Research Article
Evaluation of Serum Osteopontin as a Novel Biomarker For hepatocellular Carcinoma in Egyptian patients
1PhD Student, Tanta Fever Hospital, Egypt
2Professor, Department of Clinical Pathology, Tanta University Hospital, Egypt
3Professor, Department of Tropical Medicine, Tanta University Hospital, Egypt
4Assistant Professor, Department of Clinical Pathology, Tanta University Hospital, Egypt
*Corresponding author: Ahmed Osama Negm, PhD Student, Tanta Fever Hospital, Egypt, Tel: 01115225595, E-mail: aoen13@gmail.com
Received: October 16, 2017 Accepted: November 2, 2017 Published: November 8, 2017
Citation: Negm AO, Abd Elhaleem SM, Attia TE, Abd-Elbar ES. Evaluation of Serum Osteopontin as a Novel Biomarker for hepatocellular Carcinoma in Egyptian patients. Madridge J Oncogenesis. 2017; 1(1): 1-7. doi: 10.18689/mjo-1000101
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Hepatocellular carcinoma (HCC) is one of the most prevalent life threatening human cancers. Early detection of HCC remains a major challenge. Since alpha-fetoprotein (AFP), the most widely used biomarker for HCC surveillance and detection has limited utility for early stage disease, it is necessary to identify new serologic biomarkers with sufficient sensitivity and specificity for early detection.
Aim of the Work: The aim of the study is to evaluate serum osteopontin (OPN) as a novel biomarker for diagnosis of HCC in Egyptian patients.
Subject and Method: This study included 3 groups: Group I (control) included 30 apparently healthy subjects, group II (cirrhosis) included 30 patients with liver cirrhosis without HCC, group III (HCC) included 30 patients with HCC. All groups were subjected to the following: Full history taking, thorough clinical examination, abdominal ultrasonography, triphasic C.T, routine laboratory investigations, serum AFP, hepatitis markers and determination of serum osteopontin levels.
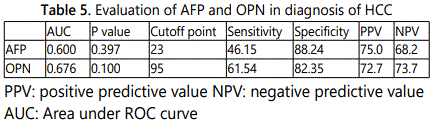
Results: This study proved a statistically significant increase in the mean values of both serum AFP and OPN levels in HCC group when compared to both control group and cirrhotic group, in contrast there was no statistically significant difference in cirrhotic group when compared to control group. OPN at an optimal cut-off of 95 ng/ml had a better performance than AFP at a cut-off of 23 ng/ml for HCC diagnosis. The sensitivity and specificity for HCC diagnosis were 61.54 and 82.35 vs. 46.15 and 88.24.
Conclusion: In the present study, we concluded that serum OPN has a better diagnostic performance than AFP for detection of HCC in Egyptian patients so, it may be considered as a promising HCC biomarker for diagnosis, particularly for those with negative AFP.
Keywords: HCC; AFP; Osteopontin.
Introduction
HCC is a frequent and deadly human disease, with 0.25-1 million new cases per year. HCC incidence varies with age, sex, and geographic region. The majority of cases coming from Asia, followed by Europe, Africa, North America and Latin America. The distribution of HCC cases among different populations may be due to the differences in the exposure to different etiological factors. Hepatitis B virus (HBV) infection is a public health problem which affects approximately 2 billion people and is considered the dominant risk factor for HCC in Eastern Asia and Sub-Saharan Africa [1].
HCC ranks almost first among all malignancies in Egypt, where it has the highest prevalence of Hepatitis C Virus (HCV) infection worldwide, with 14% of the population infected and seven% with chronic liver hepatitis [2].
HCC imposes a very high social and medical burden, especially in areas with a high incidence of viral hepatitis infection. The overall prognosis of disease is poor with a 5-year survival rate of 3-5% after initial diagnosis. This is may be due to the difficulties of early diagnosis [3].
