(1): 79-99. doi: 10.1016/0022-0981(91)90007-J
- Tipnee S, Ramaraj R, Unpaprom Y. Nutritional evaluation of edible freshwater green macroalga Spirogyra varians. Emergent Life Sci. Res. 2015; 1: 1-7.
- Saragih HT, Muhamad AAK, Alfianto A, et al. Effects of Spirogyra jaoensis as a dietary supplement on growth, pectoralis muscle performance, and small intestine morphology of broiler chickens. Vet World. 2019; 12(8): 1233-1239. doi: 10.14202/vetworld.2019.1233-1239
- Acquah C, Tibbetts SM, Pan S, Udenigwe C. Chapter 19-Nutritional quality and bioactive properties of proteins and peptides from microalgae. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes E, Maroneze MM, Queiroz MI, Zepka LQ, Eds.; Academic Press: Cambridge, MA, USA. 2020; pp. 493-531.
- Becker EW. Microalgae for Human and Animal Nutrition. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmong, A., Hu, Q., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 461-503. doi: 10.1002/9780470995280.ch18
- Koyande AK, Chew KW, Rambabu K, Tao Y, Chu D-T. Show PL. Microalgae: A potential alternative to health supplementation for humans. Food Sci Hum Wellness. 2019; 8: 16-24. doi: 10.1016/j.fshw.2019.03.001
- Ismail I, Hwang Y-H, Joo S-T. Meat analog as future food: A review. J Anim Sci Technol. 2020; 62(2): 111-120. doi: 10.5187/jast.2020.62.2.111
- Watanabe F, Takenaka S, Katsura H, et al. Dried green and purple lavers (nori) contain substantial amounts of biologically active vitamin B12 but less of dietary iodine relative to other edible seaweeds. J Agric Food Chem. 1999b; 47: 2341-2343. doi: 10.1021/jf981065c
- Belay A, Ota Y, Miyakawa K, Shimamatsu H. Current knowledge on potential health benefits of Spirulina. Journal of Applied Phycology. 1993; 5: 235-241. doi: 10.1007/BF00004024
- Ikeda K, Kitamura A, Machida H, et al. Effect of Undaria pinnatifida (Wakame) on the development ofcerebrovascular diseases in strokeprone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2003; 30(1): 44-48. doi: 10.1046/j.1440-1681.2003.03786.x
- Ortiz J, Uquiche E, Robert P, Romero N, Quitral V, Llanten C. Functional and nutritional value of the Chilean seaweeds Codium fragile, Gracilaria chilensis and Macrocystis pyrifera. Eur J Lipid Sci Technol. 2009; 111:320-327. doi: 10.1002/ejlt.200800140
- Somasekharan SP, El-Naggar A, Sorensen PH, Wang Y, Cheng H. An Aqueous Extract of Marine Microalgae Exhibits Antimetastatic Activity through Preferential Killing of Suspended Cancer Cells and Anticolony Forming Activity. Evid Based Complement Altern Med. 2016; 9730654. doi: 10.1155/2016/9730654
- Murata M, Nakazoe J-I. Production and use of marine algae in Japan. Jpn Agric Res Q. 2001; 35(4): 281-90. doi: 10.6090/jarq.35.281
- Kraan S. Algal polysaccharides, novel applications and outlook. In: Chang C-F, editor. Carbohydrates - comprehensive studies on glycobiology and glycotechnology. 2012. doi: 10.5772/51572
- Rasmussen RS, Morrissey MT. Marine biotechnology for production of food ingredients. Adv Food Nutr Res. 2007; 52: 237-92. doi: 10.1016/S1043-4526(06)52005-4
- Gutteridge MC. Hydroxyl Radicals, Iron, Oxidative Stress, and Neurodegeneration. Ann N Y Acad Sci. 1994; 54: 288-295. doi: 10.1111/j.1749-6632.1994.tb21805.x
- Prajapati VD, Maheriya PM, Jani GK, Solanki HK. Carrageenan: a natural seaweed polysaccharide and its applications. Carbohydr Polym. 2014; 105: 97-112. doi: 10.1016/j.carbpol.2014.01.067
- Richards JT, Kern ER, Glasgow LA, Overall JC Jr, Deigh EF, Hatch MT. Antiviral activity of extracts from marine algae. Antimicrob Agents Chemother. 1978; 14(1): 24-30. doi: 10.1128/AAC.14.1.24
- Baba M, Snoeck R, Pauwels R, deClerq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus and human immunodeficiency virus. Antimicrob Agents Chemother. 1988; 32: 1742-1745. doi: 10.1128/AAC.32.11.1742
- Vedros NA. Topical demulcent for viral and inflammatory diseases of the skin. US Patent No. 5198217 A. 1993.
