Research Article
Comparative Clinical and Histomorphological Evaluation of the Efficacy of the Injectable Belotero® Soft (CPM-HA S) and Radiesse® (CaHa) Combination
1Dermatovenerology and Cosmetology Department, Pacific State Medical University of Health, Moscow, Russian Federation
2Department of Anatomic Pathology, Sechenov University, Moscow, Russian Federation
3Center for the treatment of complications of Professor Yutskovskayaʼs Clinic, Moscow, Russian Federation
*Corresponding author: Ya. A. Yutskovskaya, Dermatovenerology and Cosmetology Department, Pacific State Medical University of Health, Moscow, Russian Federation, E-mail: yutsk@mail.ru
Received: November 18, 2022 Accepted: December 05, 2022 Published: December 17, 2022
Citation: Yutskovskaya YA, Kogan EA, Koroleva AY. Comparative Clinical and Histomorphological Evaluation of the Efficacy of the Injectable Belotero® Soft (CPM-HA S) and Radiesse® (CaHa) Combination. Madridge J Dermatol Res. 2022; 6(1): 118-125. doi: 10.18689/mjdr-1000132
Copyright: © 2022 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: No single technology, filler, or neuromodulator can achieve all the results desired in treating the aging face. Combined aesthetic interventions have widespread application in clinical practice, but results are infrequently reported at scientific meetings and in the medical literature, as the area is difficult to study. Expert consensus supports a combination approach using multiple modalities in specific sequence for the safe and effective treatment of the aging face. Belotero® Soft (CPM-HA S, 20 mg/mL) and Radiesse® (CaHA) being dermal fillers are Ministry of Health of the RF and FDA-approved. Their effectiveness and safety have been demonstrated in a number of studies.
Methods: The study was designed as an open-label, prospective, pilot, randomized, comparative clinical study and immunohistochemical analysis in healthy female volunteers, 35–45 years of age with BMI < 21. Fourteen study subjects lived up with inclusion/exclusion criteria and were randomly assigned to experimental groups with a 1:1 who had one CPM-HA S injection all over the face and into integumentary tissues of the periauricular area (subdermal injection) at the first Visit (D01) and followed subdermal diluted CaHA (Group I) or standard CaHA (Group II) injection at the second stage (M01). The study was comprised of three visits. As a primary endpoint the clinical efficacy in terms of a lifting effect of the combined injections was assessed by independent experts using the Merz Aesthetics Scales™. The secondary efficacy endpoints included the Global Aesthetic Improvement Scale (GAIS) completed both the study subjects and the investigators, skin elasticity testing by means of cutometry and the histomorphological evaluation based on the type I collagen, the type III collagen and the elastic fibersʼ analysis using immunohistochemistry test (IHC) in terms of the compatibility of the combination of CPM-HA S and CaHA was assessed at Stage 2 (M01) and at Stage 3 (M06) compared to the baseline data. Mild staining corresponded to marker expression of 2 points, moderate - 4 points, and high - 6 points.
Results: Patient satisfaction evaluation based on the GAIS Scale showed no difference between study subjectʼs score and practitionerʼs score due to a small sample size and a short follow-up period. A comparative analysis of histology and immunohistochemistry of samples following the combined injection of CPM-HA S and CaHA reconstituted with normal saline as well as standard showed that these interventions had a relatively greater remodeling effect on the dermis. This conclusion is confirmed by intense type I - III collagen accumulation in the dermis (p<0,05) with the preserved 1:1 ratio and intense angiogenesis.
Injection of CPM-HA Sand standard CaHA had a greater expected remodeling effect on oneʼs skin compared to combined injection of CPM-HA Sand diluted CaHA. This is confirmed by intense type I – III collagen accumulation in the dermis (p<0,05) with the preserved 1:1 ratio and intense angiogenesis (p<0,05) that provides sustained blood supply to the tissues.
There is also evidence that simultaneous injection of CPM-HA Sand CaHA at the same level is safe, confirming that there are no histologic and immunohistologic signs of inflammation and disturbance of tissue trophicity.
Conclusions: The results of this study demonstrate that the injections of CPM-HA S and CaHA supply clinical and histomorphological improvement. According to our results, the injections of CPM-HA S and standard CaHA had a relatively greater remodeling effect on oneʼs skin compared to the injections of CPM-HA S and diluted CaHA.
