Case Report
Treatment of Recalcitrant Chronic GVHD Ulcers with Combined Silver Collagen Matrix and Silver Foam Dressings
Division of Dermatology, City of Hope Comprehensive Cancer Center, USA
*Corresponding author: Jae Yeon Jung, Division of Dermatology, City of Hope Comprehensive Cancer Center, 1500 East Duarte Road, Duarte, CA 91010, USA, Tel: 626-256-4673, ext: 65621, E-mail: jjung@coh.org
Received: August 6, 2016 Accepted: October 24, 2016 Published: October 27, 2016
Citation: Almazan TH, Jung JY. Treatment of Recalcitrant Chronic GVHD Ulcers with Combined Silver Collagen Matrix and Silver Foam Dressings. Madridge J Dermatol Res. 2016; 1(1): 11-17. doi: 10.18689/mjdr-1000103
Copyright: © 2016 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Importance: Chronic ulcers in patients with sclerotic graft versus host disease are recalcitrant to treatment and treatment options are limited. These patients are immunosuppressed and frequently hospitalized due to infections. Systemic antibiotics are generally ineffective in eradicating colonized bacteria that are often multidrug resistant and local wound care is critical in the management of these difficult ulcers. We present three patients who developed rapid and sustained improvement using a combination of silver collagen matrix and silver sponge dressings with compression.
Observations: Three patients with chronic lower extremity ulcers due to sclerotic chronic graft versus host disease presented for wound care in our Dermatology clinic. The wounds were chronically colonized with multidrug resistant bacteria and these patients were frequently hospitalized due to infection and pain. Multiple topical agents were unsuccessful in treating these recalcitrant and morbid ulcerations. Significant improvement was obtained with a combination of silver collagen and silver sponge dressings.
Conclusions and Relevance: There is a paucity of published data regarding the treatment options for chronic graft versus host ulcers. These ulcers are challenging due to immunosuppressive regimens and chronic bacterial colonization and recurrent infections. We report three cases treated successfully with a combination of silver collagen matrix and silver sponge dressings.
Keywords: Recalcitrant Chronic GVHD Ulcers; Silver Collagen Matrix; Silver Foam Dressings; Sclerotic Graft vs Host Disease.
Abbreviations: GVHD: Graft Versus Host Disease; TNF: Tumor Necrosis Factor; UV A: Ultraviolent A; UV B: Ultraviolent B; IVIG: Intravenous Immunoglobulin; cGVHD: Chronic Graft Versus Host Disease; CSMD: Collagen Silver Matrix Dressing; SSD: Silver Sponge Dressing; HLA: Human Leukocyte Antigen; BSA: Body Surface Area; Mg: Milligram; STSG: Split Thickness Skin Graft; Cx: Culture; Ami: Amikacin; Tob: Tobramycin; Czn: Cefazolin; Cfx: Ceftriaxone; Cef: Cefepime; Amx: Amoxicillin; Amp: Ampicillin; Ctx: Cefoxitin; Caz: Ceftazidime; Cip: Ciprofloxacin; Cli: Clindamycin; Ery: Erythromycin; Gen: Gentamicin; Ipm: Imipenem; Lvx: Levofloxacin; Mem: Meropenem; Oxa: Oxacillin; Pen: Penicillin; Tzp: Piperacillin-Tazobactam; Tet: Tetracycline; Sxt: Trimethoprim-Sulfamethoxazole; Van: Vancomycin.
Introduction
Chronic graft versus host disease (GVHD) is a significant cause of morbidity and mortality following allogeneic hematopoetic stem cell transplantation. Approximately 50% of allogeneic transplant patients develop chronic GVHD. The skin is the most commonly affected organ (70-80%). Oral and genital mucosa, lung, liver, eye, and joint involvement are also common [1]. Of the patients with chronic cutaneous GVHD, sclerotic disease is one of the most severe manifestations and is associated with poor quality of life, functional status, and higher mortality [2]. Despite advances in transplantation methods, chronic GVHD incidence and prevalence is rising due to the increasing numbers of allogeneic transplantations, increasing use of unrelated donors, and peripheral blood transplants, as well as decreasing early transplant related mortality. These parameters predict an expanding population of long-term survivors with increasingly morbid disease [3].
