Short Communication
Radiosensitization of Prostate Cancer Cells by 2-Deoxyglucose
Radiation Oncology, Institute of Cancer Sciences, University of Glasgow, Glasgow, Scotland
*Corresponding author: Colin Rae, Institute of Cancer Sciences, University of Glasgow, Garscube Estate, Glasgow G61 1BD, Scotland, Tel: +44(0)141 330 4129, E-mail: colin.rae@glasgow.ac.uk
Received: February 8, 2018 Accepted: February 28, 2018 Published: March 6, 2018
Citation: Rae C, Sey CHC, Mairs RJ. Radiosensitization of Prostate Cancer Cells by 2-Deoxyglucose. Madridge J Oncogenesis. 2018; 2(1): 30-34. doi: 10.18689/mjo-1000105
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Prostate cancer is the most common malignancy of men. Treatment options include radiotherapy with or without hormonal manipulation and radical prostatectomy. However, there is no effective treatment for disseminated disease. A hallmark of malignancy is abnormal metabolism which also confers survival advantages and contributes to resistance to therapy. In response to exposure to ionizing radiation, metabolic pathways are activated which can protect the cell from irreversible injury. Tumor cell glycolytic activity is elevated and correlates with aggressiveness and radio resistance, indicating that targeting glucose metabolism may sensitize cancer cells to radiation. We have demonstrated that the clonogenic kill of PC3 cells induced by exposure to x-rays was enhanced by the glycolytic inhibitor 2-deoxyglucose (2DG). In contrast, treatment with 2DG failed to inhibit growth of multicellular spheroids derived from LNCaP cells. However, 2DG treatment, in the absence of irradiation, induced similar toxicity to PC3 and LNCaP cells cultured as monolayers. Radiation-induced cell cycle arrest was prevented by the simultaneous administration of 2DG in both cell lines, indicating a possible mechanism underlying sensitization. Therefore, we hypothesise that observed differences in cellular response to incubation with 2DG in the presence or absence of ionizing radiation resulted from variation in metabolic processes between tumor cell types. We conclude that inhibition of glucose metabolism by 2DG is an effective method for sensitizing prostate cancer cells to experimental radiotherapy and that this may occur by preventing DNA repair during radiation-induced cell cycle arrest.
Keywords: Radiotherapy; Prostate cancer; Glycolysis; Sensitizer.
Introduction
Prostate cancer is the most common cancer amongst men. Prostate cancer is a disease that predominantly affects men over the age of 50 and the number of deaths resulting from this disease is predicted to rise in the coming years as a result of increased life expectancy. Radiotherapy is widely used in the treatment of prostate cancer patients, with or without hormonal manipulation and radical prostatectomy. However, damage to neighbouring normal organs limits the radiation dose which can be delivered.
Although there is currently no effective treatment for disseminated disease, it is likely that the efficacy of radiotherapy can be enhanced by combination with radiosensitizing agents. A hallmark of malignancy is abnormal metabolism which generates biomass and energy for proliferation, migration and cell signalling. Such metabolic adaption by cancer cells also confers survival advantages to these cells and contributes to resistance to therapy. Furthermore, in response to exposure to ionizing radiation, metabolic pathways are activated which can protect the cell from irreversible injury [1]. Interestingly, it has been reported that radioresistant cancer cells have altered components of energy metabolism compared with their radiosensitive counterparts [2]. This suggests that targeting one or more of these metabolic pathways may sensitize cancer cells to radiation.
One characteristic of this metabolic re-programming is increased glucose consumption and lactate production, even in the presence of oxygen. This is termed aerobic glycolysis or the Warburg effect [3, 4]. Tumor cell glycolytic activity results in accumulation of pyruvate, lactate and glutathione, which counteract reactive oxygen species (ROS) [5], and correlates with tumor aggressiveness and radioresistance [6]. Targeting glucose metabolism, using the glycolytic inhibitor 2-deoxyglucose (2DG), is toxic to cancer cells and sensitises them to radiation [6-10]. 2DG competes with glucose for transmembrane transport. However, after hexokinase converts 2DG into 2-deoxyglucose-6- phosphate, it cannot be further metabolized. Cancer cells are more susceptible to inhibition of glucose metabolism in the presence of increased ROS [11], reflecting the close link between tumor glucose metabolism and altered cellular redox status, which may be further manipulated by ionizing radiation. Radiosensitization by 2DG may therefore be partially explained by the decreased antioxidant capacity after 2DG treatment [5,8,9], as well as inhibition of ATP-dependent DNA repair [12].