Keywords: Dermal fillers, Belotero® Soft, CPM-HA, standard Radiesse®, CaHA, reconstituted Radiesse®, immunohistochemistry
Abbreviations
CaHA – Calcium hydroxyapatite;
CPM-HA S - Belotero® Soft;
FDA – The United States Food and Drug Administration;
GAIS – Global Aesthetic Improvement Scale;
ICH GCP – International Conference on Harmonisation of Good clinical practice;
IHC – Immunohistochemistry test;
VEGF – Vascular endothelial growth factor
Highlights: This article highlights the clinical advantage of the combined Belotero® Soft (CPM-HA S, 20 mg/mL) and Radiesse®(CaHA) reconstituted with normal saline compared to the use of the combined CPM-HA Sand standard CaHA in remodeling effect on oneʼs skin.
1. Introduction
Belotero® Soft (CPM-HA S, 20 mg/mL) and Radiesse®(CaHA) being dermal fillers are Ministry of Health of the RF and FDA-approved to smooth moderate to severe facial wrinkles and folds, such as nasolabial folds (the creases that extend from the corner of your nose to the corner of your mouth)[1,2]. Although not approved to treat the same wrinkle or fold at the same time, each can provide immediate correction for natural-looking results. By treating wrinkles and folds at different depths of the skin, the effects of these two injectable fillers can be complementary: CaHA is effective for immediately filling deeper wrinkles and folds and adding lift to areas experiencing volume loss for a more youthful appearance; CPM-HA Sis an ideal choice to fill small wrinkles and facial lines and for lip augmentation, for more of a finishing touch. Both injectables are known for being longer-lasting and for more natural-looking results.
Since their introduction, a large quantity of clinical data has been collected on the Belotero® and Radiesse® dermal fillers. CPM-HA S has been tested in four studies, either for facial treatments[3,4] or for treatment of atrophic scars[5] and proved its effectiveness and has a favorable and wellcharacterized safety profile.
Currently, CaHA is the only biodegradable filler that immediately restores lost volume and simultaneously stimulates the production of natural skin collagen to achieve long-term results[6]. It is a versatile injectable implant and a valuable tool for short- and long-term cosmetic and reconstructive treatments. This injectable is often used in conjunction with botox, other injectables, collagen stimulators and tightening devices. The product can be reconstituted to increase its versatility and minimize adverse events[7]. Its effectiveness and safety has been also demonstrated in a number of studies[8–10].
The possibility of CPM-HA Sand CaHA combined use to potentiate the lifting effect stirs interest, but at present, there are no reports of clinical studies of this combination in the treatment of skin laxity and wrinkles on the lower face, neck and décolleté. Combined aesthetic interventions have widespread application in clinical practice, but results are infrequently reported at scientific meetings and in the medical literature, as the area is difficult to study. Expert consensus supports a combination approach using multiple modalities in specific sequence for the safe and effective treatment of the aging face[11,12].
The primary aims of this study were to evaluate the clinical efficacy, tolerability, and patient satisfaction of the combination of CPM-HA Sand standard CaHA or CaHA reconstituted with normal saline (1:1) for the correction of age-related changes in the lower face, neck and décolleté. The secondary aims of the study were to assess the pathohistomorphology following the combination of CPMHA Sand standard CaHA or CaHA reconstituted with normal saline (1:1) and assess skin elasticity over time.
2. Material and Methods
2.1. Study design
This was an open-label, prospective, pilot, randomized, comparative clinical study and immunohistochemical analysis in healthy female volunteers, 35–45 years of age. Each study subject signed the informed consent form to participate in the study. Ethical approval for the study was obtained. The study was conducted in accordance with the ethical principles of the declaration of Helsinki, and ICH GCP December 2019 to July 2020.
2.2 Study participants
In total, 14 apparently healthy female volunteers aged 35 to 45years with body mass index (BMI) <21 who had indications for lower face, neck, and décolleté lifting, participated in the study. All study subjects completed the study.
During the study, the study subjects were administered CPM-HA Sand CaHA reconstituted with normal saline (1:1) or CPM-HA Sand standard CaHA.
