Review Article
A Review on Cat Scratch Disease and itʼs Zoonotic Significance
School of Veterinary Medicine, Wollega University, P.O.Box 395, Nekemte, Ethiopia
*Corresponding author: Geremew Haile, Assistant Professor, School of Veterinary Medicine, Wollega University, Nekemte, Ethiopia, E-mail: geremewlov@gmail.com
Received: December 9, 2018 Accepted: January 8, 2019 Published: January 17, 2019
Citation: Girma G, Duguma M, Haile G. A Review on Cat Scratch Disease and its Zoonotic Significance. Madridge J Vet Med Res. 2019; 1(1): 1-7. doi: 10.18689/mjvmr-1000101
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
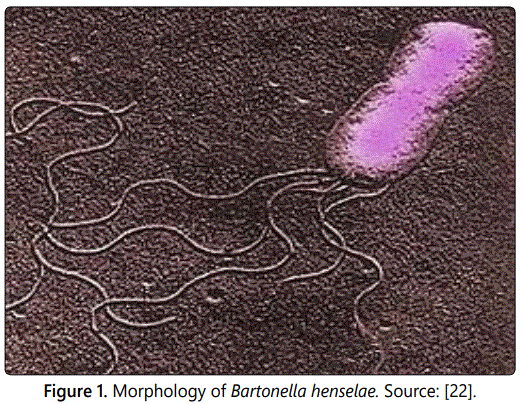
Cat scratch disease (CSD) is a zoonotic disease caused by Bartonella henselae, afastidious, hemotropic, which are rod shaped gram negative bacterium, typically presents with a localized lymphadenopathy. Bartonella henselae is maintained and spread among cats, the principal mammal reservoir species by the cat flea (Ctenocephalides felis). Transmission to humans occurs via scratches and possibly bites from cats. Asymptomatically, bacteremic cats with Bartonella henselae in their saliva serve as vectors by biting and clawing the skin. CSD occurs throughout the United States and worldwide wherever cats and their fleas are found. Diagnosis usually relies on epidemiological, clinical, histological and serological tests. The testing of cats for CSD is usually futile, since the organism is present in the blood stream only intermittently. For this reason the treatment of cats with antibiotics is usually not effective. The condition is more severe in patients whose immune system is compromised (Untreated), the disease can be fatal. The prevention for CSD is elimination of fleas which decreases transmission among cats. Avoiding contact with cats prevents the disease. Where this is not reasonable, good hand-washing after playing with a cat, avoiding scratches and bites from rough play and avoiding cat saliva will lessen the risk of infection is recommended. Despite worldwide distribution and great economic significance of the fleas, information on the occurrence of Bartonella species is unsatisfactory especially in developing countries like Ethiopia.
Keywords: Bartonella henselae; Cat fleas; Cat scratch disease; Zoonotic.
Introduction
Cats and Dogs have been human companions for more than 10,000 years. They have been sharing our environment and have gained a major status as “pets” in our modern every urbanized society [1]. The unique dynamic interaction between the humans, animals and pathogens are sharing the same environment that should be considered within the “One Health” approach, which dates back to ancient times of Hippocrates [2]. The oral cavity of healthy cats and dogs contains hundreds of different pathogenic bacteria including Bartonella species [3]. Cat scratch disease (CSD) is a systemic condition caused by the gram-negative zoonotic bacillus Bartonella henselae [4]. The disease may affect various organs producing a broad range of clinical syndromes [5].
Bartonella henselae is found in feline erythrocytes and fleas, which can contaminate saliva and then be introduced into humans through biting and clawing by cats. The cat flea or Ctenocephalides felis (Cf) is the vector responsible for horizontal transmission of the disease from cat to cat and its bite can also infect humans [6]. A recent report from Japan of a possible case of CSD caused by contact with a dog suggests that dogs could play a role in human B.henselae infection [7]. Naturally infected cats are usually asymptomatic, although they may suffer from anterior uveitis, lymphadenitis, gingivitis and stomatitis and are predisposed to neurological diseases. No major clinical signs of CSD have been reported in cats under natural conditions [8].
Among cats, casual or sexual contact and sharing of food or water dishes are not significant sources of exposure. Studies have shown that cats younger than one year old (kittens) and cats from shelters or adopted as strays are more prone to carry the infection due to a higher probability of being bacteremic [9]. Although a history of exposure to cats is important, it is not absolutely necessary to make the diagnosis after contact with an infected kitten [10]. Treatment of CSD depends on the disease presentation. Most patients, especially children have self-limited lymphadenopathy lasting two to eight weeks and do not require antibiotics [11]. In the immune suppressed patients, Bartonella henselae can cause bacillary angiomatosis and bacillary peliosis. Bacillary peliosis is caused only by B.henselaea and involves the liver and sometimes the spleen. The condition is more severe in patients whose immune system is compromised. Isolated splenic involvements as initial manifestation of CSD are extremely rare clinical occurrence; especially in patients with intact immune status [12,13].