The pathogenesis of chronic GVHD is poorly understood and its treatment is challenging. The first line therapy is corticosteroids but this treatment cannot be maintained chronically due to significant side effects. In patients who are steroid refractory, there is no second line therapy that is proven to improve survival. Currently available second-line agents such as cyclosporine, tacrolimus, sirolimus, extracorporeal photophoresis, mycophenolate mofetil, TNF inhibitors, and rituximab are used based on clinician preference, the side effect profile, and the patient's prior response to therapy. No good randomized, controlled trial data exists to guide second-line therapy, and existing studies are difficult to interpret due to the complexities of grading response [4].
More than 4000 marrow transplants are performed annually in the United States, and of these, nearly half are complicated by clinically significant GVHD [5]. The incidence of sclerotic disease is estimated at 20% of patients undergoing allogeneic hematopoetic transplantation [6]. Though a number of treatments have been used for cutaneous cGVHD including topical and systemic steroids, extracorporeal photophoresis, UV A or UV B phototherpy, IVIG, and rituximab, little data exists regarding treatment specifically for cGVHD-related skin ulcers [7]. The largest series published includes 25 patients with an incidence of 0.56% [8].
There are few guidelines for the treatment of chronic ulcers from chronic GVHD. Hematologists are reluctant to increase systemic immunosuppression due to the risk of infection. Although these patients are frequently admitted for intravenous antibiotics, these wounds are chronically colonized, often with multidrug resistant bacteria, and infection cannot be eradicated. We report successful treatment of three patients using a combination of collagen silver matrix dressing (CSMD), silver sponge dressing (SSD), and local compression.
Materials
Our choice of dressing materials was determined by the availability at our institution. We do not have any relevant financial disclosures and do not endorse the use of any specific brand of dressing. The dressings used in our patients include: silver collagen matrix dressing (Puracol® Plus AG+ Collagen Dressing, Medline Industries, Mundelein, Illinois), silver sponge dressing (Mepilex® AG, Mölnlycke Health Care, Göteborg, Sweden); self adherent compression dressing (Coban™, 3M™, Saint Paul, Minnesota); 3% bismuth tribromophenate petrolatum gauze (Xeroform, DeRoyal®, Powell, Tennessee); collagenase ointment (Santyl®, Smith and Nephew, London, United Kingdom), and gentian violet sponge dressing (Hydrofera® blue, Hollister Wound Care, Libertyville, Illinois).
Cases
Patient 1 is a man in his 40s with a history of AML treated with allogeneic stem cell transplantation from his HLA-matched sibling 9 years prior to presentation. He was admitted to our hospital with right lower extremity ulcerations. Cultures at that time demonstrated pan-sensitive Pseudomonas aeruginosa. He was treated with vancomycin, ceftazidime and metronidazole. At his initial presentation to the dermatology clinic, his foot was erythematous and there was a green-yellow necrotic base in the ulcers (figure 1A). His sclerotic GVHD was extensive, involving over 50% BSA with restricted range of motion in his wrist and ankles. The skin over his distal lower extremities demonstrated hide bound sclerotic plaques and he was unable to move his toes. He had poorly controlled type 2 diabetes with a hemoglobin A1c of 9.6%. He was on sirolimus and mycophenolate mofetil for his chronic GVHD. He had been treated with negative pressure wound therapy without significant improvement. Local therapy was initiated with clobetasol 0.05% ointment, silver sponge dressings and compression. After 2 months without improvement, he was switched to collagenase ointment and 3% bismuth tribromophenate petrolatum gauze. No improvement was noted after an additional 2 months. He was then switched to a combination dressing consisting of collagen silver matrix, silver sponge dressing and compression. At each visit, the necrotic base was gently debrided with a sharp, disposable curette, the CSMD was cut to fit in the ulcer base, and the SSD was applied over it. A self-adherent wrap was used to hold the dressing in place and provide compression. The patient was instructed to change the dressing every 3-4 days and was seen in clinic once a week. After 2 months of treatment, the ulcers had granulated and the surface started to re-epithelialize (figure 1B). His pain also significantly improved.