We sought to determine the ability of 2DG to sensitize prostate cancer cells to experimental radiotherapy in monolayer and 3-dimensional cultures and to assess the role of cell cycle regulation in 2DG-induced radiosensitization.
Materials and Methods
Reagents
All cell culture media and supplements were purchased from Life Technologies (Paisley, UK), unless stated otherwise. All other chemicals, including 2DG, were from Sigma-Aldrich (Dorset, UK).
Tissue Culture
Human prostate cancer cell lines, PC3 and LNCaP, were obtained from American Type Culture Collection (Manassas, VA, USA) and were used in this study for less than 6 months after resuscitation. PC3 cells were maintained in F12K medium supplemented with 10% (v/v) fetal bovine serum (Autogen Bioclear, Wiltshire, UK), 2 mM L-glutamine, 0.1 mM sodium pyruvate and 10 µg/ml gentamicin. LNCaP cells were maintained in RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum (Hyclone, Fisher Scientific, UK), 1% (v/v) HEPES, 1% (v/v) D-glucose, 1 mM sodium pyruvate, 4 mM L-glutamine, 10 µg/ml gentamicin.
Clonogenic Survival Assay
PC3 cells were seeded in 25 cm2 flasks at 105 cells/flask. When cultures were in exponential growth phase, medium was removed and replaced with fresh medium containing drug. Cells were irradiated using an X-Strahl RS225 X-ray irradiator at a dose rate of 1.6 Gy per minute. After treatment for 24 h, cells were seeded for clonogenic survival assay as previously described [13,14]. Cells were incubated at 37°C in 5% CO2 for 13 days. Colonies were fixed in methanol, stained with crystal violet solution and colonies of at least 50 cells were counted.
Multicellular Spheroid Growth Assay
Multicellular tumor spheroids consisting of LNCaP cells were obtained using the liquid overlay technique [15]. Spheroids were initiated by inoculating cells into a plastic flask coated with 1% (w/v) agar. After 3 days, aliquots of spheroids were transferred to sterile plastic tubes and centrifuged at 12 x g for 3 min. Thereafter, spheroids were irradiated or re-suspended in serum-free culture medium containing 2DG. After treatment, the spheroids were washed twice and those of approximately 100 µm in diameter were transferred individually into agar-coated wells of 24-well plates, as previously described [16]. Individual spheroid growth was monitored twice per week for three weeks using an inverted phase-contrast microscope connected to an image acquisition system. Two perpendicular diameters, dmax and dmin, were measured using image analysis software (Image J) and the volume, V (µ3) was calculated, as previously [16], using the formula: V = π × dmax × dmin² / 6. The area under the V/V0 versus time curve (AUC) was calculated for individual spheroids using trapezoidal approximation.
Cell Cycle Analysis
Following treatment for 6 h, LNCaP or PC3 cells were trypsinized, washed twice with PBS then fixed with ice cold 70% (v/v) ethanol. After fixation, cells were washed twice with PBS and re-suspended in PBS containing propidium iodide (10 μg/ml) and RNase A (200 μg/ml). Cells were stained for 30 min before flow cytometric analysis using a FACSVerse analyzer (BD Biosciences, Oxford, UK). Data were analyzed using FlowJo software.
Statistical Analysis
Data are presented as means ± standard error of the mean (SEM), unless otherwise stated, with the number of independent repetitions provided in the legend to each figure. Statistical significance was determined using Student's t test. A P value < 0.05 was considered to be statistically significant.