Cat scratch disease is more common in autumn and winter months especially in warm and humid climates. Cats are considered to be the main reservoir of B. henselae and can transmit the pathogen to humans via scratches or bites, although naturally infected cats are asymptomatic. In worldwide B. henselae is reported in cats and fleas. However the presence of Bartonella species in fleas was not reported in Ethiopia until now, 11% prevalence of antibodies against Bartonella species was reported in cats from Addis Ababa [14]. However the information on the occurrence of Bartonella species in fleas that is collected from domestic animals with regard to public health importance of the disease in developing country is so poor and no more research has been conducted on cat scratch disease.
Therefore the objective of this seminar paper is:
: To review and provide information on cat scratch
disease and its zoonotic significance. Cat Scratch Disease and itʼs Zoonotic Significance
Historical background of cat scratch disease
Cat Scratch Disease (CSD) also known as Cat Scratch Fever, was first discovered in 1889 by Henri Parinaud. The cat was recognized as the natural reservoir of the disease in 1950. The causative organism was first thought to be “Afipiafelis” but this was disproved by immunological studies demonstrating that CSD patients developed antibodies to two other organisms, Bartonella henselae and Bartonella clarridgeiae1, which are rodshaped Gram negative bacteria [15].
Etiology
Cat scratch disease is an infection following the scratch of a cat (usually a kitten) with the organism Bartonella henselae, formerly known as Rochalimaea henselae; it is characterized by self-limited regional lymphadenopathy. The bacteria may infect cats and be spread to humans by bites or scratches. Cats rarely show signs of illness but humans can develop skin lesions, fever or in severe cases systemic (whole body) infection [16]. The response to infection with B. henselae depends on the immune status of the infected host. The species of Bartonella are fastidious, hemotropic, which are rod-shaped Gram-negative and aerobic bacilli bacterial belonging to the class Alphaproteobacteria. Several species have been implicated in causing human diseases ranging with short-term fever to severe endocarditis [17].
The bacteria are widespread in nature with several animal reservoirs (mainly cats, dogs, and rodents) and insect vectors (mainly fleas, sand flies, and human lice) [18]. The genus Bartonella have recently become associated with an increasing spectrum of diseases. Since the last 20 years, the number of Bartonella species or subspecies identified from a wide range of mammals has increased considerably [19]. Recently, Bartonella quintana was isolated from a pet cat [20]. B. bacilliformis, B. quintana and B.henselae are responsible for the majority of infections in humans [11,21].
Epidemiology
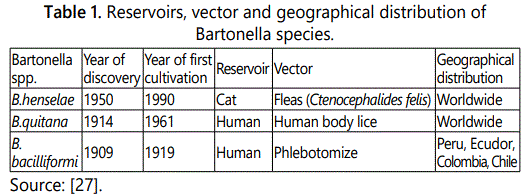
Geographical distribution and occurrence: The incidence of CSD is usually seasonal and with peaks in the autumn and winter, which may be explained by the breeding pattern of cats or the acquisition of pets at these times of year [23]. B. henselae has a worldwide distribution, with cases of classic Bartonella infection reported in the United States, Europe, Japan, New Zealand and Australia [24]. Seroepidemiologic studies have revealed worldwide distribution of B. henselae infection in domestic cats, with 4% to 80% of cats having antibodies against B. henselae (according to the geographic location) [15] (Table 1).
Additional epidemiologic studies in different animal and arthropod species and the public health significance of these zoonotic bacteria in different agroecologic zones are urgently needed in developing countries like Ethiopia [14].
The disease is more prevalent in areas with warm humid climates, In temperate climates, cat scratch disease predominantly occurs in autumn and winter; in the tropics, seasonal changes in frequency of the disease are not observed. However, it is not a reportable disease in humans in most countries. Therefore, sufficient data to determine the exact incidence or prevalence of Bartonella infection are not available. In United States, it was estimated that 22,000 to 24,000 humans developed cat scratch disease during 1992, of whom 2,000 were hospitalized [25]. Bacillary angiomatosis and bacillary peliosis are unusual vascular proliferative lesions observed in immune compromised humans as a result of infection by B.henselae or B.quintana. The Study show that of Bartonella infection among HIV-infected patients with fever, 68 of 382 (18%) patients had evidence of Bartonella infection detected via bacteriologic culture, indirect immunofluorescent antibody (IFA) testing, or PCR assay [26].
