Research Article
Efficacy Evaluation of a Novel Home-Used Device Based on a Multiple Stimulation Technology on Hair Growth in Patients with Androgenetic Alopecia
1Dermatology, Edith Wolfson Medical Center, Holon, Israel
2Dermatology, G Marconi University of Rome, Rome, Italy
3Dermatology, Pacific State Medical University, Vladivostok, Russia
*Corresponding author: Yana Yutskovskaya, MD, Dermatology, Pacific State Medical University, Vladivostok, Russia, E-mail: yutsk@mail.ru
Received: July 3, 2018 Accepted: August 26, 2018 Published: August 31, 2018
Citation: Landau M, Lotti T, Yutskovskaya Y. Efficacy Evaluation of a Novel Homeused Device Based on a Multiple Stimulation Technology on Hair Growth in Patients with Androgenetic Alopecia. Madridge J Dermatol Res. 2018; 3(2): 70-74. doi: 10.18689/mjdr-1000116
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Androgenetic Alopecia (AGA) is a common hair disorder, affecting both men and women worldwide. Few medications and a single device have been approved by FDA to treat AGA with variable efficacy. Pilogics Hairegen device is a novel homeused appliance, combining mechanical, electrical and biochemical stimulations. It has been released to the market in Israel in 2016. We summarize the preliminary results in 345 patients with AGA who used the device for 20 weeks in average.
Methods: A group of patients diagnosed with AGA used Pilogics Hairegen device for 20 (14-25) weeks at home. Treatment protocol included daily use of the device according to the protocol. Adherence to the protocol was recorded by specific monitoring software installed on a device. Only patients who adhered with the treatment protocol were included in data analysis. Hair count was performed using TrichoScan before the initiation and at the end of the treatment.
Results: A total of 289 subjects (243 males and 46 females) were included in the final data analysis. Tricho Scan results in these patients demonstrated 23.2% increase in total hair count and 21.1% increase in terminal hair count. Higher efficacy was noticed at more advanced ages. No adverse effects were reported.
Conclusions: Pilogics Hairegen device induces positive effect on the hair counts in men and women suffering from AGA after average of 20 weeks of use.
Keywords: Multiple Stimulation Technology; Androgenetic Alopecia; Pilogics Hairegen Device.
Introduction
Androgenetic Alopecia (AGA) is a common hair disorder, affecting both men and women world-wide [1, 2]. In predisposed persons, under effect of dihydrotestosterone, progressive miniaturization of the hair follicles occurs in a specific pattern distribution. Without treatment, patients undergo progressive hair loss, reaching prevalence rates of 73% in men and 57% of women over the age of 80 [3]. Only few drugs and a single device have been proven as effective, and cleared by the FDA to treat AGA [4]. The efficacy of the approved therapies varies between 30% and 60%. This has led to a large number of unsatisfied patients who demand for a better cosmetic coverage over the scalp. Therefore, biotechnology companies and academic research centers continue further to investigate new technologies to treat AGA [5].
Pilogics Hairegen device is a novel home-used appliance that combines electrical, biochemical and mechanical stimulation of the scalp. The combination of specific stimuli was chosen based on previous publications, demonstrating their efficacy in promoting hair growth [6, 7, 8, 9, 10, 11].
Materials and Methods
Between November 2016 and April 2017 800 patients, diagnosed with AGA, had purchased Hairegen device for home use. All the patients underwent standard medical intake and initial screening to rule out other than AGA etiologies of hair loss. They were further classified according to Norwood-Hamilton (for males) and Ludwig (for females) scores for hair loss severity. Male patients were accepted for the treatment if classified as Norwood-Hamilton II to VI. Female patients were accepted if classified as Ludwig I to II. The hair count was performed using TrichoScan computerized analysis before initiation of the treatment and after 16-20 weeks of using the device.
As a part of commercial agreement, the clinic, in which the patients were followed up, provided a "performance money-back guarantee". The essence of the agreement was full financial reimbursement in case the device was used according to the given instructions, yet the patient witnessed insufficient results. The cutoff level of the adherence to the instructions was set to 80%. Only patients, who fulfilled 80% of the pre-set treatment time, as retrieved from the personal usage log of the device, were included in the study group.
Treatment protocol consisted of adaptation time of one week, during which the patients used the device twice a day for 3 minutes to get used to a slight discomfort produced by the contact of the device's spikes with the skin. This was followed by twice a day 5 minutes' treatment for short-haired patients and twice a day 8 minutes' treatment for longer-haired patients.