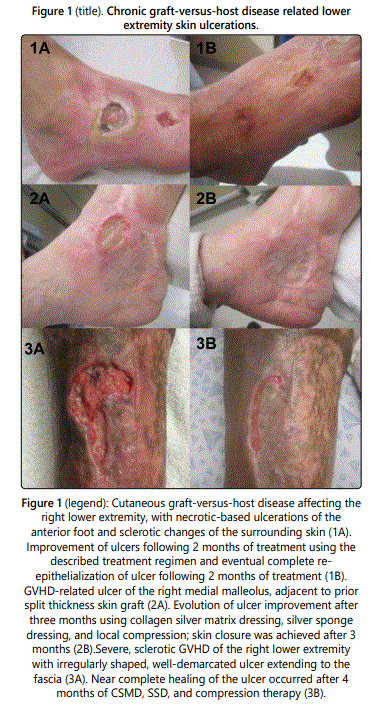
Patient 2 is a woman in her 70s with a history of myelodysplastic syndrome and subsequent allogeneic hematopoietic transplant from her HLA-matched sibling 4 years prior to presentation. She had sclerotic GVHD of the skin affecting over 50% BSA and recurrent ulceration of her right medial malleolous that was treated previously by split thickness skin graft. She also had chronic lower extremity edema with recurrent hospitalizations for lower extremity cellulitis. She also had poorly controlled diabetes with a hemoglobin A1c of 8.1%. Immunosuppressive medications included tacrolimus, sirolimus and prednisone (20 mg daily). Additionally, photopheresis was attempted, but was held due to neutropenia. She presented with a new ulceration that developed adjacent to her previously well-healed STSG on her right medial ankle (figure 2A) that had been slowly enlarging for 10 months. She had numerous admissions for infection and pain control and cultures demonstrated methicillinresistant Staphylococcus aureus and Morganella morganii. She had been treated with collagenase ointment and gentian violet impregnated dressing. The patient noted an enlarging wound with increasing pain despite this treatment. Local therapy was initiated with compression wraps, topical 1% clindamycin, 0.05% clobetasol ointment and silver sponge dressings; these were unsuccessful in promoting ulcer regression. Treatment was changed to include the CSMD and SSD with compression and resulted in gradual improvement and eventual closure of the ulcer after 3 months (figures 2B).
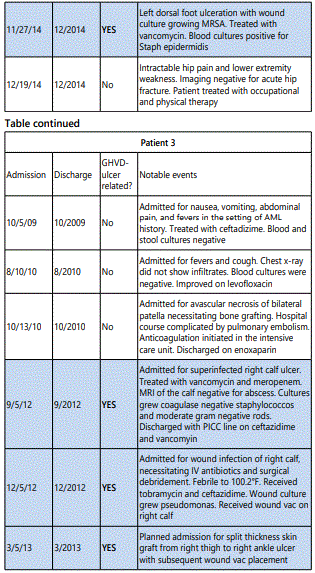
Patient 3 is a man in his 30s with a history of AML who received a hematopoietic cell transplant from a matched unrelated donor 5 years prior to presentation. He developed sclerotic GVHD of the skin that was largely confined to his right lower extremity. He developed a lower extremity ulceration that was treated with split thickness skin graft (figure 3A). Immunosuppressive regimen included prednisone, tacrolimus and sirolimus. Failed treatments included collagenase ointment and gentian violet containing dressing. We initiated treatment with CSMD, SSD and compression. The dressings were changed every 3-4 days and the wound base was debrided every 1-2 weeks using a disposable sharp 5 mm curette. The ulcer was noted to heal to near complete resolution over the course of 4 months (figure 3B).
Discussion
The treatment of sclerotic disease is particularly difficult when chronic ulcerations develop because the immunosuppressive medications that are used to treat the GVHD contribute to the recurrent infections common to these patients. Although the exact mechanisms are unknown, inflammation and fibrosis are thought to play a key role in the etiology of chronic GVHD related ulcer formation. While only 10% of patients with cGVHD present with sclerotic skin, nearly all patients with cGVHD-related ulcers develop them on areas of prior sclerosis [9, 10]. Ulcers are usually preceded by bullous lesions, where skin detachment between the epidermis and the dermis facilitates erosion and provides a highly prone substrate for ulcers to form following the slightest trauma. Additionally, skin edema, a non-specific finding associated with cGVHD, may lead to inappropriate endothelial activation and exacerbation of the dermal-epidermal detachment. Once erosions are formed, a background of sclerotic tissue along with local lymphatic and microvascular derangement may lead to both ulcer enlargement and poor wound healing.