Risk factors: Risk factors that make cats more likely to have flea infestation and thus come infected with Bartonella are: originating as a stray, coming from a shelter or humane group, living in a multi cat household, going outdoors often, and living in a hot and humid area. Tick exposure was reported as a risk factor associated with CSD in humans [3]. Similarly, the tick exposure was determined to bea risk factors associated with the Bartonella vinsonii subspecies berkhoffi seropositivity in dogs. The specific role of ticks in Bartonella transmission requires additional studies. However, several recent publications have reported a high prevalence of Bartonella species infection in ticks from various parts of the world [28].
Source of infection: Cat fleas are the main vector and transmit infection through flea feces containing infective bacteria, which can survive several days in the environment. Stray and young cats are more likely to be bacteremic and being a source of human infection. Cats are known to be the most important source of infections for B. henselae [29]. Among cats, casual or sexual contact, sharing of food or water dishes are not significant sources of exposure, Bartonella species have also been found in other blood sucking arthropods such as ticks and flies but the role of these vectors in transmission of infection is still ill defined. Studies have shown that cats younger than one year old (kittens), cats from shelters or adopted as strays are more prone to carry the infection due to a higher probability of being bacteremic [9].
The vast majority of humans with cat scratch disease, bacillary peliosis or bacillary angiomatosis, B. henselae or B. quitana have been isolated as the agents. On the basis of serological tests, B. clarridgeiae has been suspected as the source of infection similar to cat scratch disease [30].
Transmission
Cats transmit the infection to humans through scratches when infective flea feces contaminate their claws and play critical role in the horizontal cat to cat transmission of B.henselae, means direct horizontal transmission from cat to cat and the possible vertical transmission from infected queens to their offspring [31]. The infection is transmitted to humans by cats or dogs and Cat fleas are the main vector and transmit infection through flea feces containing infective bacteria, which can survive several days in the environments and inoculated through the bite or the scratch of an infected cat thus configuring a zoonotic infection [32]. All known Bartonella species are transmitted by a spectrum of arthropod vectors, by animal bites or scratches, by blood transfusion from an infected donor, or mechanically by needle sticks [33]. Susceptible cats housed in an arthropod-free environment in prolonged intimate contact with infected cats remained non-bacteremic and seronegative [34].

Pathogenesis
The pathogenesis of CSD remains poorly understood [36]. Early in the clinical course of CSD in immunocompetent persons, histological examination of skin lesions and lymph nodes reveals lymphoid hyperplasia and arteriolar proliferation [37]. The disease manifestations result from either local infection, such as lymphadenopathy, or from blood borne disseminated infection, such as occurs with neuroretinitis or visceral organ involvement. B. henselae and the organism cause intraerythrocytic bacteremic that can persist for a year or longer in some cats. In the mammalian reservoir, Bartonella initially infect a yet unrecognized primary niche, which seeds organisms into the blood stream leading to the establishment of a long lasting intra erythrocytic bacteraemia as the hall mark of infection [19].
Furthermore, the bacterial adhesions mediate a critical, the early step in the pathogenesis of the Bartonella by binding to extracellular matrix components of host cells, which leads to firm bacterial adhesion to the cell surface as a prerequisite for the efficient translocation of type IV secretion effect or proteins [38]. It remains unknown why some patients have infection that remains localized whereas others develop disseminated disease Infection that remains localized [39].
Clinical Signs
In cats: Because of the high prevalence of infection in cats, it has been difficult to associate infection with specific clinical signs [40]. Even though, the despite clinical of the manifestations of Bartonella infection are expanding with the improved ability to recognize the presence of the bacteria. Naturally infected cats are usually asymptomatic, although they may suffer from anterior uveitis, lymphadenitis, gingivitis and stomatitis and are predisposed to neurological diseases. No major clinical signs of CSD have been reported in cats under natural conditions [8].
In experimentally infected cats, some research groups found no clinical signs, whereas others reported fever, moderate neurological symptoms and lymphadenopathy [15]. Cats those inoculated with B. henselae experience nonspecific febrile illness and transient anemia [27]. Additionally, reproductive disorders (inability to become pregnant, pregnancy achieved only after repeated breedingʼs, and stillbirths) have been observed in queens infected by test [41].
In Dogs: Bartonella vinsonii subspecies berkhoffi (Bvb) has been identified as an important cause of endocarditis in dogs and human was reported. The known clinical spectrum of this infection in dogs continues to expand and includes cardiac arrhythmias, myocarditis, granulomatous, lymphadenitis and granulomatous rhinitis [42]. Nevertheless Bartonella vinsonii subspecies berkhoffi has also been isolated from clinically healthy dogs, which may be long term carriers of the bacterium. Dogs may also be infected with several other Bartonella species, including B.clarridgeiae which has been isolated from a blood sample obtained from a dog with inflammation of endocardium and detected in a liver sample [43].
