Case Report
Cutaneous Metastasis from Squamous Cell Carcinoma Lung
1Consultant, Care hospital, Villivakkam, Chennai, India
2Assistant professor, ACS medical college, Velappanchavadi, Chennai, India
*Corresponding author: Jayabal Pandiaraja, Consultant, Care hospital, Chennai, Pin: 600049, India, E-mail: dr.pandiaraja@gmail.com
Received: November 29, 2017 Accepted: December 4, 2017 Published: December 9, 2017
Citation: Pandiaraja J, Shalini A. Cutaneous Metastasis from Squamous Cell Carcinoma Lung. Madridge J Dermatol Res. 2017; 2(1): 23-25. doi: 10.18689/mjdr-1000106
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Cutaneous metastasis may be the first sign of internal malignancy. In lung carcinoma skin is 13th common site of metastasis. Other sites includes hilar node, liver, adrenal gland, bones, brain are the most frequent site of metastasis. Cutaneous metastasis occurs as an initial presentation in 0.8% of patient with internal malignancy. Here we report a case of squamous cell carcinoma of right lung with left side back metastasis. This case was reported due to uncommon site and uncommon side of lung metastasis.
Keywords: Squamous Cell Carcinoma; Cutaneous Growth; Metastasis; Lung.
Introduction
Cutaneous nodule occurs following internal malignancy is very rare. Cutaneous metastasis is defined as group of malignant cells in the skin due to internal malignancy. Most of the time cutaneous nodule was detected after initial diagnosis of primary lesion. But in sometime it may be detected before or same time of detection of primary malignancy [1]. Incidence of Cutaneous metastasis is varies from 0.7% to 9%. In male lung (24%) is the most common site for cutaneous metastasis followed by colon (19%), melanoma (13%) and oral cavity (12%). In women breast (69%) is the most common site followed by colon (9%), melanoma (5%), ovary (4%) and lung (4%) [2]. But recent trial showed higher incidence of cutaneous metastasis following melanoma (32.3%) in male.
Case Report
A 35-year-old male who is a chronic smoker for 20 years, presented with complaint of ulcerated growth over the left side of back for 1 month duration. There was history of pain over the growth for 1 month duration. Patient said there is a history of loss of appetite and loss of weight. Patient was initially treated for squamous cell carcinoma right cheek with curative radiotherapy. Local examination of back revealed an ulcerated growth of 4x3 cm present on the left side of back. The growth covered with necrotic base with fresh bleeding and foul smelling discharge. The growth was hard in consistency. There was evidence of left axillary lymphadenopathy [Figure 1].
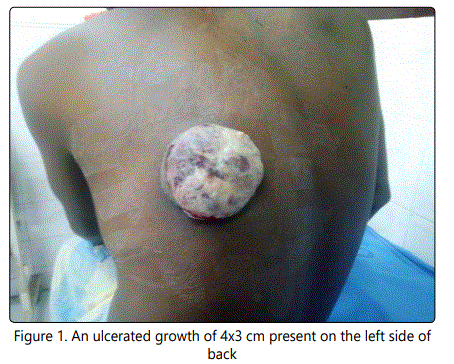
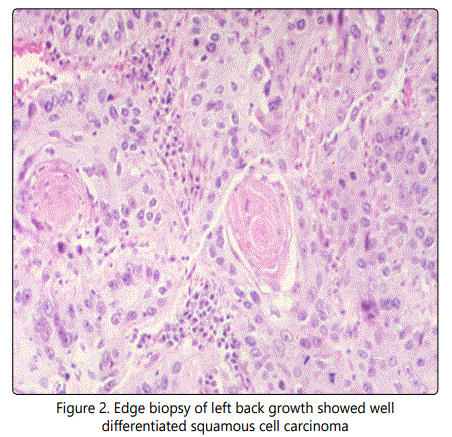
Routine blood examination like complete blood count, renal function test and liver function test were normal. Edge biopsy of left back growth showed well differentiated squamous cell carcinoma [Figure 2].