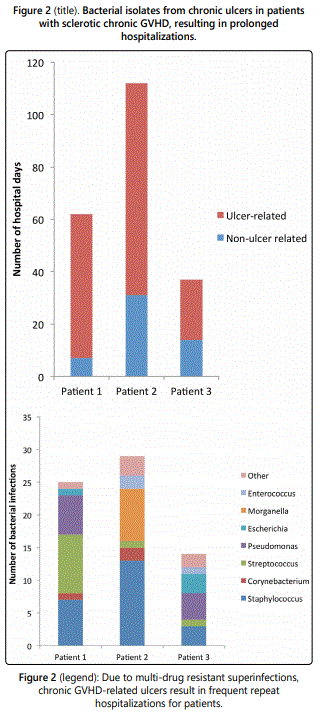
All patients described in this case series developed ulcerated lesions of the lower extremities. Two patients initially responded well to split thickness grafting, however, both developed subsequent ulcerations adjacent to the skin graft. Patient 1 and 2 were hospitalized 3 times for ulcer-related infection and patient 3 was hospitalized 8 times (table 1). These patients had a total of 211 hospital days in our electronic medical record and 75% (159) of those days were ulcerrelated (Figure 4). With increased costs associated with health care, outpatient management of these chronic ulcers will be increasingly desirable [11].
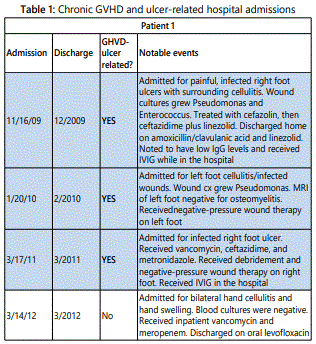
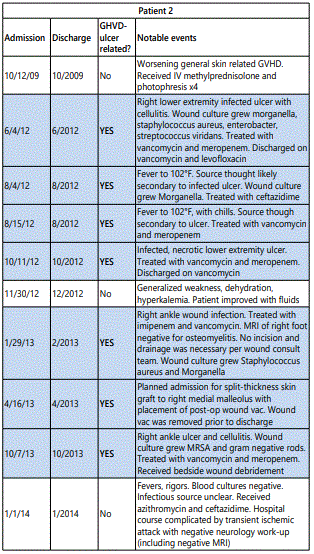
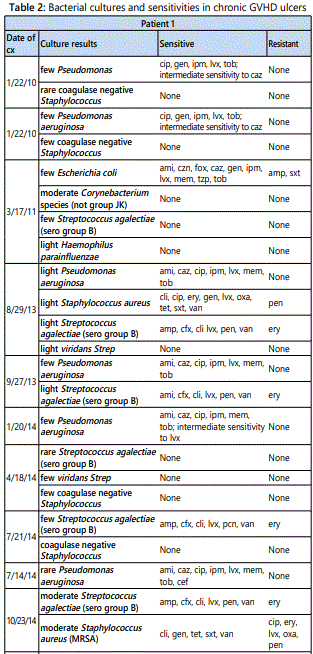
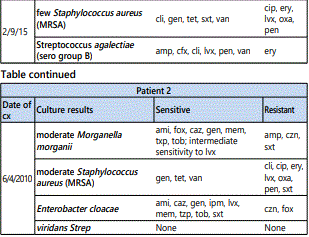
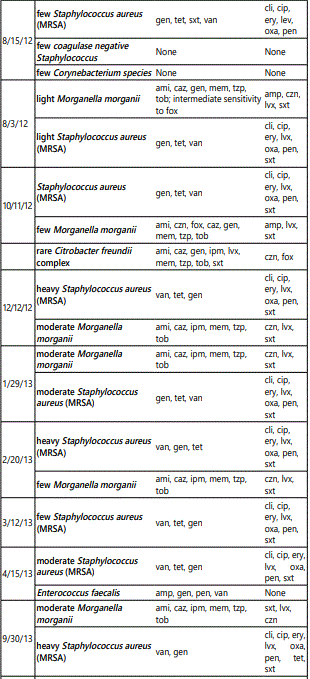
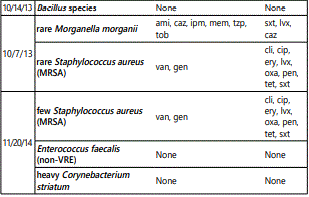
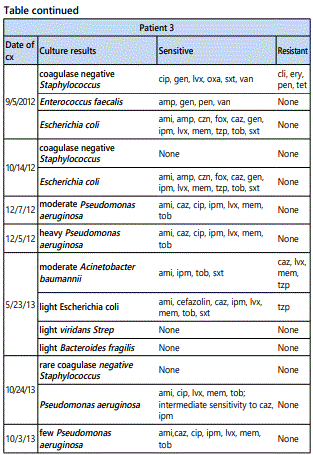
All three patients developed persistent colonization and recurrent super-infection of the ulcers (Table 2). Cultures of the right lower extremity ulcer from Patient 1 demonstrated Streptococcus agalectiae resistant to erythromycin on 3 different cultures spanning nearly 2 years (2013-2015). He also developed colonization of multidrug resistant MRSA with an identical drug resistance pattern. Patient 2 had multi-drug resistant Morganella morganii cultured 8 times and multidrug resistant MRSA cultured 10 times over a 3-year period. The sensitivities were nearly identical suggesting chronic colonization and the formation of recalcitrant bacterial biofilms [12] (Table 1). Cultures are underutilized and too often, empiric antibiotic use leads to inappropriate treatment and contributes to antibiotic resistance [13]. The most prevalent species in our patients were Staphylococcus, Streptococcus, Pseudomonas, Escherichia, and Morganella which comprised over 80% of the isolates in our patients (Figure 2).
Chronic wounds, such as those implicated in cGVHD, contain a persistently high amount of inflammatory exudate, a milieu that impedes the proliferation of fibroblasts [14]. Similar to ulcerations of other causes (such as venous stasis ulcers, or diabetic foot ulcers), local therapy plays an important role in diminishing local inflammation and promoting wound healing. Ideal dressings are those that control exudate, prevent bacterial proliferation, and absorb excessive wound drainage while preventing the ulcer from drying out. The efficacy of silver impregnated