- Eccles R, Meier C, Jawad M, Weinmüllner R, Grassauer A, Grassauer EP. Efficacy and safety of an antiviral iotacarrageenan nasal spray: a randomized, double-blind placebocontrolled exploratory study in volunteers with early symptoms of the common cold. Respir Res. 2010; 11(1): 108. doi: 10.1186/1465-9921-11-108
- Talarico LB, Noseda MD, Ducatti DRB, Duarte MER, Damonte EB. Differential inhibition of dengue virus infection in mammalian and mosquito cells by iota-carrageenan. J Gen Virol. 2011; 92: 1332-1342. doi: 10.1099/vir.0.028522-0
- Levendosky K, Mizenina O, Martinelli E, et al. Griffithsin and carrageenan combination to target herpes simplex virus 2 and human papillomavirus. Antimicrob Agents Chemother. 2015; 59(12): 7290-7298. doi: 10.1128/AAC.01816-15
- Luo Z, Tian D, ZhouM, et al. Lambda-Carrageenan P32 is a potent inhibitor of rabies virus infection. PLoS One. 2015; 10(10): 0140586. doi: 10.1371/journal.pone.0140586
- Halliwell B. Reactive Oxygen Species in Living Systems: Source, Biochemistry and Role in Human Disease. Am J Med. 1991; 91: S14-S22. doi: 10.1016/0002-9343(91)90279-7
- Feig DI, Reid TM, Loeb LA. Reactive Oxygen Species in Tumorigenesis. Cancer Res. 1994; 54(7): 1890-1894.
- Valko M, Leibfritz D, Mancol J, Cronin MTD, Mazur M, Telser J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int J Biochem Cell Biol. 2007; 39(1): 44-84. doi: 10.1016/j.biocel.2006.07.001
- Matanjun P, Mohamed S, Mustapha NM, Muhammad K, Cheng HW. Antioxidant Activities and Phenolics Content of Eight Species of Seaweeds from North Borneo. J Appl Phycol. 2008; 20(4): 367-373. doi: 10.1007/s10811-007-9264-6
- Yuan YV, Walsh NA. Antioxidant and Antiproliferative Activities of Extracts from a Variety of Edible Seaweeds. Food Chem Toxicol. 2006; 44(7): 1144-1150. doi: 10.1016/j.fct.2006.02.002
- Zhang QB, Li N, Liu XG, Zhao ZQ, Li ZE, Xu ZH. The Structure of a Sulfated Galactan from Porphyra haitanensis and Its in vivo Antioxidant Activity. Carbohydr Res. 2004; 339(1): 105-111. doi: 10.1016/j.carres.2003.09.015
- Zhang Q, Li N, Zhou G, Lu X, Xu Z, Li Z. In vivo Antioxidant Activity of Polysaccharide Fraction from Porphyra haitanensis (Rhodephyta) in Aging Mice. Pharmacol Res. 2003; 48: 151-155. doi: 10.1016/s1043-6618(03)00103-8
- Micheline RS, Cybelle M, Celina GD, Fernando FS, Hugo OR, Edda L. Antioxidant Activities of Sulfated Polysaccharides from Brown and Red Seaweeds. J Appl Phycol. 2007; 19(2): 153-160. doi: 10.1007/s10811-006-9121-z
- Li Y, Qian ZJ, Ryu B, Lee SH, Kim MM, Kim SK. Chemical Components and Its Antioxidant Properties in Vitro: An Edible Marine Brown Algae, Ecklonia cava. Bioorg Med Chem. 2009; 17(5): 1963-1973. doi: 10.1016/j.bmc.2009.01.031
- Li DY, Xu RY, Zhou WZ, Sheng XB, Yang AY, Cheng JL. Effects of Fucoidan Extracted from Brown Seaweed on Lipid Peroxidation in Mice. Acta Nutrimenta Sinica. 2002; 24: 389-392.