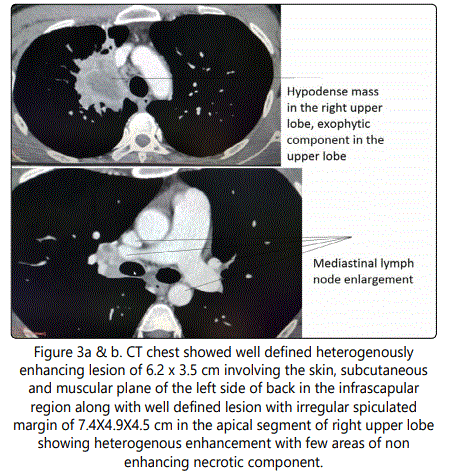
Whether squamous cell carcinoma left back is primary or metastatic we are not sure. So we took biopsy form oral cavity. The biopsy from the oral cavity showed reactive inflammatory tissue and no evidence of residual malignancy. Computed tomography chest showed well defined heterogenously enhancing lesion of 6.2 x 3.5 cm involving the skin, subcutaneous and muscular plane of the left side of back in the infrascapular region along with well defined lesion with irregular spiculated margin of 7.4X4.9X4.5 cm in the apical segment of right upper lobe showing heterogenous enhancement with few areas of non enhancing necrotic component on contrast study [Figure 3a & b].
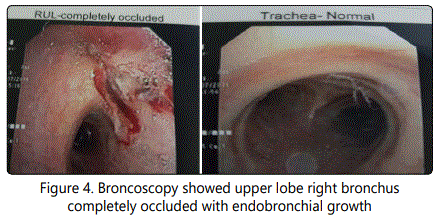
Broncoscopy showed upper lobe right bronchus completely occluded with endobronchial growth which bleeds on touch [Figure 4].
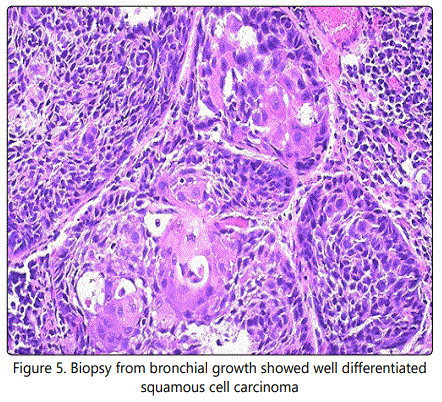
Biopsy from bronchial growth showed well differentiated squamous cell carcinoma [Figure 5].
Patient was offered palliative chemotherapy. Patient died after 4 months following chemotherapy.
Discussion
Cutaneous metastasis of internal malignancy may be nodular or ulcerated, mobile or fixed, single or multiple and painful or painless lesion [3]. There is lot of controversy in which type of lung carcinoma has high incidence of metastasis. Kanazawa &Hidaka et al showed high incidence of cutaneous metastasis in large cell carcinoma than squamous or small cell carcinoma. Dreizen et al showed adenocarcinoma has highest tendency for cutaneous metastasis, whereas Brown stein & Helwing et al reported adenocarcinoma and squamous cell carcinoma has equal incidence of cutaneous metastasis.
The incidence of cutaneous metastasis in lung cancer varies from1% to 12% [4]. Most of the cases nodule occurs in the same side of lesion. But in our case it is different. It occurred on the opposite side of the primary lesion. Nodule commonly seen in the chest, abdomen, scalp, head and neck, extremities and rarely over back [5]. In our case it occurred over the back. In most of the cases cutaneous nodule is indistinguishable from the primary cutaneous malignancy or metastatic nodule.
Adenocarcinoma lung has high incidence of cutaneous metastasis than squamous cell carcinoma [6]. Metastatic deposit from squamous cell carcinoma mostly moderately differentiated or poorly differentiated. Cutaneous metastasis occur either lymphatic or hematogenous route. Carcinoma lung mostly spread via hematogenous route [7]. This is the reason for occurrence of cutaneous nodule in lung carcinoma anywhere in the body. Most of the cases managed with combined chemo radiation. But the prognosis is very poor for malignancy with cutaneous deposit. The median survival following cutaneous metastasis varies from 3 months- 6 months [8].
Conclusion
Cutaneous metastasis should be suspected in patient who is a chronic smoker or previous history of squamous cell carcinoma. It may be the only manifestations of internal malignancy, when internal malignancy is silent. The prognosis of internal malignancy with cutaneous metastasis is always poor. Even with best available combination chemotherapy or radiotherapy the chance of survival is poor.
References