14study subjects met inclusion/exclusion criteria and were randomly assigned to either experimental or control group with a 1:1 allocation as per a computer-generated randomization schedule into two groups: Group I (CPM-HA Sand CaHA reconstituted) – 7subjects and Group II (CPM-HA Sand standard CaHA) – 7subjects.Subjects were followed up for 5 months.
Each subject had a case report form (CRF) which included information on the date and frequency of procedures, gender, age, area of product injection, biopsy area marking, procedure tolerability assessment score, and assessment of any side effects.
2.3. Study treatments
The study was comprised of three visits.
Injections were carried out under topical anaesthesia with the Acriol Pro cream. The cream was applied for 30-45 minutes prior to the procedure. The injection site was disinfected with chlorhexidine bigluconate 0,05%.
Volunteers had intradermal CPM-HA S injections all over the face and into integumentary tissues of the periauricular area using a microdroplet technique at Stage 1 (D01). The 30G 13 mm needle is inserted at an angle of 35–40 degrees into the dermis.
CaHA was injected subdermally in a standard form using a vector technique, reconstituted with 0.9% physiological saline, at the dermo-hypodermal junction. Drug-to-saline ratio 1:1, 3 ml of the product for 3 ml of normal saline, respectively. Areas to be treated using the product — mid and lower face, the periauricular area at Stage 2 (M01). Subdermal injections are made using a linear retrograde technique along skin tension lines (Langerʼs lines). A full length of a 22G x 50 mm cannula was inserted into the skin at the least angle to the skin surface.
Punch biopsy from the treated periauricular area was performed at all visits (Figure 1).
2.4. Study endpoints
2.4.1. Efficacy: As a primary endpoint the clinical efficacy in terms of a lifting effect of the combined injections of CPM-HA Sand CaHA and CPM-HA Sand CaHA reconstituted with normal saline was assessed at Stage 2 (M01) and at Stage 3 (M06) compared to the baseline by independent experts using the Merz Aesthetics Scales™. The secondary efficacy endpoints included the comparison of the following parameters between the treatment groups:
• patient satisfaction evaluation based on the Global Aesthetic Improvement Scale(GAIS) completed both the study subjects and the investigators,
• skin elasticity testing by means of cutometry at Stage 2 (M01) and at Stage 3 (M06) compared to baseline,
• histomorphological evaluation based on the type I collagen, the type III collagen and the elastic fibersʼ analysis using immunohistochemistry test (IHC).
For the IHC test the patient biopsies samples were fixed in 10% neutral formalin and paraffin-embedded using a standard technique. Series of paraffin sections 4 µm each were prepared and stained using hematoxylin and eosin, Van Giesonʼs and Weigertʼs elastic stain. ICH reactions were carried out by antigen retrieval in a retriever according to the standard protocol. Monoclonal anti-collagen I antibodies (murine-derived monoclonal antibodies produced by Santa Cruz (sc-293182), clone 3G3, dilution 1:100), Collagen III (murine-derived monoclonal antibodies produced by Santa Cruz sc-166316), clone B-4, dilution 1:100), VEGF (rabbit polyclonal antibodies produced by Abcam (ab-183100), dilution 1:100) were used. Reactions were performed with positive and negative controls in the absence of primary antibodies.
A comparative analysis of type I and type III collagen, elastin and other histomorphologic characteristics (presence of an inflammatory reaction, angiogenesis) included 3 zones of the sample (sub-epithelial, superficial and deep dermal layers). Staining intensity (on a point-based scale) of samples for histology and immunohistochemistry was assessed using a semi-quantitative method. Mild staining corresponded to marker expression of 2 points, moderate - 4 points, and high - 6 points.
2.4.2. Safety: Tolerability of the procedures was evaluated using a 4-pointsafety scale: 0 (no reaction), 1 (mild), 2 (moderate), 3 (severe:pain, edema, erythema, bruising). Adverse events were assessed and recorded at each of three visits.
2.5. Statistical analysis
The study was planned as a pilot one and the sample size was not determined. Descriptive statistics are given for each studied parameter. Mann-Whitney test was used to analyze the between-group differences for the study efficacy endpoints. Within the group comparison of the study efficacy endpoints was conducted using Wilcoxon test.