In Egypt, HCC represents 75% of all malignant liver tumors. Liver cancer is considered the 5th most common cancer in both genders, the 6th in female representing (3.4%) of cancers, while it is the 2nd in males after urinary bladder cancer representing (11.5%) of all cancers [4]. Primary liver cancer is the second leading cause of cancer related death worldwide and therefore a major public health challenge. HCC alone accounts for 90% of all cases of primary liver cancer, with nearly 800,000 new cases annually [5].
Liver cancer exhibits no symptoms in the early stage, whereas clinical symptoms are evident in the advanced stage, leading to an unsatisfactory curative effect [6]. Early diagnosis of HCC and effective treatment help to prolong the lifetime of liver cancer patients [7].
AFP together with imaging and pathology detection are commonly used in clinical early diagnosis for liver cancer. However, AFP does not yield satisfactory results in the early diagnosis of HCC, particularly AFP-negative HCC. These false-negative results limit its application as a tumor marker for HCC. Thus, the identification of new, high sensitive and specific markers for HCC is essential to improve the early diagnostic rate, treatment effect in addition to curative satisfaction [6].
The utility of OPN in early HCC detection may be relevant to many reports suggesting that beside its important role in metastasis, OPN expression is also critical for tumor growth of human HCC, and that down-regulation of OPN suppresses growth of HCC via induction of apoptosis [8, 9].
OPN attracted attention as a promising biomarker for HCC diagnosis in patients with virus-related cirrhosis with better sensitivity than AFP in differentiating HCC cases from cirrhosis and controls [10, 11].
Aim of the Work
The aim of the study is to evaluate serum osteopontin as a novel biomarker in diagnosis of HCC in Egyptian patients.
Subjects and Method
After ethical committee approval from Research Center in Tanta University and written consent from all subjects, this study was conducted on 3 groups: Group I (Control), this group included 30 apparently healthy subjects, they were sero-negative for hepatitis B surface markers and HCV antibodies and with normal liver function tests. Group II (Cirrhosis), this group included 30 patients with Liver cirrhosis, they were diagnosed by ultrasonographical findings and biochemical evidence of parenchymal damage as well as liver biopsy in some cases. Group III (HCC), this group included 30 patients with HCC, the diagnosis of HCC was by clinical examination, radiological investigations including abdominal ultrasonography, triphasic C.T, and laboratory investigations. All patients were selected from Tropical medicine department Tanta University Hospital.
All patients and controls were subjected to the following: Full history taking, thorough clinical examination, abdominal ultrasonography, triphasic C.T (for patients only, routine laboratory investigations (liver function tests including ALT, AST, serum albumin, total and direct bilirubin, rothrombin time and INR, complete blood count, serum urea and creatinine, fasting and 2-hours post prandial blood glucose levels, serum AFP, hepatitis markers (HCV-Abs, HBs Ag)and determination of serum osteopontin levels (by ELISA Kit for osteopontin).
The analysis of liver functions, fasting and 2-hours post prandial blood glucose levels, serum urea and creatinine were performed on Konelab Thermo Scientific (Thermo Scientific–Finland) auto analyzer. Hepatitis markers (HCV-Abs, HBsAg) were done by ELISA and confirmed by quantitative PCR for positive cases. HCV antibody was done by anti-HCV ELISA kit which is provided by WKEA med suppliescorp. (www.wkeamedsupplies.com). HBsAg was done by Hepatitis B Surface Antigen ELISA Kit which is provided by Prechek Bio Inccompany USA (www.prechecbio.com). Full blood count was done using ERMA PCE-210 cell counter.
Serum alpha-fetoprotein was estimated by a direct solid phase sandwich assay based on the ELISA, supplied by Assay Pro LLC. Human (α-Fetoprotein ELISA Kit, catalogue number EF6011). Osteopontin was done by Human OPN ELISA Kit, Catalogue No. 201-12-1526 supplied by PELOBIOTECH GmbH – Am Klopferspitz 19 – 82152 Planegg – Germany.