Results
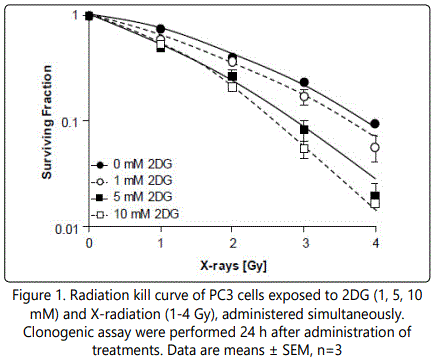
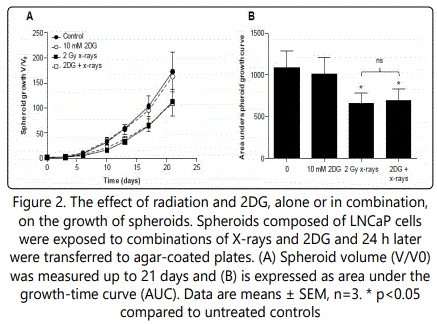
The ability of 2DG to enhance the radiation-induced clonogenic kill was assessed by clonogenic assay of PC3 cells (LNCaP cells did not form colonies). Simultaneous administration of 2DG (1-10 mM) with radiation (1-4 Gy x-rays) resulted in a concentration- dependent decrease in clonogenic survival. Surviving fractions after 2 Gy (SF2) were 0.40, 0.37, 0.27 and 0.21 for 0, 1, 5 and 10 mM 2DG, respectively. The divergence of the survival curves in Figure 1 suggests that the cells were sensitized to radiation-induced clonogenic kill by the administration of 2DG. The efficacy of the combination of 2DG with x-radiation was further evaluated in a three-dimensional model of metastatic prostate tumors (multicellular tumor spheroids) comprised of LNCaP cells (PC3 cells did not form spheroids). Radiation alone (2 Gy x-rays) decreased spheroid growth (Figure 2A), manifest by an area under the growth-time curve (AUC) of 64% of untreated control spheroids (Figure 2B). However, 2DG alone (10 mM) did not significantly reduce spheroid growth nor did it enhance the radiation- induced inhibition of growth as indicated by AUC values of 96% and 65% for 2DG alone and in combination with x-rays, respectively.
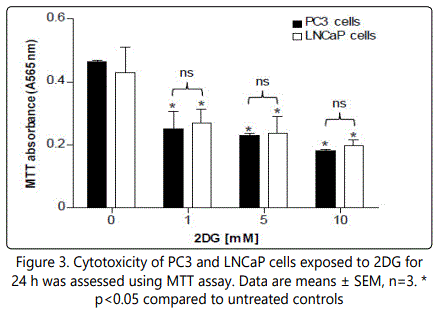
Cytotoxicity of 2DG was assessed in monolayer cultures of PC3 and LNCaP cells using the MTT assay. Our results demonstrated that the concentrations of 2DG used in this study were significantly toxic to both cell lines (Figure 3). Importantly, the response to 2DG did not differ significantly between cell lines.
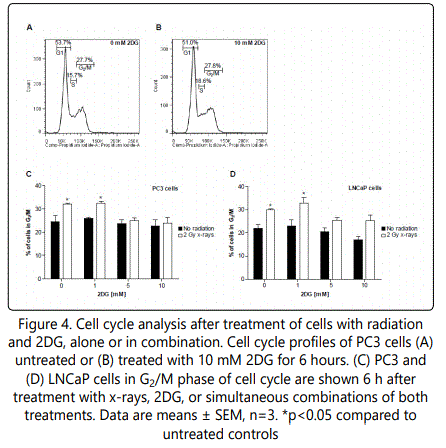
Cell cycle analysis revealed that 2DG had no significant effect on cell cycle distribution of PC3 cells or LNCaP cells (Figure 4). However, radiation (2 Gy x-rays) induced a significant increase in the proportion of cells in the G2/M phase of cell cycle in both cell lines 6 h after treatment (Figures 4C and 4D). This radiation-induced arrest was prevented by simultaneous administration of 2DG (5 or 10 mM) in both PC3 and LNCaP cell lines.
Discussion
It has previously been reported that inhibition of glycolysis by 2DG enhanced the clonogenic kill induced by radiation treatment in several cancer cell lines [6-10]. Although prostate cancer is not characterized by glycolytic activity as high as many other solid cancers, increased glycolysis is observed in the advanced stages of the disease [17], which is the target of combination radiotherapy being investigated here.