The statistical analysis was performed with the Stata application software (StataCorp, USA) version 14[28].
3. Results
3.1. Study population
Fourteen screened healthy female volunteers met the inclusion/exclusion criteria and were randomized 1:1 into two groups. All study subjects completed the study without significant protocol deviations. Safety population includes all the patients, received at least one dose of study medications (Figure 2).
All the enrolled subjects were divided into 2 groups:
• Group I (7 patients who received CPM-HA Sand CaHA reconstituted with normal saline in a 1:1 ratio), mean age 40,6±5,83 years.
• Group II (7 patients who received CPM-HA Sand standard CaHA), mean age 38,3±4,61 years (p=0,4432, Mann-Whitney test).
Group allocation was based on indications for combined CPM-HA S+ CaHA, 1:1 dilution, or CPM-HA S+ standard CaHA.
Available demographic and clinical data show no difference between the two groups before treatment; the two groups are comparable in terms of the tested parameters.
3.2. Efficacy outcomes
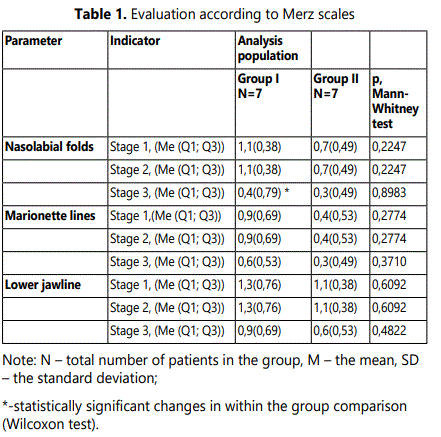
3.2.1. Evaluation according to the Merz scale: Evaluation using a validated Merz scale was based on the parameters of nasolabial folds, marionette lines and lower jawline changes. In Group I statistically significant (p=0,0082, Wilcoxon test) changes over time according to the Merz scale were observed for nasolabial folds (Table 1). In Group II a trend towards changes was observed. Marionette linesʼ evaluation showed a trend towards changes in both groups. A bigger sample size is required to obtain a statistically significant result. Lower jawline also showed a trend towards changes in both groups. As to results a bigger sample size is required to obtain statistically significant results.
3.2.2. Patient satisfaction evaluation using GAIS Scale:
Study subjectʼs satisfaction evaluation based on the GAIS Scale showed no difference between subjectʼs score and practitionerʼs score due to a small sample size and a short follow-up period (evaluation was performed twice, after Stage 1 and Stage 2). A trend towards increased satisfaction with the results of the procedures reported both by the subject and the practitioner was observed following Stage 2. The changes of the study practitionerʼs score in thegroups were quantified as follows: in the Group I at the Stage 2 (M01)– 2,1 ±0,38 and at the Stage 3 (M06)– 2,3 ±0,49 points (p=0,3359, Wilcoxon test);and the Group II at the Stage 2 (M01)–1,9 ±0,38 and at the Stage 3 (M06)– 2,3 ±0,49 points (p=0,0781, Wilcoxon test).
The changes of the study subjectʼs score in the Group I was 2,3 ±0,95 at the Stage 2 (M01)and2,6 ±0,79 pointsat the Stage 3 (M06) (p=0,1723, Wilcoxon test);and in the Group II they were 1,7 ±0,76 at the Stage 2 (M01)and2,6 ±0,98 pointsat the Stage 3 (M06)(p=0,0781, Wilcoxon test). 3.2.3. Evaluation of skin elasticity: Skin elasticity testing by means of cutometry showed statistically significant (p<0,05) changes over time in the buccal area and the T-zone in the Group I. The Group II showed a trend towards elasticity changes in the buccal area and the T-zone.
The changes of the skin elasticityin the buccal area in thegroups were quantified as follows: in the Group I at the Stage 1 (D01) – 56,1 ± 10,93, at the Stage 2 (M01)–61,0 ± 14,08(p=0,5390, Wilcoxon test) and at the Stage 3 (M06)– 72,6 ± 10,06(p=0,0464, Wilcoxon test);and the Group II at the Stage 1 (D01) – 57,7 ± 5,47, at the Stage 2 (M01)–54,3 ± 17,90(p=0,6979, Wilcoxon test) and at the Stage 3 (M06)– 71,9 ± 13,79 (p=0,1083, Wilcoxon test). There were no statistically significant changes between the groups.