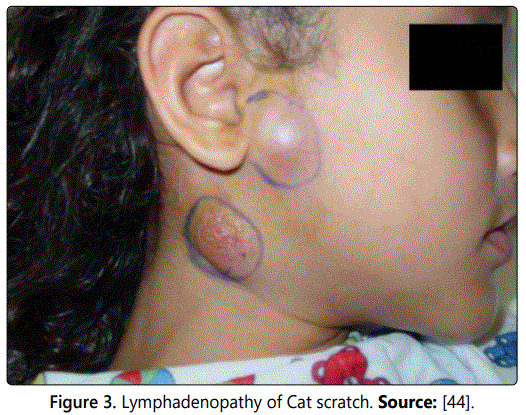
In humans: In some individuals, the organisms disseminate and infect the liver, spleen, eye, or central nervous system. Patients with localized disease generally have a self-limited illness; whereas those with disseminated disease can have life threatening complications. The most common clinical manifestation of CSD is chronic lymphadenopathy. Affected node is often warm, tender and erythematous (you can see the figure 3). Fever occurs in approximately half of patients and systemic symptoms such as fatigue, malaise, anorexia and headache may occur and are generally mild. Most patients (approximately 50-85%) have only single node involvement, with the axillary and epitrochlear, head and neck, and inguinal nodes most commonly affected [44].
Bartonella henselae is a vascular proliferative disease of the skin, characterized by multiple, blood filled and cystic nodules. The cutaneous lesion evolves from a vesicle to a pustule, and finally to a papule, or more frequently from a macule to a papule [45]. The lesions resolve within a few days to several weeks. Inoculation lesions are non-pruritic and heal without scar formation [46].
In immune competent patients: Cat scratch disease caused by B.henselae is mainly characterized by a benign regional Lymphadenopathy [47]. Seven to 12 days after receiving a cat scratch (or a bite), a papule and then a pustule develop at the inoculation site. Regional lymphadenopathy develops 1 to 3 weeks after the inoculation and can persist for a few weeks to several months. Patients with cat scratch disease encephalopathy usually completely recover within 1 year without any sequelae. Bartonella henselae was also recently identified as a frequent cause of prolonged fever and fever of unknown origin (FUO) in children. Rheumatic manifestations of Bartonella infection have also been described in children, arthritis and skin nodules [48].
In immunocompromised patients: The bacillary angiomatosis is one of the most common clinical manifestations of Bartonella infections [49]. In affected individuals, chronic vascular proliferative lesions, which are clinically and histologically similar caused by B bacilliformis, are observed. Persons infected with HIV that have CD4+ cell counts of <50/mm3 are likely to develop lesions of bacillary angiomatosis [50]. Histologically, cutaneous bacillary angiomatosis is characterized by a tumor like growth pattern with proliferation of capillaries that have protuberant epithelioid endothelial cells [51].
Diagnosis
The DNA of B.henselae and B.quitana were also detected in the cardiac tissue [52]. The Bartonella species are difficult to make culture and culture is not routinely recommended. Furthermore, blood samples from most cats with B.henselae associated endocarditis yield no growth of the organism in bacteriologic culture but yield positive results for the bacteria via DNA amplification [53]. Diagnosis of CSD usually requires three of the following four criteria: a history of contact with a cat and the presence of a scratch or primary lesion of the skin, eye or mucous membrane, a positive cat scratch skin test reaction, negative laboratory testing for other causes of lymphadenopathy and characteristic histopathological findings in a lymph node biopsy specimen or at a site of systemic involvement [27].
Serology: It is the best initial test and can be performed by indirect fluorescent assay (IFA) or Enzyme-linked Immunosorbent assay (ELISA). Although more sensitive than culture, serologic tests lack specificity because many asymptomatic cats have positive serology because of previous (often asymptomatic) exposure [54]. A scratch or injury and a history of contact with a cat indicate that cat scratch disease is a possible cause of the lymph node swelling. In some cases, physical examination also shows an enlarged spleen or splenomegaly [16].
Polymerase chain reaction (PCR): Can detect different Bartonella species; specificity is very high, but the sensitivity is lower than with serology [10]. The PCR technique is a highly specific and rapid method for definitive species identification in the diagnosis of Bartonella species Infections [55].
Differential diagnosis: The differential diagnosis of cat scratch disease is lymphadenopathy includes other causes of sub-acute or chronic lymphadenopathy [56]. Primarily, this means other granulomatous infections such as Mycobacterium tuberculosis as well as non tuberculous mycobacteria, fungi, nocardia, and actinomyces. Non-infectious diseases that may present with similar manifestations include malignancies and autoimmune diseases. In the first few days of illness, the differential diagnosis includes acute bacterial lymphadenitis [44].
