Research Article
Treatment of Leprosy with Ofloxacin - containing Combined Drug Regimens in Vietnam
1National Hospital of Dermato-Venereology, Hanoi and Hanoi Medical University, Vietnam
2World Health Organization & Global Leprosy Programme
3Hospital of Dermato-Venereology, Khanh Hoa province, Vietnam
4Hồ Chí Minh City Hospital of Dermato Venereology, Ho Chi Minh City, Vietnam
*Corresponding author: Tran Hau Khang, National Hospital of Dermato-Venereology, Hanoi Medical University, Hanoi, Vietnam, E-mail: khangquocduc@fpt.vn
Received: March 26, 2019 Accepted: May 10, 2019 Published: May 20, 2019
Citation: Khang TH, Panikar V, Lanh PH, Minh TT, Hai PH. Treatment of Leprosy with Ofloxacin - containing Combined Drug Regimens in Vietnam. Madridge J Dermatol Res. 2019; 4(1): 96-99. doi: 10.18689/mjdr-1000125
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Development and implementation of multidrug therapy (MDT) were the most important achievements in the history of leprosy control. The regimens have proved to be highly effective and well tolerated by the patients. However, the duration of the treatment is too long, especially the duration for multibacillary leprosy therapy which causes serious operational difficulties in certain areas.
Objectives: The main objective of the study was to evaluate the therapeutic efficacy in terms of the rate of relapse among leprosy patients of two new regimens containing ofloxacin and standard WHO/MDT applied for one year in comparison with the standard two year duration WHO/MDT regimen. In addition, the feasibility of and tolerance to the new regimens was measured.
Methodology: The comparison of different regimens was conducted in a double blind field trial with 3 centers carrying it out through a standard protocol.
Result: The trial started in 1992. A total of 349 active untreated leprosy patients (159 PB and 190 MB) were allocated for the study. Among them, 322 successfully completed the different regimens. After release from treatment (RFT), all of them were followed-up for 15 years. The total duration of follow up of the patients was 4,890 persons-years.
Moderate to marked clinical and bacterial improvement has been observed in 100 percentage of patients treated with ofloxacin-containing regimens. However, relapse rate was very high among patients treated with regimen C (Ofloxacin and Rifampicin daily for only one month). All the 6 regimens were well tolerated. Only 6(1.7%) patients was removed from the trial due to severe complication with erythroderma.
Keywords: Leprosy; Multidrug therapy; Paucibacillary; Multibacillary.
Introduction
Development and implementation of multidrug therapy (MDT) were the most important achievements in the history of leprosy control. The regimens have proved to be highly effective and well tolerated by the patients. However, the duration of the treatment is too long, especially the duration for multibacillary leprosy therapy which causes serious operational difficulties in certain areas [1-3]. Therefore, it will be of great benefit to leprosy control if a shorter duration MDT regimen can be developed by strengthening the regimen with a powerful companion bactericidal drug to rifampicin.
Over many years, many important trials have been implemented in identifying highly effective drugs and designing multidrug regimens for treatment of the disease. According to the results from both in mice and in clinical trials, ofloxacin, a new fluoroquinolones has proved the most active compound against M. leprae. More importantly, this drug provides an effective tool with which to deal with the rifampicin resistant organisms [4-6]. Thus, the addition of ofloxacin to multidrug regimens may make a considerable shortening of the required duration of MDT possible. It is very important to determine whether a regimen consisting primarily of rifampicin and/or ofloxacin for treatment of leprosy patients and administered for a shorter duration.
In regard to this reason, Vietnam was selected as a center to perform the WHO clinical trial. The objective of the trial was to determine in both MB and PB leprosy patients the therapeutic efficacy of six new multidrug regimens containing ofloxacin in comparison with the standard WHO/MDT. The feasibility and tolerance to the new regimens were also measured.
Materials and Methods
Patients
The patients recruited into the trial were selected from provinces with experiences in implementing the WHO/MDT.