The changes of the skin elasticityin the T-zone in the Group I was 52,0 ± 25,89 at the Stage 1 (D01), 73,1 ± 10,51 at the Stage 2 (M01) (p=0,1610, Wilcoxon test) and 72,9 ± 15,23 and at the Stage 3 (M06) (p=0,0248, Wilcoxon test); and in the Group II they were 53,7 ± 23,85; 60,9 ± 22,32 (p=0,5095, Wilcoxon test) and 63,4 ± 16,30 (p=0,39980, Wilcoxon test) respectively. There were no statistically significant changes between the groups.
3.2.4. Histomorphological evaluation of the efficacy
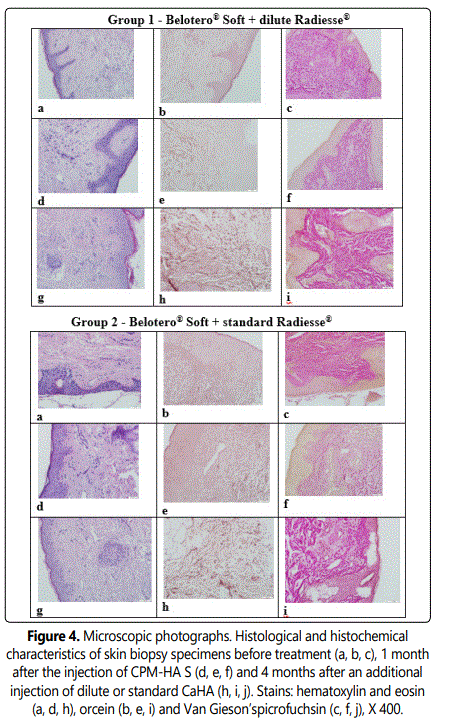
3.2.4.1. Comparative histology and histochemistry changes: Comparative analysis of skin biopsy histomorphology prior to, 1 month and 4 months after the injection of the combination of CPM-HA Sand CaHA reconstituted with normal saline or standard CaHA was performed. Fourteen punch skin biopsy specimens from the retroauricular area of all 14 study subjects were analyzed.
At the baseline in both groups elastic stain showed no elastic fibers in the sub-epithelial and superficial layers of the dermis; instead, these were observed as individual fibers within the perivascular and periglandular tissues in the deep dermal layers (0 points).
One month after the injection of CPM-HA Shematoxylin and eosin staining and Van Giesonʼs staining showed the dermis extracellular matrix accumulation with deposition of collagen bundles. Few lymphocytes show somewhat uneven distribution in the field of view and are typically located in perivascular spaces within the walls of small blood vessels as well as close to some hair follicles, and these are limited in number, which is not indicative of an inflammatory reaction at the site of filler implantation.
There are slightly more vessels (small veins, venules, capillaries, and arterioles) in the dermis compared to Stage 1 (p<0,05). They show lumen enlargement and relatively even distribution in the sample. These findings may also be considered morphological signs of active connective tissue remodeling at the site of dermal filler injection (2–4 points). Elastic stain revealed elastic fibers mainly in the superficial and deep dermal layers located around vessels and skin appendages (2–4 points), which is significantly higher compared to baseline (p<0,05).
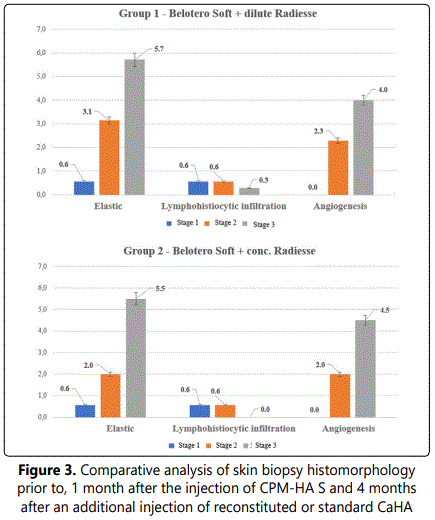
Four months after the injection of CaHA reconstituted with normal saline morphologic changes in the skin were different from those observed at Stage 2 in terms of angiogenesis and accumulation of extracellular matrix and elastic fibers. With hematoxylin and eosin staining and Van Giesonʼs staining, the dermis shows increased extracellular matrix accumulation with deposition of collagen bundles.