- Vo TS, Kim SK. Fucoidans as a Natural Bioactive Ingredient for Functional Foods. Journal of Functional Foods. 2013; 5: 16-27. doi: 10.1016/j.jff.2012.08.007
- Li Y, Qian ZJ, Kim MM, Kim SK. Cytotoxic Activities of Phlorethol and Fucophlorethol Derivatives Isolated from Laminariaceae Ecklonia cava. Journal of Food Biochemistry. 2011; 35: 357-369. doi: 10.1111/j.1745-4514.2010.00387.x
- Heo SJ, Lee KW, Song CB, Jeon YJ. Antioxidant Activity of Enzymatic Extracts from Brown Seaweeds. Bioresour Technol. 2005; 96: 1613-23. doi: 10.1016/j.biortech.2004.07.013
- Ahn CB, Jeon YJ, Kang DS, Shin TS, Jung BM. Free Radical Scavenging Activity of Enzymatic Extracts from a Brown Seaweed Scytosiphon lomentaria by Electron Spin Resonance Spectrometry. Food Research International. 2004; 37(3): 253-258. doi: 10.1016/j.foodres.2003.12.002
- Skrovankova S. Seaweed vitamins as nutraceuticals. In: Kim SK (ed) Marine medicinal foods: implications and applications, macro and microalgae. Elsevier, San Diego. 2011; 357-369. doi: 10.1016/B978-0-12-387669-0.00028-4
- Barra L, Chandrasekaran R, Corato F, Brunet C. The challenge of ecophysiological biodiversity for biotechnological applications of marine microalgae. Mar Drugs. 2014; 12: 1641-1675. doi: 10.3390/md12031641
- Brunet C, Chandrasekaran R, Barra L, Giovagnetti V, Corato F, Ruban AV. Spectral radiation dependent photoprotective mechanism in diatom Pseudo-nitzschia multistriata. PLoS ONE. 2014; 9(1): 87015. doi: 10.1371/journal.pone.0087015
- Xu Y, Harvey P. Carotenoid Production by Dunaliella salina under Red Light. Antioxidants. 2019; 8(5): 123. doi: 10.3390/antiox8050123
- Xu Y, Harvey P. Red Light Control of β-Carotene Isomerisation to 9-cis β-Carotene and Carotenoid Accumulation in Dunaliella salina. Antioxidants. 2019; 8(5): 148. doi: 10.3390/antiox8050148
- Nègre D, Aite M, Belcour A, et al. Genome–Scale Metabolic Networks Shed Light on the Carotenoid Biosynthesis Pathway in the Brown Algae Saccharina japonica and Cladosiphon okamuranus. Antioxidants. 2019; 8(11): 564. doi: 10.3390/antiox8110564
- Maroneze M, Zepka L, Lopes E, Pérez-Gálvez A, Roca M. Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus. Antioxidants. 2019; 8: 600. doi: 10.3390/antiox8120600
- Zhang H, Tang Y, Zhang Y, et al. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid Based Complement Alternat Med. 2015; 723515. doi: 10.1155/2015/723515
- Iwamoto C, Minoura K, Hagishita S, Nomoto K, Numata A. Penostatins F–I, novel cytotoxic metabolites from a Penicillium species separated from an Enteromorpha marine alga. J Chem Soc Perkin Trans. 1998; 3: 449-456. doi: 10.1039/A706853K
- Iwamoto C, Minoura K, Oka T, Ohta T, Hagishita S, Numata A. Absolute stereostructures of novel cytotoxic metabolites, penostatins A-E, from a Penicillium species separated from an Enteromorpha alga. Tetrahedron.1999; 55: 14353-14368. doi: 10.1016/S0040-4020(99)00884-4
- Shimizu Y. Microalgal metabolites: A new perspective. Annu Rev Microbiol. 1996; 50: 431-465. doi: 10.1146/annurev.micro.50.1.431
- Dhargalkar VK, Pereira N. Seaweed: Promising Plant of the Millennium. Sci Cult. 2005; 71: 60-66.
- Markou G, Nerantzis E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol Adv. 2013; 31: 1532-1542. doi: 10.1016/j.biotechadv.2013.07.011
- Pérez-López P, González-García S, Ulloa RG, Sineiro J, Feijoo G, Moreira MT. Life cycle assessment of the production of bioactive compounds from Tetraselmis suecica at pilot scale. J Clean Prod. 2014; 64: 323-331. doi: 10.1016/j.jclepro.2013.07.028
- Soeda S, Shibata Y, Shimeno H. Inhibitory Effect of Oversulfated Fucoidan on Tube Formation by Human Vascular Endothelial Cells. Biol Pharm Bull. 1997; 20: 1131-1135. doi: 10.1248/bpb.20.1131
- Wang HM, Pan JL, Chen CY, et al. Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010; 45: 1865-1872. doi: 10.1016/j.procbio.2010.05.023
- Guedes ÉAC, da Silva TG, Aguiar JS, de Barros LD, Pinotti LM, SantʼAna AEG. Cytotoxic activity of marine algae against cancerous cells. Rev Bras Farmacogn. 2013; 23(4): 668-673. doi: 10.1590/S0102-695X2013005000060
- Smerilli A, Balzano S, Maselli M, et al. Antioxidant and Photoprotection Networking in the Coastal Diatom Skeletonema marinoi. Antioxidants. 2019; 8: 154. doi: 10.3390/antiox8060154
- Deniz I, Ozen MO, Yesil-Celiktas O. Supercritical fluid extraction of phycocyanin and investigation of cytotoxicity on human lung cancer cells. J Supercrit Fluids. 2016; 108: 13-18. doi: 10.1016/j.supflu.2015.10.015
- Tan LT. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry. 2007; 68: 954-979. doi: 10.1016/j.phytochem.2007.01.012
- World Cancer Research Fund (2013). www.wcrf.org.
