Research Article
Polymorphism of 8-Oxoguanine DNA N-Glycosylase 1 Gene in Egyptian patients with Larynx Cancer
1Professor, Department of Clinical Pathology, Faculty of medicine, Tanta University, Egypt
2Professor, Department of Tropical Medicine, Faculty of medicine, Tanta University, Egypt
3Assistant Professor, Department of Clinical Pathology, Faculty of medicine, Tanta University, Egypt
4PhD Student
*Corresponding author: Amal Helmy Abd Elhameed, Professor, Clinical Pathology department, Faculty of medicine, Tanta University, Egypt, Tel: 01001856164, E-mail: amalhelmy2015@gmail.com
Received: October 16, 2017 Accepted: November 10, 2017 Published: November 16, 2017
Citation: Abd Elhameed AH, Tawfik HA, Ibrahim WS, El-Kabbany SM. Polymorphism of 8-Oxoguanine DNA N-Glycosylase 1 Gene in Egyptian patients with Larynx Cancer. Madridge J Oncogenesis. 2017; 1(1): 8-11. doi: 10.18689/mjo-1000102
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective: To investigate the human 8-Oxoguanine DNA N-Glycosylase 1 gene polymorphism susceptibility in Egyptian patients with cancer larynx.
Methods: DNA samples from 240 patients with larynx cancer and 240 age-matched controls were genotyped using a PCR-RFLP.
Results: The frequencies of the genotypes were Ser/Ser89%, Ser/Cys9%, and Cys/Cys2% in the controls, and in patients were 58%, 32.9% and 9.1%, respectively. The Ser/Cys and Cys/Cys genotypes were significantly associated with the increased risk of cancer larynx and these genotypes increased the risk percent of larynx cancer among moderate and heavy smokers.
Conclusion: The Ser/Cys and Cys/Cys genotypes are significantly associated with the increased risk of larynx cancer in Egyptian population.
Keywords: hOGG1; Polymorphism; Ser326Cys; Larynx cancer.
Introduction
Carcinoma of the larynx forms an important group of malignancies as it accounts for approximately 1% of new cancer diagnosis [1]. The highest incidence rates have been registered in more developed countries of the world and the lowest in the developing countries. In Egypt, laryngeal cancer is considered the most common head and neck cancer because cigarette smoking, an indisputable risk factor, is on the rise in the community [2].
The incidence of laryngeal cancer in Kasr El-Aini Center of Radiation Oncology and Nuclear Medicine, Faculty of Medicine, Cairo University is 3.1% per year. Advanced laryngeal cancer is generally considered as the disease in stages III and IV based on the primary tumor extension and/or the presence of metastatic lymph node(s) in the neck and it accounts for roughly 40% to 50% of patients with laryngeal cancer [3].
A variety of genes can be involved in the multi-stage process of carcinogenesis, including oncogenes and suppress or genes, genes involved in the metabolism of carcinogen, DNA repair genes [4]. The human 8-oxoguanine DNA N-glycosylase 1 (hOGG1) gene maps to 3p26chromosome and expresses two major mRNAs encoding proteins composed of 345 and 424 amino acid residues (α-hoGG1 and β-hoGG1, respectively) [5].
8-Oxoguanine is a major form of mutagenic base damage caused by reactive oxygen species (ROS) and is a hallmark and marker of oxidative damage to DNA. This lesion is recognized by the hOGGl protein, which is a glycosylase of DNA base excision repair (BER) pathway. It has an endo-nuclease activity allowing it to excise oxidized base from DNA [6].
Because malignant tumors often show an increased level of oxidation of the DNA bases, this may suggest the implication of oxidative DNA damage in the etiology of at least some cancers [4]. It seems that this be the case of fraction of head and neck cancers (HNCs), in which smoking is involved in their pathogenesis, because tobacco smoking products can induce (ROS) [7].
The human OGG1 gene is located on chromosome 3 (3p26), and encodes a bifunctional DNA glycosylase endowed with an AP lyase activity. This is a region frequently lost in various types of cancers. Loss of the gene would abrogate OGG1 activity imposing an increased risk of mutagenicity on the cell due to accumulation of 8-oxoG in DNA. A common polymorphism of this gene, Ser326Cys a C→G polymorphism in codon 326 at exon 7 is associated with an increased risk of cancer [8].
It was suggested that the Ser326Cys polymorphism of the hOGG1 gene might play an important role in risk for smoking and alcohol-related cancers of the aero digestive tract, including HNCs [9, 10]. This study aimed to investigate the association of the variability in the hOGG1genepolymorphism, and there is k of larynx cancer in Egyptian patients.