There are slightly more vessels (small veins, venules, capillaries and arterioles) in the dermis (4 points) compared to Stage 2 (p<0.05), showing lumen enlargement and relatively even distribution in the sample.
Elastic stain revealed an increase in the number of elastic fibers (6 points) mainly in the superficial and deep dermal layers located around vessels and skin appendages (p<0,05). The same changes showed 4 months after the injection of standard CaHA. The results are shown in Figures 3-4.
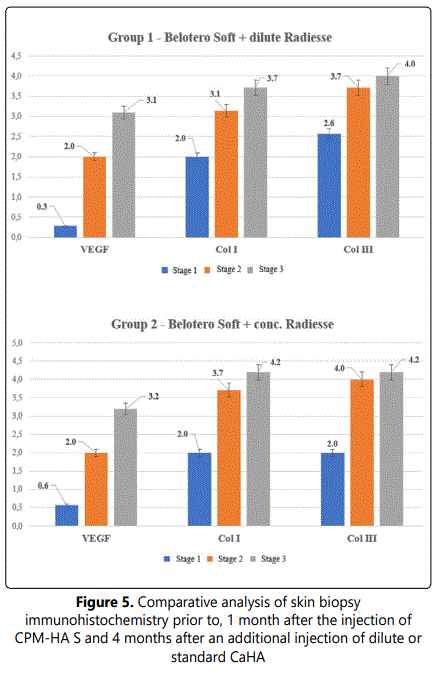
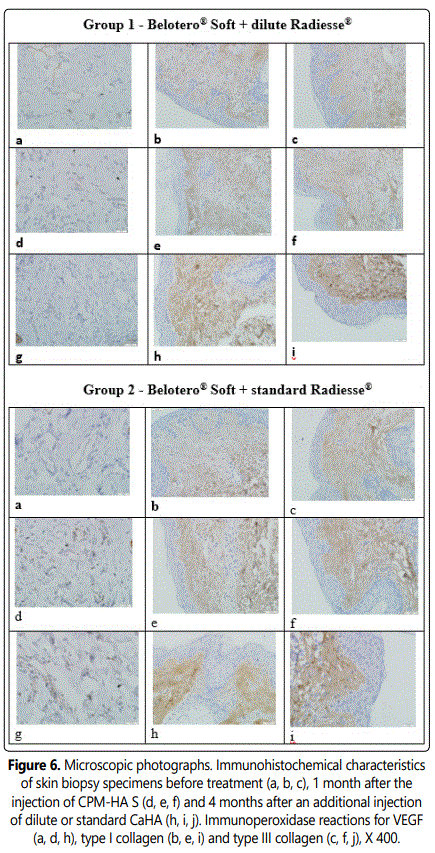
3.2.4.2. Comparative immunohistochemistry changes: At stage 1 before products injection type I collagen is observed as thin interlacing fibers in the dermis found mainly in the sub-epithelial and superficial layers. Type III collagen is observed as thicker interlacing fibers of similar location in the dermis and in an amount similar to that of type I collagen. Collagen I/III Ratio = 1.0. VEGF is observed as cytoplasmic staining in isolated vascular endothelial cells.
A comparative analysis of immunohistochemical characteristics of the tissues following the combined injection of CPM-HA S and CaHA reconstituted with normal saline as well as standard CaHA showed that these interventions had a relatively greater remodeling effect on the dermis. This conclusion is confirmed by intense type I - III collagen accumulation in the dermis (p<0,05) with the preserved 1:1 ratio and intense angiogenesis that provides sustained blood supply to skin tissues.
The results are shown in Figures 5-6.
3.3. Safety outcomes
Erythema, ecchymoses, and petechiae at the injection site were reported in the study, as well as occasional hematomas, which spontaneously resolved within a short time after injection. During the 5-month follow up, no further adverse events were reported.
Reaction evaluation showed no statistically significant changes following the procedures.