Participation in the measurements was consensual and voluntary. The collected data included age, gender, general medical condition, concomitant drug use and hair counts of total hair and terminal hair. Data on patients younger than 18 years was excluded from analysis. Concomitant use on minoxidil or finasteride, initiated before the use of Pilogics Hairegen device (10% of the study group), was registered but did not exclude the data from the analysis. None of the patients started using the medications during the study period.
Pilogics Hairegen is a personal, hand-help home-used electrical device. The user holds the handle and moves the device on the scalp, rolling the disks back and forward on the treated area. While in contact with the scalp, the device applies multiple pinpoint stimulation technology (MPST) in highly-localized, high-gradient fashion.
The device includes 8 uniaxial disks that are 32mm in diameter and 0.2mm thick. Along each disk's circumference, there are about 100 protrusions ("spikes") that are the main contact points of the device with the scalp as it is rolled by the user. The disks have some space between them to allow plowing through the hair for improved contact with the skin. The disks are metallic, and alternately coated by zinc (Zn) or brass (Cu-Zn). (Fig. 4).
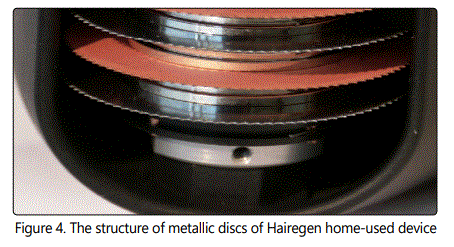
The spikes deliver mechanical, electrical and ionic stimulations to the scalp in the following fashion. Hairegen device includes a chargeable applicator that applies AC current of up to 100Hz via the disks. All the zinc-coated disks are equi-potential, and all the brass-coated disks are equipotential, thus the current flows between disks of dissimilar material. The voltage is up to 32V, chosen as below electroporation threshold. The current is limited to 200μA to minimize patients' discomfort during the treatment.
In addition, the device includes a vibration element that moves the entire device, including the spikes, in an orthogonal direction to the scalp surface (in/out) and in sideways direction relatively to the movement path of the spikes on the scalp. This vibration augments the mechanical stimulation created by the spikes as the disks are rolled back and forth.
The metals, zinc and copper, were chosen due to their ability to produce inhibition of 5-alpha-reductase, a major factor of AGA onset.8Since human skin, when slightly humid, has electrolytic solution properties, this naturally occurring electro-chemical interaction and the electric current, both cause the trace amounts of zinc and copper ions to depose into the skin at the contact points of the metallic spikes with the scalp.
Thus, the mechanical, electrical and biochemical stimulation - all occur in highly localized points of the spikes. As the user rolls the disks back and forth for several minutes, thousands of points are stimulated over the target scalp area.
The device is controlled by a programmable microcontroller, which shuts off the device after pre-determined treatment time. When the electrical contact of the disks with the scalp is poor, the user receives audible feedback, indicating that the treatment becomes ineffective. The treatment dates and times are kept in the personal log on the device.
Treatment
After the intake and hair count, each user received treatment regimen instructions requiring twice a daily treatment. Hairegen device offers 3 treatment options, differing in duration (3, 5 and 8 minutes). All the users were instructed to start with 3 minutes' treatment during the first week to be customized with the device. Short-hair patients, then, switched to twice a day 5 minutes and long-hair patients-twice a day 8 minutes' program. Patients with longer hair required longer treatment time to adjust the hair position in order to create a full contact of the device with the scalp.
At the second visit (14-25 weeks after treatment initiation), the device's internal logs were reviewed, to ascertain proper use per instructions. Only the results of the users, who employed the device at least 80% of the required accumulating time, were included in the data analysis.
Data Analysis
The hair analysis was performed using TrichoScan. TrichoScan software provides, among others, measurement of total hair density and terminal hair density. The correlation between evaluation of hair parameters using manual identification of hairs and the fully-automated TrichoScan method has been validated in previous studies [12, 13]. After the clinical assessment of the balding area, a dot was marked by a permanent tattoo ink at its border to allow re-assessment to be performed at exactly the same area.The hair near the dot was trimmed. Dyeing of the hair was performed in individuals with white hairs. Images of the assessment area were recorded and analyzed. The detection limit of the TrichoScan is any hair above 5 μmdiameter. Hair less than 40 μm in diameter is defined as vellus hair [14]. (TRICHOLOG GmbH). (Fig. 5).