Treatments
In Cats: antimicrobial agents are not commonly used or recommended for the treatments or for prevention of infection with B.henselae. Thus, antimicrobial (enrofloxacin or doxycycline) treatments evaluated in cats to date may reduce the level of bacteremic but do not eliminate the infection [57]. Additionally, the minimal effectiveness of these antimicrobial agents could be explained by the fact that Bartonella species are intracellular (especially intraerythrocytic) organisms [50].
In dogs: When amino glycosides are administered to dogs with endocarditis, renal function should be monitored carefully. Antimicrobial treatment may not be effective when the lesions of endocarditis are already well established. The use of antimicrobials that achieve high intracellular concentrations, such as doxycycline, fluoroquinolones, or azithromycin, would be required to eliminate intracellular infection [58].
Human: Most patients, especially children, have self-limited lymphadenopathy lasting two to eight weeks and do not require antibiotics. Up to 14 percent of persons develop dissemination to the liver, spleen, eye, or central nervous system (CNS) and antibiotics may help [11]. The therapeutic approach to Bartonella infection varies on the basis of the clinical manifestations and immune status of the patient. There is a paucity of data in the literature as to the most effective therapy in all cases of Bartonella infection, with most data presented as part of case series rather than randomized, controlled trials. There is a significant divide in the literature between in vitro efficacy of antibiotics and the ability to successfully treat in clinical practice. For patients with significant lymphadenopathy, treatment with azithromycin at doses of 10 mg/kg on day 1 and 5 mg/kg per day on days 2 to 5 can be considered [27].
In vitro Bartonella species have been found to be susceptible to a number of antimicrobial agents, including macrolides (azithromycin, clarithromycin, erythromycin), amino glycosides, lactase (penicillin G, amoxicillin) [24]. Supportive therapy with antipyretics and analgesics should be given as needed and local heat may relieve the pain of enlarged lymph nodes. Aspiration of fluctuant tender nodes may help to relieve pain but incision and drainage should be avoided, as this may leave scars and fistulae. A minority of patients may, however, require surgical treatment. The condition is usually self-limiting in immune competent patients and, in the majority of patients; the lymph nodes gradually regress over weeks or months without antibiotics being needed. So, Patients should follow up in 2-6 months for confirmation of symptom resolution [5].
Prognosis: Prognosis varies from grave to good, depending up on the disease manifestations and response to antibiotics [59]. Complete recovery is usual in 2-5 months. Rarely, there is severe hepatic or neurological involvement, in which case granulomatous hepatitis, therefore neuroretinitis and peripheral neuritis can occur. The condition is more severe in patients whose immune system is compromised (Untreated); the disease can be fatal [16].
Control and Prevention
Prevention of CSD includes elimination of cat fleas from cats and avoidance of traumatic injury from cats, which is particularly important for immune compromised patients. If possible, the cat should be an adult and obtained from a flea controlled environment. Cats could be serologically tested, so that prospective owners could adopt only a seronegative animal [15]. Declawing the cats has also been suggested. Therefore, flea control appears to be one of the major measures needed to prevent cats from becoming infected and B.henselae being spread from cat to cat. The most effective means of preventing infection by B.henselae are common sense; keep the hygiene of cat and possibly changing the behavior of cat owners themselves. Peoples wash their hands after handling pets, and clean any cuts, bites or scratches promptly with soap and water. Infections with Bartonella species in dogs are likely to be vector borne. A tick vector is strongly suspected for B. vinsonii subspecies berkhoffi. Never force your attentions on a cat that does not welcome them. Most importantly, always do what is necessary to control fleas. Persons should avoid playing roughly with cats and kittens to minimize cat induced scratches and bites [33].
Development of a feline vaccine to prevent the spread of infection in cat populations and to reduce the risk of infection of humans ,will be difficult, given the diversity of Bartonella Species and types harbored by cats ,and the lack of cross-protection between species. Use insect controls measures for people and pets. Avoid direct contact with feral cats and wild animals [60].
Status of Cat Scratch disease in Ethiopia
The presence of Bartonella species in fleas was not reported in Ethiopia until recently. However, an 11% (5 of 46) prevalence of antibodies against Bartonella species was reported in cats from Addis Ababa [61] and Bartonella species related to B. Elizabethan were reported in small mammals in northern Ethiopia. As a result, studies of the role of fleas as vectors of pathogens of veterinary and medical importance in Ethiopia are lacking [14]. Current study was conducted to determine the presence of Bartonella and Rickettsia species in fleas collected from domestic dogs and cats in central Oromia, Ethiopia [62].