dressings stems from their ability to reduce wound bioburden. The silver impregnated foam dressingsare absorbent and have broad-spectrum antimicrobial activity. They are slightly adherent on one side, facilitating application and allowing the dressing to be left on wounds for up to seven days. It can also be removed non-traumatically during dressing changes and in between dressing changes. The dressing consists of a silicone-based wound contact layer, an absorbent polyurethane foam pad comprised of silver sulfate and activate carbon, and a vapor-permeable waterproof film. Once in contact with wound exudate, silver ions are released. The use of silver in wound management is not new and can be traced to the 18th century for the treatment of skin ulcers. Silver ions target thiol groups on bacterial enzymes causing unraveling; the ions also inhibit bacterial growth, damage the cell wall, and cause structural abnormalities in bacterial nucleic acids. The bactericidal properties of silver have lead to its common use in burn patients and wound dressings.
Addition of a silver-containing collagen scaffold dressing may assist in replenishing some of the structure, components, and signaling mechanisms needed for wound healing and regeneration[15]. Compression therapy is a critical component to healing most chronic wounds by reversing tissue edema and improving blood and lymphatic flow to the wound bed. The use of self-adherent dressings for compression is useful for these patients because they are easily applied, can be changed frequently and are disposable. Traditional compression stockings are very difficult for these patients because of their restricted range of motion.
While the small number of patients in our case series (3 patients total) is a limiting factor, we report the successful treatment of three recalcitrant ulcers from sclerotic GVHD using a combination of silver collagen matrix dressings, silver sponge dressing and compression. All three patients demonstrated wound closure, decreased pain, and increased mobility following 2 to 4 months of therapy. Data on localized treatment of cGHVD ulcers is scare, and no randomized control trials investigating specific dressings or wound care regimens have been performed on patients with cGHVD ulcers. Chronic GVHD ulcers represent the most severe form of cutaneous disease and further knowledge is needed to identify effective treatments.
Funding: This article has no funding sources
Disclosures: The authors have no conflict of interest to declare
References