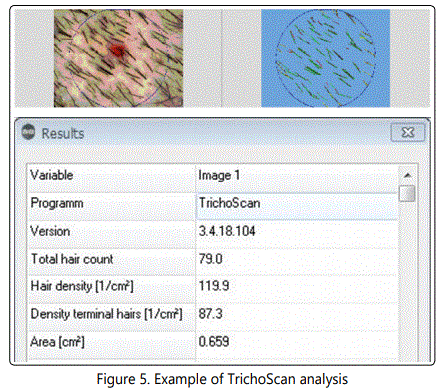
The parameters assessed per each patient included total hair density, and the density of terminal hair. Data analysis was performed only on patients who: 1. Completed 80% of the treatment time; 2. had their second visit within 20 (14-25) weeks; 3. Aged over 18.
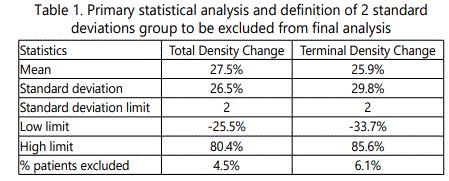
To exclude the possibility of imprecision in hair density measurements, after receiving the initial data analysis, patients with recorded change in hair density of more than 2 standard deviations from the mean, were excluded from the final data analysis.
Results
35% of the patients (345 in total, 288 males and 57 females), who purchased the Pilogics Hairegen device, and came for the second visit, were found to be adherent with the pre-set treatment protocol. 313 of them (260 males, 53 females) came for the second visit within 14-25 weeks after the initiation of the treatment and were older than 18. The average age for male patients was 35 (15-75), and for females- 57 (23-75). 8 patients (1 female and 7 males) refrained from disclosing their age.
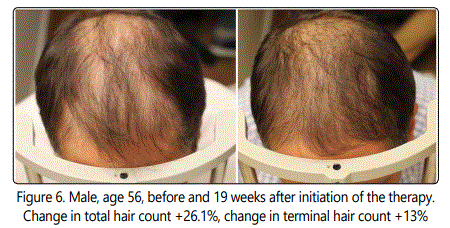
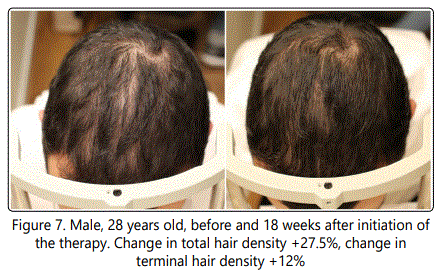
The change in cosmetic appearance of some of the patients were visibly significant (Fig.6, 7, 8, 9, 10).
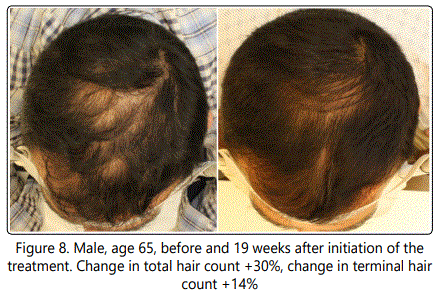
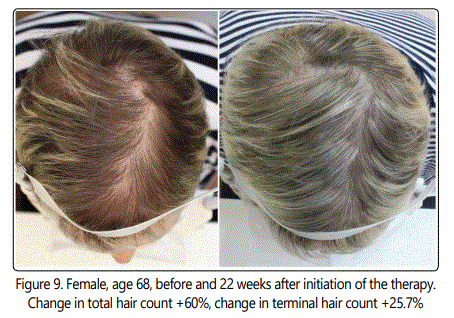
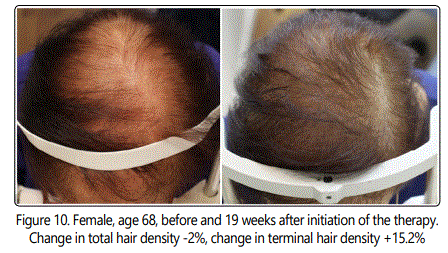
7.7% of the patients with hair density changes of more than 2 standard deviations from the mean were excluded from the final data analysis (Table 1).
The final group consisted of 289 patients. TrichoScan results in this group demonstrated the increase in total hair densityof 23.25% and increase in terminal hair densityof21.2% (Table 2).

The change for male patients was 22.7% increase for total hair density and 20.8% for terminal hair density, while for females-25.7% increase in total hair density and 22.4% in terminal hair density.