Conclusion and Recommendations
Cat scratch disease is caused by Bartonella henselae, a fastidious, slow-growing Gram-negative bacillus. The organism is transmitted from cats (and, rarely, dogs) to humans via scratches, licks and bites. Approximately 80% of cats are asymptomatically infected (and even bacteremic) with B. henselae at some point in their lives, and kittens and outdoor cats are more likely to be infected. Nonetheless, epidemiologic studies suggest that contact with flea-infested kittens increases the risk of CSD. Although CSD occurs in persons of all ages, the vast majority of cases occur in children younger than 10 years of age. CSD is more common during rain fall and winter months for reasons that is unclear, but may reflect seasonal fluctuations in transmission of the organism among cats or in animal behavior. Infected cats are usually asymptomatic and there are no commercially available vaccines to prevent B.henselae infection in cats. Therefore as a prevention eliminating cat fleas from cats and avoidance of traumatic injury is important for immune compromised patients.CSD in immune competent persons generally is self-limited and requires supportive care in most patients.
Based on the above conclusion the following recommendations are forwarded:
- The bite or scratch sites should be washed immediately by soap.
- Care should be given when handling animals and cleaning up feces, urine, or vomit.
- The child should be kept away from areas that may be contaminated with pet feces.
- Insect controls measures should be done and Number of cat should be limit in the home.
- Cat and dogs should be discouraged from licking a personʼs skin, particularly eyes, mucous membranes and broken skin.
Acknowledgements
First and foremost, I give all the praise and glory to almighty God and Kidanemeheret for their unfailing faithfulness and grace that kept me safe and floating in all stages of my academic struggle and even beyond and for their infinite love and care throughout my life.
Secondly, my special thanks goes to my advisor Dr. Misgana Duguma (Assistant professor) for his earnest and constructive comments and guidance throughout the preparation of this paper and also I would never forget Dr. Girma Kebede (Assistant professor, I have great respect and thanks for his noble hearten help and encouragement.
My thanks also extend to Wollega University, School of Veterinary Medicine for their preparation of this interesting and educative Senior Seminar presentation.
Finally, my honorable appreciation goes to my family and all classmates who support me during all my work especially, my lovely friend Aster (Meti) Siyoum for their endless ideological and financial support mainly during these stressful and difficult moments.
References
- Calistri P, Iannetti S, Danzetta L, et al. The components of ‘One World One Health’ approach. Transbound Emerg Dis. 2013; 60: 4–13. doi: 10.1111/tbed.12145.
- Zambori C, Cumpanasoiu C, Mladin B. Biofilms in oral cavity of dogs and implication in zoonotic infections. Anim Sci Biotechnol. 2013; 46(1): 83.
- Oray M. Diverse Clinical Signs of Ocular Involvement in Cat Scratch Disease. Turk J Ophthalmol. 2017; 47(1): 9–17. doi: 10.4274/tjo.28009
- Florin TA, Zaoutis TE, Zaoutis LB. Beyond cat scratch disease: widening spectrum of Bartonella henselae infection. Pediatrics. 2008; 121(5): 1413-1425. doi: 10.1542/peds.2007-1897
- Zangwill KM, Hamilton DH, Perkins BA. Cat scratch disease in Connection. Epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med. 1993; 329(1): 8-13. doi: 10.1056/NEJM199307013290102
- Tsukahara M, Tsuneoka H, Lino H, Ohno K, Murano I. Bartonella henselae infection from a dog. Lancet. 1998; 352(9141): 1682.
- Lappin MR, Kordick DL, Breitschwerdt EB. Bartonella species antibodies and DNA in aqueous humour of cats. J Feline Med Surg. 2000; 2(1): 61-68. doi: 10.1053/jfms.2000.0067
- Guptill L, Wu CC, HogenEsch H, et al. Prevalence, risk factors and genetic diversity of Bartonella henselae infections in pet cats in four regions of the United States. J Clin Microbiol. 2004; 42(2): 652-659.
- Massei F, Gori L, Macchia P, Maggiore G. The expanded spectrum of bartonellosis in children. Infect Dis Clin North Am. 2005; 19(3): 691-711. doi: 10.1016/j.idc.2005.06.001
- Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004; 48(6): 1921-1933. doi: 10.1128/AAC.48.6.1921-1933.2004
- Koehler JE, Tappero JW. Bacillary angiomatosis and bacillary peliosis in patients infected with human immunodeficiency virus. Clin Infect Dis. 1994; 17(4): 612-624.
- Gilad J, Wolak A, Borer A, et al. Isolated Splenic scratch disease in an immunocompetent adult woman. Clin Infect Dis. 2003; 36(1): 10-13. doi: 10.1086/344771
- Kumsa B, Parola P, Raoult D, Socolovschi C. Molecular Detection of Rickettsia fleas and Bartonella henselae in Dog and Cat Fleas in Central Oromia, Ethiopia. Am J Trop Med Hyg. 2014; 90(3): 457-462. doi: 10.4269/ajtmh.13-0010
- Chomel BB. Cat scratch disease. Rev Sci Tech. 2000; 19(1): 136-150.