The patients were diagnosed and classified according to bacteriology and clinical manifestations. Diagnosis of leprosy was made if a person has one of the following conditions: (a) definite loss of sensation in a pale or reddish skin patch or (b) if there is enlarged and thickened peripheral nerve with loss of sensation and/or weakness of the muscles supplied by that nerve or (c) the presence of acid-fast bacilli in a split skin smear. Classification was decided by the number of skin lesions: Paucibacillary (PB) if cases have up to five skin lesions and Multibacillary (MB) if cases have six or more skin lesions. All patients with positive skin smears were classified as MB whatever the number of skin lesion [2,3].
Treatment
Both MB and PB patients were randomly allocated to 6 regimens from A to F:
For MB:
- Regimen A: MDT/WHO: for one year.
- Regimen B: MDT/WHO for one year plus Ofloxacin 400 mg daily for only the 1st month.
- Regimen C: Rifampicin 600 mg daily and Ofloxacin 400 mg daily for only one month.
- Regimen D: MDT/MB/WHO for 2 years.
For PB:
- Regimen E: MDT/PB/WHO for 6 months.
- Regimen F: Rifampicin 600 mg daily, Ofloxacin 400 mg daily for only one month.
These were done through a system of re-coded packs by WHO. The comparison of different regimens was conducted in a double blind field trial according to the WHO procedures.
Examination procedures
All patients were examined and followed-up by dermatologists.
For the first month, the patients were admitted as inpatients in leprosy wards/hospitals/centers (NIDV, Hanoi, Nha Trang and Ho Chi Minh City) and examined daily. During 5 consecutive months of treatment for PB and 23 consecutive months for MB, the patients were seen monthly at their home for monitoring and following-up the intake of drugs/placebo, as well as any complications.
After completion of the scheduled therapies, the patients were examined once every 6 months for clinical changes and any evidence of relapse for at least 15 years.
The resulting data was analyzed by using statistical software (SPSS version 13, SPSS Inc). Statistical difference was assumed at p less than 0,05.
All patients agreed to participate in the trial with their signatures in the consent forms. Moreover, their names were coded in the files, records of the trial.
Results
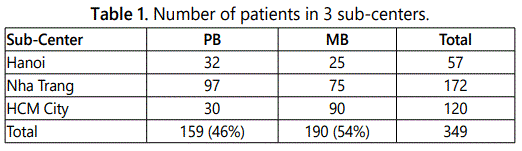
159 PB and 190 MB leprosy cases were recruited into the trial. All patients were allocated into 3 sub-centers: Hanoi, Khanh Hoa and Ho Chi Minh City. The number of patients of each sub-center was shown in table 1.
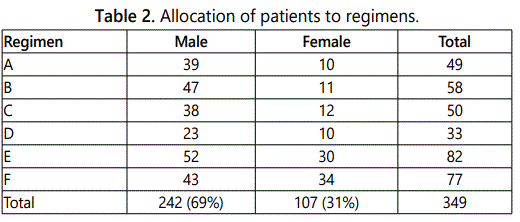
The patients were allocated randomly to 6 regimens A, B, C, D, E, and F (given in table 2).
Clinical responses
PB patients: Definite improvement occurred in 50% cases by the day 28 and 100% of patients showed marked improvement by the 6th month. All lesions as tuberculoids, infiltrations had resolved remarkably. The response was not different significantly among the patients treated with regimen E and regimen F.
MB patients: Only 35 cases (18%) had definite improvement by day 28, 80% showed either definite or marked improvement at the end of the month 12 and 100% patients presented marked improvement by the month 24. The clinical responses did not differ significantly among patients treated with the different regimens (P>0.05).
Changes of Bacterial Index (BI) in skin smears: The mean BI among the MB patients of 4 regimens appeared to have declined by an average of 0.8+ per year.