- Kato I, Tominaga S, Matsuura A, Yoshii Y, Shirai M, Kobayashi S. A Comparative Case-Control Study of Colorectal Cancer and Adenoma. Jpn J Cancer Res. 1990; 81: 1101-1108. doi: 10.1111/j.1349-7006.1990.tb02520.x
- Aceves C, Anguiano B, Delgado G. Is Iodine a Gatekeeper of the Integrity of the Mammary Gland? J Mammary Gland Biol Neoplasia. 2010; 10: 189-196. doi: 10.1007/s10911-005-5401-5
- Garcia-Solis P, Alfaro Y, Anguiano B. Inhibition of N-Methyl-NNitrosourea- Induced Mammary Carcinogenesis by Molecular Iodine (I2) but Not by Iodide (I2) Treatment Evidence that I2 Prevents Cancer Promotion. Mol Cell Endocrinol. 2005; 236: 49-57. doi: 10.1016/j.mce.2005.03.001
- Setchell KD, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal Estrogens of Dietary Origin, Possible Roles in Hormone Dependent Disease. Am J Clin Nutr. 1984; 40: 569-578. doi: 10.1093/ajcn/40.3.569
- Eskin BA, Grotkowski CE, Connolly CP. Different Tissue Responses for Iodine and Iodide in Rat Thyroid and Mammary Glands. Biol Trace Elem Res. 1995; 49: 9-19. doi: 10.1007/BF02788999
- Pangestuti R, Kim SK. Biological Activities and Health Benefit Effects of Natural Pigments Derived from Marine Algae. Journal of Functional Foods. 2011; 3: 255-266. doi: 10.1016/j.jff.2011.07.001
- Wijesekara I, Pangestuti R, Kim SK. Biological Activities and Potential Health Benefits of Sulfated Polysaccharides Derived from Marine Algae. Carbohydrate Polymers. 2011; 84: 14-21. doi: 10.1016/j.carbpol.2010.10.062
- Li YX, Wijesekara I, Li Y, Kim SK. Phlorotannins as Bioactive Agents from Brown Algae. Process Biochemistry. 2011; 46: 2219-2224. doi: 10.1016/j.procbio.2011.09.015
- Kong CS, Kim JA, Yoon NY, Kim SK. Induction of Apoptosis by Phloroglucinol Derivative from Ecklonia cava in MCF-7 Human Breast Cancer Cells. Food Chem Toxicol. 2009; 47: 1653-1658. doi: 10.1016/j.fct.2009.04.013
- Koyanagi S, Tanigawa N, Nakagawa H, Soeda S, Shimeno H. Oversulfation of Fucoidan Enhances Its Antiangiogenic and Antitumor Activities. Biochem Pharmacol. 2003; 65: 173-179. doi: 10.1016/s0006-2952(02)01478-8
- Smith CD, Zhang X, Mooberry SL, Patterson GML, Moore RE. Cryptophycin: A New Antimicrotubule Agent Active against Drug-resistant Cells. Cancer Res. 1994; 54: 3779-3784.
- Teruya T, Konishi T, Uechi S, Tamaki H, Tako M. Anti-Proliferative Activity of Oversulfated Fucoidan from Commercially Cultured Cladosiphon okamuranus Tokida in U937 Cells. Int J Biol Macromol. 2007; 41(3): 221-226. doi: 10.1016/j.ijbiomac.2007.02.010
- Haneji K, Matsuda T, Tomita M, et al. Fucoidan Extracted from Cladosiphon okamuranus Tokida Induces Apoptosis of Human T-Cell Leukemia Virus Type 1-Infected T Cell Lines and Primary Adult T-Cell Leukemia Cells. Nutr Cancer. 2005; 52(2): 189-201. doi: 10.1207/s15327914nc5202_9
- Alekseyenko TV, Zhanayeva SY, Venediktova AA, et al. Antitumor and Antimetastatic Activity of Fucoidan, a Sulfated Polysaccharide Isolated from the Okhotsk Sea Fucus evanescens Brown Alga. Bull Exp Biol Med. 2007; 143(6): 730-732. doi: 10.1007/s10517-007-0226-4