Interestingly to note, in spite of small number in the study, both females and males of a more advanced age (>60) performed better than patients of younger ages (Table 2). A correlation was found between the total density change and terminal density change (Fig. 1).
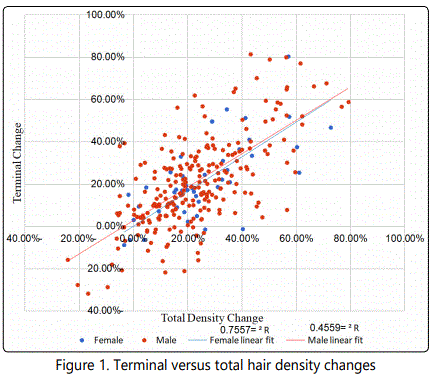
When both indicators were displayed along linear fitting, women showed stronger correlation (R2=0.75579), while male patients demonstrated weaker, but visible fit (R2= 0.4559).
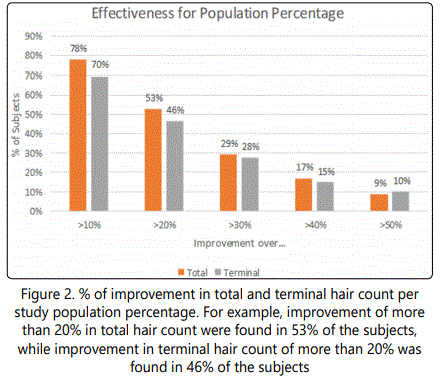
When change in total and terminal hair density was analyzed per study population percentage, it was found that terminal hair density increased by at least 78% in 10% of population, by at least 20% in 53%, by at least 29% in 30%, by at least 17% in 40% and by at least 9% in 50% of the study population (Fig. 2). None of the patients reported on adverse events during the treatment period.
Discussion
In this retrospective "real life" study, we showed that 14- 25 weeks of treatment with Pilogics Hairegen device resulted in increase of total and terminal hair density in men and women diagnosed with AGA. The device was found to be useful and harmless in treatment of AGA.
The major limitation of the device was a relatively high percentage of non-compliance to the daily treatment schedule, probably resulting in a reduced efficacy, not assessed in this study.
The relationship between electrical effect and the growth of mammalian tissue has been a subject of interest for a long time [6]. A non-invasive, non-thermal technique with pulsed electric fields can lead to proliferation of the epidermis, formation of microvasculature, release of growth factors, and stimulation of new collagen formation at treated areas [7]. Reduction in hair shedding was demonstrated by using a commercially available transcutaneous electrical neural stimulation (TENS) device, or specifically designed devices [15, 16]. It was suggested that follicular stem cells are electrically sensitive and respond to specific exogenous current levels, similar to that demonstrated in the healing of soft tissue wounds and fractures [9, 16].
Cationic ions of different metals interrupt with enzymatic activity in the tissue. It has been shown that cations of Cd, Cu, and Zn strongly inhibit the activity of type I 5 alpha- reductase in transfected mammalian cells [8].
Mechanical stimulation or trauma to the scalp skin is one of the oldest "traditional" techniques to enhance hair growth. Microneedling, also called dermarolling, has recently become a popular therapeutic approach for AGA.While the mechanism of efficacy has not been investigated in any detail, microneedling may increase related gene expression and localized growth factor release [10, 11].
Hairegen combines the three aforementioned modalitiesmechanical, electrical and biochemical stimulation, making the device more efficient. The results of this study, analyzing the effect of Hairegen device on total and terminal hair density, demonstrated a marked improvement in both parameters for both genders, across all the age groups, and especially on patients that are more elderly.
Conclusions
Currently, only topical minoxidil, systemic finasteride and low level laser therapy are FDA-approved modalities for the treatment of AGA. Hair density improvement following these therapies is expected to become visible 6 months after the initiation. Recommended administration of minoxidil twicedaily may result in erythema, dryness and irritation of scalp. Among the commonly mentioned finasteride associated adverse effects, erectile and ejaculation dysfunction, and decreased libido are mentioned. Due to teratogenicity, this drug is forbidden to use by women of child bearing potential.
Given their partial efficacy and potential adverse events, many patients seek for alternative and complementary treatments. Hairegen home-used device, based on electrical, biochemical and mechanical stimulation, demonstrated safety and efficacy in this retrospective "real life" study.
Conflict of Interest
The authors declare no conflicts of interest.
References