Subjects and Methods
Blood samples were obtained from 240 patients (192 men and 48 women) with histological confirmed laryngeal carcinoma aged from 46 to 82 years. Patients were selected from Clinical Oncology Department of Tanta University Hospitals, Egypt, and 240 normal healthy controls were matched by age, gender and geographic origin with the patients group from June 2015 to September 2017.The study was approved by the Ethical Committee of Tanta University, and a written informed consent was obtained from all participants.
Complete personal and family history, smoking, clinical examination and routine laboratory investigations (CBC, ESR, LFT and KFT) were performed. Genotyping of the hOGG1 gene was performed by PCR/RFLP. All investigations were done in Clinical Pathology Department, Faculty of medicine, Tanta University, Egypt.
DNA isolation
Peripheral blood lymphocytes (PBLs) were isolated by centrifugation in a density gradient of 15 min, 280 g. The pellet containing PBLs was re-suspended in Tris-EDTA buffer, pH 8, to give 1-3 × 103 cells/ml. Genomic DNA was extracted from PBLs by phenol/chloroform extraction and proteinase K digestion. The final samples were kept in Tris-EDTA buffer, pH 8, at –20°C until use.
Genotype determination
The genotypes of the Ser326Cys polymorphism of the hOGG1 gene were determined by restriction fragment length polymorphism polymerase chain reaction (PCR) with the following primers: sense5´-GGAAGGTGCTTGGGGAAT-3´, ntisense5´- ACTGTCACTAGTCTCACCAG -3´. Individual PCR reaction were Performed in a total volume of 25 μl using 10 ng of genomic DNA, 0.4μ Mol of oligonucleotide primer specific for hOGG1 gene (exon 7), 0.2m Mol dNTPs (dATP, dCTP, dGTP, dTTP), 1 U of Taq DNA polymerase and PCR buffer [75mMol Tris-HCl pH 9.0, 50m Mol KCl, 2m Mol MgCl2 and 20m Mol (NH4)2SO4] to make up the volume. DNA amplification was carried out using light cycler instrument.
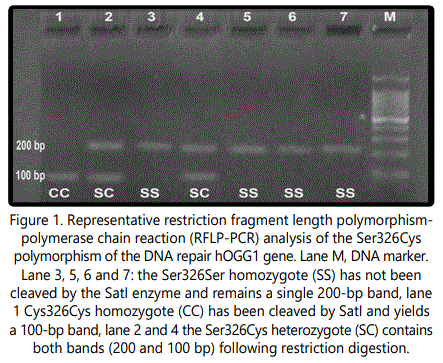
The amplification protocol was as follows: Initial denaturation step at 95°C for 5 min, 30 cycles at 95°C for 30 sec and 30 sec at 57°C annealing temperature and at 72°C for 45 sec. The final extension step was performed at 72°C and was carried out using light cycler instrument. The 200 bp PCR product was digested 3 hours with 2 U of the restriction enzyme SatI (Fermentas, Vilnius, Lithuania). The Cys allele was digested into 100 bp fragments whereas the Ser variant remained intact.
Data were coded and entered using the statistical package SPSS version 23. Data was summarized using mean and standard deviation for quantitative variables and frequencies (number of cases) and relative frequencies (percentages) for categorical variables. Comparisons between groups were done using unpaired t test [11].
Results
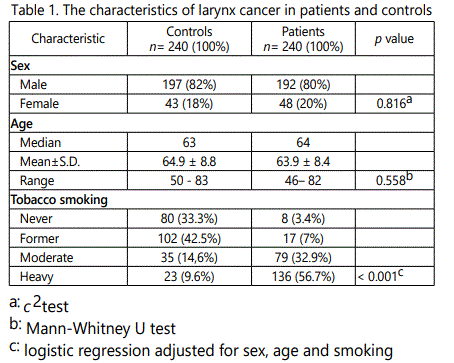
Characteristics of the study subjects. The characteristics of 240 patients with larynx cancer and 240 controls are presented in Table 1. Overall, there was no a statistically significant difference between the cases and controls in the distribution of age or gender while as regards to smoking status, there was a statistically significant difference in patients group when compared to control group (p value < 0.05). The frequency of moderate and heavy smokers was higher in the patients (32.9% and 56.7%, respectively) than in the controls (14.6 and 9.6%) (p < 0.001 in both cases).