Several mechanisms of radiation sensitizing action by 2DG have been suggested, including alterations in ROS levels, inhibition of ATP generation, reduced antioxidant capacity [2,6,8,9,12]. Alterations in cell cycle progression may also affect sensitivity to radiation. In the Kelly neuroblastoma cell line, 2DG caused accumulation of cells in G1 phase of cell cycle, but did not affect the cycling of SK-N-SH cells [18]. We observed that six hours after radiation treatment, the proportion of cells in G2/M cells was significantly increased in both PC3 and LNCaP cell lines. We previously reported that PC3 and LNCaP cells differ in their cell cycle response to ionizing radiation [19]. Specifically, 24 h after irradiation of PC3 cells, the cell cycle distribution returned to control levels whereas in LNCaP cells, radiation caused an increase in G1 phase and a decrease in G2/M phase 24 h after irradiation. As a single treatment, 2DG alone did not significantly alter the proportion of G2/M cells 6 h after administration, but the radiation-induced arrest was returned to control levels by 2DG. Furthermore, reduced DNA repair in response to radiation has previously been reported after administration of 2DG [20]. Although cells in the G2/M phase are considered more sensitive to radiation than cells in other phases [21], it is possible that the prevention of cycle arrest by 2DG may result in entry of cells into mitosis with unrepaired DNA, resulting in mitotic catastrophe and cell death. This is consistent with the observed enhancement of radiation-induced clonogenic kill.
PC3 and LNCaP cells differ with regards to their p53 status, inasmuch as PC3 cells harbour non-functional p53, whereas LNCaP harbour wild-type p53 [22]. However, the observed effect of radiation on cell cycle distribution (G2/M arrest 6 h after treatment) was similar in both cell lines, suggesting that the ability of 2DG to prevent radiationinduced G2/M arrest is not affect by p53 status in these cells. This is in contrast to a previous report suggesting that expression of wild-type p53 in lung cancer cell lines is necessary for 2DG-mediated radiosensitization [10]. Unfortunately, the latter report did not describe the effects of 2DG on cell cycle. The scheduling of treatment may also influence the role of p53. It is noteworthy that simultaneous administration of treatment was used in the current study whereas in the investigation by Sinthupibulyakit et al [10] 2DG treatment preceded irradiation by 24h. The importance of drug scheduling in radiosensitization has previously been demonstrated by ourselves and others [13,14,19,23]. The cytotoxicity of 2DG to both cell lines also suggested that, when cultured as monolayers, the two cell lines responded in a similar manner.
Previous reports of the radiosensitizing effects of 2DG were carried out in monolayer cultures. Therefore, we sought to evaluate the tumor growth delay in response to the combination therapy in a 3-dimensional model. Multicellular tumor spheroids are representative of malignant metastases in their prevascular growth phase. The use of this model system can enable the determination of the dependence of therapeutic strategies on cycling and oxygenation status because they are composed of cells in various proliferative states - necrotic core, quiescent middle layer and rapidly proliferating rim [24]. We observed that concentrations up to 10 mM (Figure 2) and 30 mM 2DG (data not shown) had no growth inhibitory effect. This suggests that further work is required to determine the reasons for this lack of effect of 2DG on spheroids and whether 2DG is the most appropriate glycolytic inhibitor to be used in combination with radiotherapy. It is possible that the observed lack of inhibition of spheroid growth by 2DG was caused by differences in drug penetration, oxygenation and other microenvironmental factors of the various layers within the spheroid. This is a commonly observed feature of multicellular spheroids and results in their relative resistance to experimental therapies [25].
Conclusion
In conclusion, we report that the glycolytic inhibitor 2DG sensitized prostate cancer cells to experimental radiotherapy in monolayer cell culture and suggest a possible mechanism of interaction involving the prevention of cell cycle arrest. However, our preliminary experiments indicate that the combination was not effective in 3-dimensional tumor models and that further work is required in order fully assess the potential for this means of radiosensitization.
Acknowledgments
This work was supported by a grant from Chief Scientist Office, Scotland (TCS/16/38).
Conflict of Interest
The authors report no financial disclosure, or conflict of interest.
References