4. Discussion
The results of this study demonstrate that the injections of the injectable CPM-HA S and CaHA combination supply clinical and histomorphological improvement. Clinical efficacy evaluation according to a Merz scale showed a trend towards positive changes in both study groups. Although bigger sample size is required to obtain statistically significant results.
Patient satisfaction evaluation based on the GAIS Scale showed no difference between study subjectʼs score and practitionerʼs score due to a small sample size and a short follow-up period.
A comparative analysis of histomorphological changes in tissues showed that the products cause remodeling in the skin, with the combined use of CPM-HA S and CaHA reconstituted with normal saline and standard CaHA having a considerable impact on aging skin remodeling resulting in trend towards ECM accumulation and accumulation of elastic fibers (p<0,05).
The previous studies reported an increase in collagen type III with a gradual equalizing of the ratio toward collagen type I[9,13]. This study showed an increase in the amount of both collagen type I and collagen type III with the same ratio. According to our results, the use of CPM-HA S and standard CaHA had a relatively greater remodeling effect on oneʼs skin compared to the use of CPM-HA S and CaHA reconstituted with normal saline. This is confirmed by intense elastic fiber formation (p<0.05) and type I - III collagen accumulation in the dermis (p<0.05) with the preserved 1:1 ratio. However, the differences between histologic and immunohistologic parameters evaluated were not statistically significant (p>0,05).
We assume the fact is due to introduction of the same volumes of CaHA (standard) and CaHA diluted with saline (1:1), the number of calcium hydroxyapatite microspheres of CaHA (standard) having a stimulating effect on fibroblasts was more in the ratio 1:2.
The absence of histological and immunohistochemical signs of inflammation in the tissues confirms that the stimulation processes as a result of the combined injections of CPM-HA S and CaHA diluted with saline (1:1) as well as CPM-HA S and CaHA (standard) are controlled and safe.
5. Conclusion
In conclusion, the data from this randomized, open, prospective, pilot, clinical study correspond to the aim that allows the researchers to fulfill their tasks: to display clinical efficacy in terms of a lifting effect and patient satisfaction of the combined injections of CPM-HA S and CaHA and CPM-HA S and CaHA reconstituted with normal saline. A comparative histomorphological analysis following the combined injection showed that the use of CPM-HA S and standard CaHA had a relatively greater remodeling effect on oneʼs skin compared to the use of CPM-HA S and CaHA reconstituted with normal saline.
6. Other Information
Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany, provided financial support for the manuscript preparation. None of the authors received any financial support for the writing of this manuscript. All listed authors approved the article for submission.
7. CRediT Authorship Contribution Statement
Ya.A. Yutskovskaya- Conceptualization, Methodology, Project administration, Writing - original draft;
E.A. Kogan, A.Yu. Koroleva - Investigation, Recourses; Writing - review & editing.
8. Conflict of Interest
Dr. Ya. A. Yutskovskaya is a consultant and researcher for Merz Pharmaceuticals. Dr. E.A. Kogan, and Dr. A. Yu. Koroleva have no conflicts of interest to declare in this work.
References
- Radiesse, Merz Pharmaceuticals. Federal Service for Surveillance in Healthcare. Accessed: 16.06.2021
- BELOTERO Soft, Merz Pharmaceuticals. Federal Service for Surveillance in Healthcare.
- Prasetyo AD, Prager W, Rubin MG, Moretti EA, Nikolis A. Hyaluronic acid fillers with cohesive polydensified matrix for soft-tissue augmentation and rejuvenation: a literature review. Clin Cosmet Investig Dermatol. 2016; 9: 257-280. doi: 10.2147/CCID.S106551
- Pavicic T, Few JW, Huber-Vorländer J. A novel, multistep, combination facial rejuvenation procedure for treatment of the whole face with incobotulinumtoxin A, and two dermal fillers- calcium hydroxylapatite and a monophasic, polydensified hyaluronic acid filler. J Drugs Dermatol. 2013; 12(9): 978-984.
- Hasson A, Romero WA. Treatment of facial atrophic scars with Esthélis, a hyaluronic acid filler with polydense cohesive matrix (CPM). J Drugs Dermatol. 2010; 9(12): 1507-1509.
- Loghem JV, Yutskovskaya YA, Philip Werschler W. Calcium hydroxylapatite: over a decade of clinical experience. J Clin Aesthet Dermatol. 2015; 8(1): 38-49.