Association between hOGG1 Ser326Cys polymorphism and larynx cancer
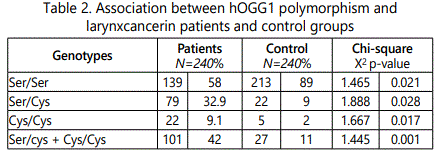
The observed genotypes and allele frequencies of hOGG1among cases and controls and their associations with risk of cancer larynx in this work were demonstrated in Table 2. There were 139(58%) patients with ser/ser genotype, 79(32.9%) patients with ser/cys genotype and 22(9.1%) patients with cys/cys genotype, while in control group 213(89%)subjects were with ser/ser genotype, 22 (9%) with ser/cys genotype and 5(2%) with cys/cys genotype. There was aa statistically significant difference between patients and control groups as regard Ser/Ser, Ser/Cys, Cys/Cys and (Ser/Cys + Cys/Cys) genotypes of the hOGG1 gene (P value < 0.05). Moreover, there was a stronge association for the Ser/Cys heterozygotes than Cys/Cys homozygotes with larynx cancer.
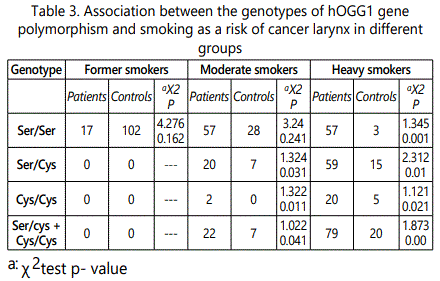
The association between smoking asa risk for larynx cancer and genotypes of hOGG1 gene polymorphism wasdemonstrated inTable 3. This study revealed that the Ser326Cys polymorphism was increased in patients with cancer larynx in bothmoderate and heavy smokers but had more potentiated effect in heavy smokers. So, this Cys allele of the Ser326Cys polymorphism increased the risk of larynx cancer in both moderate and heavy smokers.
Discussion
DNA repair process play an important role in stability of the genome and their adequate functioning could prevent cancer development. The hOGG1proteinis a line of the cellular defense against oxidative DNA damage, so any change in the gene encoding this protein may influence the ability of the cell to eliminate the oxidative DNA damage.
Cancer larynx is often smoke-related so, this study aimed to investigate the association of the variability in thehOGG1 genepolymorphism, and there is k of larynx cancer in Egyptian patients.
In this study, statistical analysis suggested that tobacco smoking consumption is a risk factor for development of larynx cancer. Heavy smokers had a much higher risk ratio than former and moderate smokers' groups, which displayed a higher risk than never- smokers. This was agreed with results of other authors and researchers [12, 13].
A link between 8-oxoG formation and tobacco smoke carcinogenesis has also been suggested with a correlation observed between levels of 8-oxoG and the number of cigarettes smoked. In addition, increased levels of 8-oxoG were detected in both peripheral leukocyte DNA and the nuclei of oral mucosa from smokers as compared to nonsmokers. Furthermore, a 50% increase in the levels of 8-oxoG was detected in the urine of smokers as compared with the urine of non-smokers [14].
These data strongly implicate 8-oxoG formation in tobacco smoke-induced carcinogenic pathways. These data are also consistent with the fact that G> T a transversions, which are the primary mutational event induced by 8-oxoG, are also the primary mutational event occurring in the p53 tumor suppressor gene, a common occurrence for upper aero digestive tract cancers [9].
In this study, there was a significant difference between patients and control groups as regard the genotypes of the hOGG1 where, Ser/Ser 89%, Ser/Cys 9%, and Cys/Cys 2% in the controls and those in patients were 58%, 32.9% and 9.1%, respectively. The role of the Ser-326Cys SNP and HNC risk was investigated in six studies [15]. The first case-control study, with a small sample size (167 cases and 331 controls), reported a positive association with HNC risk [9], which was confirmed by Pawlowska et al. [6]. Moreover, Sliwinski et al. [16] showed that the Ser326Cys SNP might modify the risk of SCCHN associated with smoking.
Pawlowska et al. [6] searched for an association between the Ser326Cys polymorphism and the risk of larynx cancer in a Polish population and studied also the association between genotypes of this polymorphism and smoking-related cancers. They concluded that the Cys326 allele of the hOGG1 gene may increase the risk of larynx cancer associated with smoking or alcohol. Cys326 allele had a potential effect in the group of heavy smokers. However, this allele did not affect the groups of former and moderate smokers. These differences may be due to small sample size [6].
Conclusion
From this study it could be concluded that the Ser/Cys and Cys/Cys genotypes are significantly associated with the increased risk of larynx cancer in Egyptian population.
Acknowledgements
References