Lepra reactions
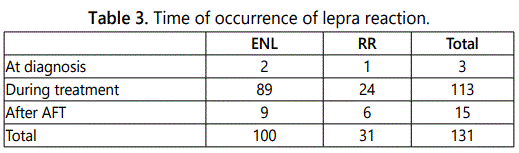
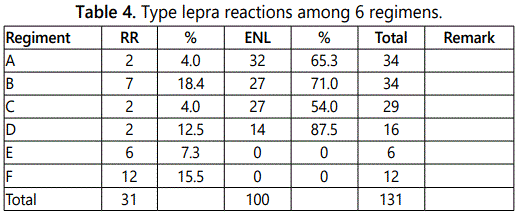
During treatment, 131 patients developed lepra reactions, among them 31 had reversed reaction (RR) and 100 with erythema nodosum leprosum (ENL). Table 3 shows the type of reactions in regimens. The table 4 shows the time of their occurrence in relation to the treatment. 15 patients developed post-treatment reactions during surveillance (6 had RR and 9 ENL). Reactions occurred up to 4 years after release from treatment (RFT) and all reactions were successfully treated with steroid as recommended.
Side effects: During 1st week of treatment, 12 (3.5%) patients developed light side effects: itching, vomitting and these were generally not serious and were not related to the treatment. These problems disappeared within 2-3 days. However, 6 patients (1.7%) developed erythroderma. Because of serious problems, these patients were removed from the trial (Table 5).
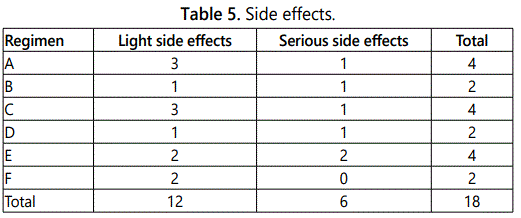
Relapse: Of 349 patients, 322 successfully completed the different regimens. However, 27 cases were removed from the trial, among them 7 died, 14 migrated and 6 suffered from severe side effects. 322 patients were eligible for 15 years of follow-up. From 1998–2008, 13 patients were detected as relapse. Among them, there were 7 from regimen C, 4 from regimens A, B, D and 2 from regimens E and F. From 2009 until now, no relapse was found.
Discussion
Clinical improvement
159 PB cases were randomly allocated to regimens E and F. All patients were strictly assessed with dermatologists. At the end of the first month of treatment, only 50% of cases showed clinical improvement. However, after finishing 6 months of therapy, all skin lesions, such as: tuberculoids, infiltration disappeared and no deformity was detected. 100% of patients demonstrated definite improvement. More interesting, the clinical responses did not differ significantly among 2 groups treated with regimens E and F.
Among 190 MB cases allocated to 4 regimens from A to D, in the first month of treatment, the clinical response was very slowly. However, after finishing 12 months of treatment, 80% of cases showed either definite or considerable improvement and 100% of patients presented marked improvement by the month 24. The clinical responses did not differ significantly among 4 groups of regimens A,B,C,D. The result was similar to other reported in the literatures [6,7].
This result can be explained that ofloxacin with the powerful bactericidal drug in treatment of leprosy for only one month [8-10].
BI changes: The decline of Bacterial Index (BI) was in accordance with the clinical response. The mean of BI among MB patients of all regimens appeared to have decreased by an average of 0.8 plus per year. The difference of BI changes was significantly differ among 4 regimens (P<0,05). The BI of patients treated with regimen C was still positive at the end of 60 months of the trial. This seemed that a short duration for treatment of one month with Rifampicin and Ofloxacin was not enough for killing completely M. leprae.
Lepra reactions: During treatment, 131 patients developed lepra reactions, among them 31 had reversal reaction (RR) and 100 with erythema nodosum leprosum (ENL). Table 4 showed the type of reactions in each regimen. The proportion of lepra reaction was not different among the different regimens. Moreover, the result was not significantly different in comparison with the result of the previous researches [8,11].
However, 5 patients treated with regimen C had very severe reaction. The reaction accompanied by fever (39°C–40°C) and acute neuritis. This seemed to be of that the shortage of Clofazimine in this regimen cannot control inflammation of the reaction [12-14]. Fortunately, all symptoms were rapidly controlled by the administration of corticosteroid.