- Klotz SA, Iianas V, Elliott SP. Cat scratch Disease. Am Fam Physician. 2011; 83(2): 152-155.
- Houpikian P, Raoult D. Western immunoblotting for Bartonella endocarditis. Clin Diagn Lab Immunol. 2003; 10(1): 95-102. doi: 10.1128/CDLI.10.1.95-102.2003
- Lamas C, Curi A, Bóia MN, Lemos ERS. Human bartonellosis: seroepidemiological and clinical features with an emphasis on data from Brazil - A Review. 2008; 103(3): 62. doi: 10.1590/S0074-02762008000300001
- Chomel B, Boulouis H, Breitschwerdt EB, et al. Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet Res. 2009; 40(2): 29. doi: 10.1051/vetres/2009011
- Breitschwerdt EB, Maggi RG, Duncan AW, Nicholson WL, Hegarty BC, Woods CW. Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Infect Dis. 2007; 13(6); 938-941. doi: 10.3201/eid1306.061337
- Eskow E. Concurrent infection of the centeral nerve system by Barrollia burgdorferib and Bartonella henselae infection. Evidence for a novel tickborne disease complex. Arch Neurol. 2001; 58(9): 1357-1363.
- Windsor JJ. Cat-scratch disease: epidemiology, etiology and treatment. Br J Biomed Sci. 2001; 58(2): 101-110.
- Florin TA, Zaoutis TE, Zaoutis LB. Beyond Cat Scratch Disease Widening Spectrum of Bartonella henselae Infection. Pediatrics. 2008; 121(5): e1413-e1425. doi: 10.1542/peds.2007-1897
- Chomel B, Boulouis H, Maruyama S, Breitschwerdt EB. Bartonella species in pets and effect on human health. Emerg Infect Dis. 2006; 12(3): 389-394. doi: 10.3201/eid1203.050931
- Koehler JE, Sanchez MA, Tye S, et al. Prevalence of Bartonella infection among human immune deficiency virus infected patients with fever. Clin Infect Dis. 2003; 37(4): 559-566. doi: 10.1086/375586
- Mavrouli M, Vrioni G, Papaparaskevas J, Kapsimali V. Bartonella infections: clinical manifestations, diagnostic techniques and treatment. Bartonella INFECTIONS. 2016; 61(1): 7-15.
- Chomel BB, Boulouis H, Maruyama S, Breitschwerdt EB. Bartonella species in pets and effect on human health. Emerg Infect Dis. 2006; 12(3): 389-394. doi: 10.3201/eid1205.050931
- Skerget M, Wenisch C, Daxboeck F, Krause R, Haberl R, Stuenzner D. Cat or Dog Ownership and Seroprevalence of Ehrlichiosis, Q Fever, and CatScratch Disease. Emerg Infect Dis. 2003; 9(10): 1337-1340. doi: 10.3201/eid0910.030206
- ESCCAP. Control of Vector Borne Diseases in cat and dog. 2nd Edition. ESCCAP Guideline 05. 2012.
- Moavero C, Giuffré G, Mangione A, et al. Atypical Bartonella henselae neuroretinitis in an immunocompetent patient. Acta Medical Mediterranea. 2014; 30(2): 429-433.
- Centers for Disease Control and Prevention (CDC). Cat Scratch Disease in children. JAMA. 2002; 287(20): 2647-2649. doi: 10.1001/jama.287.20.2647-JWR0522-2-1
- Cotté V, Bonnet S, Le Rhun D, et al. Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis. 2008; 14(7): 1074-1080. doi: 10.3201/eid1407.071110
- Jacomo V, Kelly PJ, Raoult D. Natural history of Bartonella infections (an exception to Kochʼs postulate). Clin Diagn Lab Immunol. 2002; 9(1): 8-18. doi: 10.1128/CDLI.9.1.8-18.2002
- English R. Cat-Scratch Disease. Pediatrics in Review. 2006; 27(4): 123.
- Zhang P, Chomel BB, Schau MK, et al. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and auto aggregation in Bartonell aquintana. Proc Natl Acad Sci U S A. 2004; 101(37): 13630-13635. doi: 10.1073/pnas.0405284101
- Dehio C. Molecular and cellular basis of Bartonella pathogenesis. Annu Rev Microbiol. 2004; 58: 365-390. doi: 10.1146/annurev.micro.58.030603.123700
- Regnery RL, Rooney JA, Johnson MA, et al. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am J Vet Res. 1996; 57(12): 1714-1719.