- Eviatar J, Lo C, Kirszrot J Radiesse. Advanced Techniques and Applications for a Unique and Versatile Implant. Plastic and Reconstructive Surgery. 2015; 136: 164-170. doi: 10.1097/PRS.0000000000001825
- Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008; 34 Suppl 1: S64-67. doi: 10.1111/j.1524-4725.2008.34245.x
- Yutskovskaya YA, Kogan EA. Improved Neocollagenesis and Skin Mechanical Properties After Injection of Diluted Calcium Hydroxylapatite in the Neck and Décolletage: A Pilot Study. J Drugs Dermatol. 2017; 16(1): 68-74.
- Yutskovskaya Y, Kogan E, Leshunov E. A randomized, split-face, histomorphologic study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J Drugs Dermatol. 2014; 13(9): 1047-1052.
- Carruthers J, Burgess C, Day D, Fabi SG, Goldie K, Kerscher M, et al. Consensus Recommendations for Combined Aesthetic Interventions in the Face Using Botulinum Toxin, Fillers, and Energy-Based Devices. Dermatologic surgery. 2016; 42(5): 586-97. doi: 10.1097/DSS.0000000000000754
- Fabi S, Pavicic T, Braz A, Green JB, Seo K, Loghem JAJ van. Combined aesthetic interventions for prevention of facial ageing, and restoration and beautification of face and body. Clin Cosmet Investig Dermatol. 2017; 10: 423-429. doi: 10.2147/CCID.S144282
- Suh D.H. et al. Intense focused ultrasound tightening in Asian skin: clinical and pathologic results. Dermatol Surg. 2011; 37(11): 1595-1602. doi: 10.1111/j.1524-4725.2011.02094.x
- Bhagra S, Ganju SA, Sood A, Guleria RC, Kanga A. Microsporum gypseum dermatophytosis in patient of acquired immunodeficiency syndrome: A rare case report. Indian Journal of Medical Microbiology. 2013; 31(3): 295-298. doi:10.4103/0255-0857.115656
- Choudhary SV, Bisati S, Koley S. Efficacy and safety of terbinafine hydrochloride 1% cream vs. sertaconazole nitrate 2% cream in tinea corporis and tinea cruris: A comparative therapeutic trial. Indian J Dermatol. 2013; 58(6): 457-460. doi: 10.4103/0019-5154.119958
- Dave P, Mahendra R, Pal M. Growing significance of Microsporum canis in tinea of animal handlers. Journal of Environmental and Occupational Science. 2014; 3: 193-195. doi: 10.5455/jeos.20141009114534
- Ely JW, Rosenfeld S, Stone MS. Diagnosis and management of Tinea infections. Am Fam Physician. 2014; 90(10): 702-711.
- Niranjan HP, Padmaja N, Priyanka BV. Study of onychomycosis at a tertiary care hospital in South India. Journal of Evolutionary Medicine and Dental Science. 2012; 1(5): 823-829.
- Pal M. Efficacy of Narayan stain for morphological studies of mould, yeasts and algae. Revista Iberoamericana de Micologia. 2004; 21(4): 219.
- Pal M. Veterinary and Medical Mycology. 2007. First Edition, Indian Council of Agricultural Research, New Delhi, India.
- Peerapur BV, Inamdar AC, Pushpa PV, Srikant B. Clinicomycological study of dermatophytosis in Bijapur. Indian Journal of Med Microbiology. 2004; 22(4): 273-274.
- Ramaraj V, Vijayaraman RS, Rangrajan S, Kindo AJ. Incidence and prevalence of dermatophytosis in and around Chennai, Tamilnadu, India. International Journal of Research in Medical Sciences. 2016; 4: 695-700. doi: 10.18203/2320-6012.ijrms20160483
- Romano C, Massai L, Gallo A, Fimiani M. Microsporum gypseum infection in the Siena area in 2005-2006. Mycoses. 2009; 52: 67-71. doi: 10.1111/j.1439-0507.2008.01543.x
- Weitzman I, Summerbell R. The dermatophytes. Clinical Microbiology. 1995; 8: 240-259. doi: 10.1128/CMR.8.2.240