Side effects of drugs: During the first week of treatment, 12 (3,5%) patients developed light side effects with itching, erythema, vomitting. These symptoms were not generally serious and not related to the treatment. The problems disappeared within 2-3 days. However, 6 patients (1,7%) developed erythroderma. Because of this serious problem, these patients were removed from the trial. The frequency of adverse drug was not significantly greater than that stated in the literature [13,15].
Relapse: Relapse is the most important indicator for evaluation of the effectiveness of the trial. The diagnosis of relapse leprosy was based on the following evidences: appearance of new lesions, and/or significant increase in bacterial index over the previous values. Among 349 patients, 322 successfully completed the different regimens, 13 died, 14 migrated. All patients after release from treatment were followed-up for 15 years. By the end of 2008, the total of relapse detected was 13. More interesting, no further relapse has been detected since 2009.
The number of relapse was highest in regimen C. After 5 years of follow-up, 7 cases were diagnosed as relapse in the group treated with regimen C. This unacceptably high relapse rate indicated that 4 weeks of treatment of daily Rifarmpicin and Ofloxacin was unable to reduce/to kill the number of M. leprae.
Conclusion
Ofloxacin has proved to be a powerful bactericidal drug. 6 regimens were well tolerated. Only 6 patients (1.7%) were removed from the trial due to severe complication with erythroderma. All leprosy patients treated with ofloxacincontaining regimens showed moderate to marked clinical and bacterial improvements. However, relapse rate was very high among patient treated with regimen C of Rifamficin combined with Ofloxacin for only one month.
Acknowledgments
This trial was financially supported by the World Heath Organization (WHO). We thank Dr. P. Pannikar, Leprosy Expert from India for his technical help during the research.
Conflict of Interest
The authors declare no conflict of interest.
References
- World Health Organization. WHO technical report series No. 847. WHO Geneva, Switzerland 1994.
- World Health Organization. MDT for leprosy. WHO Geneva, Special report. 1982.
- Franco Raul J, Marcia B, Isabel S, VirgÃnia Y. Bactericidal activity of Ofloxacin against Mycobacterium Leprae. 14th International Leprosy Congress. Florida. 1993: 13-14.
- Ganapati R, Verma SN. Ofloxacin-Rifampicin trials in multibacillary leprosy. Preliminary observations 14th International Leprosy Congress. Florida. 1993: 8-9.
- Ji B, Grosset J. Ofloxacin for the treatment of leprosy. Acta Leprol. 1991; 7(4): 321-326.
- Franzblau SG, White KE. Comparative in vitro activities of 20 fruoloquinolones against Mycobacterium leprae. Antimicrob Agents Chemother. 1990; 34(2): 229-231.
- Ji B, Grosset JJ. Recent advances in the chemotherapy of leprosy. Lepr Rev. 1990; 61(4): 313-329.
- Shepard CC. A brief review of experiences with short-term clinical trials monitored by mouse-foot-pad-inoculation. Lepr Rev. 1981; 52(4): 299-308.
- Rao PN, Jain S. Newer management options in leprosy. Indian J Dermatol. 2013; 58(1): 6-11. doi: 10.4103/0019-5154.105274
- World Heath Organization. WHO guidelines for the management of severe ENL reaction. Use of Clifazimine for treating Erythema Nodosum Leprosum reaction in leprosy. 2000.
- Fajardo TT, Villahermosa LG, Cruz EC, et al. A clinical trial of pefloxacin and ofloxacin in lepromatous leprosy. Lepr Rev. 2004; 75(4): 389-397.
- Halkin H. Adverse effects of the fluoroquinolones. Rev Infect Dis. 1988; 10: S258-S261.
- Costa PDSS, Fraga LR, Kowalski TW, Daxbacher ELR, Schuler-Faccini L, Vianna FSL. Erythema Nodosum Leprosum: update and challenges on the treatment of a neglected condition. Acta Trop. 2018; 183: 134-141.
- Grosse JH, Guelpa-Lauras CC, Perani EG, Beoletto C. Activity of Ofloxacine against Mycobacterium leprae in the mouse. Int J Lepr Other Mycobact Dis. 1998; 56(2): 259-264.