- Guptill L, Slater LN, Wu CC, et al. Evidence of reproductive failure and lack of prenatal transmission of Bartonella henselae in experimentally infected cats. Vet Immunol Immunopathol. 1998; 65(2-4): 177-189.
- Pappalardo BL, Brown T, Gookin JL, Morrill CL, Breitschwerdt EB. Granulomatous disease associated with Bartonella infection in 2 dogs. J Vet Intern Med. 2000; 14(1): 37-42.
- Chomel B, MacDonald KA, Kasten RW, et al. Aortic valve endocarditis in a dog due to Bartonella clarridgeiae. J Clin Microbiol. 2001; 39: 3548–3554. doi: 10.1128/JCM.39.10.3548-3554.2001
- Lieberman JM. Cat Scratch Disease. Cancer Therapy Advisor. 2017.
- Margileth AM. Cat scratch disease. Adv Pediatr Infect Dis. 1993; 8: 1-21.
- Koehler JE, Sanchez MA, Garrid CS, et al. Molecular epidemiology of Bartonella infections in patients with bacillary angiomatosis- peliosis. N Engl J Med. 1997; 337(26): 1876-1883. doi: 10.1056/NEJM199712253372603
- Jacobs RF, Schutze GE. Bartonella henselae as a cause of prolonged fever and fever of unknown origin in children. Clin Infect Dis. 1998; 26: 80-84.
- Chian CA, Arrese JE, Piérard GE. Skin manifestations of Bartonella infections. Int J Dermatol. 2002; 41(8): 461-466.
- Chomel BB, Boulouis HJ, Breitschwerdt EB. Cat scratch disease and other zoonotic Bartonella infections. J Am Vet Med Assoc. 2004; 224(8): 1270-1279 doi: 10.2460/javma.2004.224.1270
- Krause R, Auner HW, Daxbock F, et al. Monoclonal and biclonal gammopathy in two patients infected with Bartonella henselae. Ann Hematol. 2003; 82(7): 455-457. doi: 10.1007/s00277-003-0675-4
- Wesslen L, Ehrenborg C, Holmberg M, et al. Subacute Bartonella infection in Swedish orienteers succumbing to sudden unexpected cardiac death or having malignant arrhythmias. Scand J Infect Dis. 2001; 33(6): 429-438.
- Fournier PE, Lelievre H, Eykyn SJ, et al. Epidemiologic and clinical characteristics of Bartonella Quintana and Bartonellahenselae endocarditis: a study of 48 patients. Medicine (Baltimore). 2001; 80(4): 245-251.
- Podsiadły E, Sapiejka E, Dąbrowska-Bień J, Majkowski J, TylewskaWierzbanowska S. Diagnostics of cat scratch disease and present methods of bartonellosis recognition a case report. Pol Merkur Lekarski. 2009; 26(152): 131-135.
- Dauga C, Miras I, Grimont PA. Identification of Bartonella henselae and B. quintana 16s rDNA sequences by branch-, genus- and species-specific amplification. J MedMicrobiol. 1996; 45(3): 192-199. doi: 10.1099/00222615-45-3-192
- Klein JD. Cat scratch disease. Pediatr Rev. 1994; 15(9): 348-353.
- Kordick DL, Papich MG, Breitschwerdt EB. Efficacy of enrofloxacin or doxycycline for treatment of Bartonella henselae or Bartonella clarridgeiae infection in cats. Antimicrob Agents Chemother. 1997; 41(11): 2448-2455.
- Breitschwerdt EB, Blann K, Stebbins ME, et al. Clinic pathologic abnormalities and treatment response in 24 dogʼs seroreactive to Bartonella vinsonii subsp. Berkhoffi antigens: a retrospective study. J Am Anim Hosp Assoc. 2004; 40(2): 92-101. doi: 10.5326/0400092
- Yamamoto K, Chomel BB, Kasten RW, et al. Homologous protection but lack of heterologous-protection by various species and types of Bartonella in specific pathogen free cats. Vet Immunol Immunopathol. 1998; 65(2-4): 191-204.
- Tiao N, Darrington C, Molla B, Saville WJ, Tilahun G. An investigation into the seroprevalence of Toxoplasma gondii, Bartonella spp., feline immunodeficiency virus (FIV), and feline leukaemia virus (FeLV) in cats in Addis Ababa, Ethiopia. Epidemiol Infect. 2013; 141(5): 1029-1033. doi: 10.1017/S0950268812001707
- Meheretu Y, Leirs H, Welegerima K, et al. Bartonella prevalence and genetic diversity in small mammals from Ethiopia. Vector Borne Zoonotic Dis. 2013; 13(3): 164-175. doi: 10.1089/vbz.2012.1004