Results
Demographic data of the studied group
Group I (control) included 30 apparently healthy subjects, their ages ranged from 30 to 60 years with mean value 44.60±9.62, they were 24 males and 6 females with males to females ratio 4:1. Group II (cirrhosis) included 30 patients with Liver cirrhosis without HCC. Their ages ranged from 40 to 62 years with mean value 45.76±9.23, there were 22 males and 8 females with males to females ratio 2.75:1.Group III (HCC), this group included 30 patients with HCC, their ages ranged from 40 to 65 years with mean value 49.73±7.47, there were 23 males and 7 females with males to females ratio 3.28:1.There were no statistically significant differences in the mean values of ages or gender in all studied groups (P > 0.05).


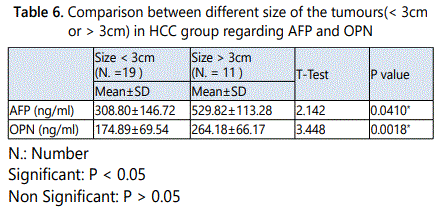
It was shown that the mean values of both serum AFP and OPN in HCC patients with size of the tumour<3cmwere 308.80±146.72ng/ml and 174.89±69.54ng/ml respectively, while their mean values in HCC patients with size of the tumour>3cm were 529.82±113.28ng/ml and 264.18±66.17ng/ ml, respectively. It was shown that, there were a statistically significant increases in the mean values of serum AFP and serum OPN in HCC patients with the tumour size >3cm when compared to patients with the tumour size < 3cm (p = 0.0410*, 0.0018* respectively).While the mean values of both serum AFP and OPN in HCC patients with no PVT (17 patients) were 328.34±141.86ng/ml and 184.53± 73.04ng/ml, while in patients with PVT (13 patients) were 470.26±133.31ng/ml and 237.85±81.69ng/ml. There were no statistically significant differences in both mean values of serum AFP and OPN in patients with PVT when compared to patients with no PVT (p = 0.186, 0.070 respectively).
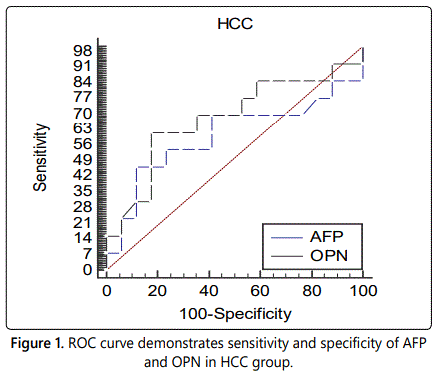
Our results demonstrated that OPN at an optimal cut-off value (95 ng/ml) had a better performance than AFP at a cutoff value (23 ng/ml) for HCC diagnosis. The sensitivity and specificity for HCC diagnosis were 61.54 and 82.35 vs. 46.15 and 88.24 with an area under the ROC curve of 0.676 vs. 0.600 for OPN vs. AFP, respectively.
Discussion
HCC is frequently detected in pre-existing liver cirrhosis, but can also develop without such pre-conditions. There is an increasing trend of HCC incidence worldwide. In patients with liver cirrhosis, HCC has become the leading cause of death. At diagnosis the tumor has very often reached an advanced stage and curative treatment options are missing. Thus, early diagnosis would help the patient and prevent increasing healthcare costs [12].
Accurate detection and differential diagnosis of early HCC can significantly improve patient survival. Currently, detection of HCC in clinical practice is performed by diagnostic imaging techniques and determination of serum biomarkers, most notably α-AFP, fucosylated AFP and des-γ-carboxyprothrombin. However, these methods display limitations in sensitivity and specificity, especially with respect to early stages of HCC. Recently, high-throughput technologies have elucidated many new pathways involved in hepatocarcinogenesis and have led to the discovery of a plethora of novel, non-invasive serum biomarkers. In particular, the combination of AFP with these new candidate molecules has yielded promising results [13].
Identification of new specific and sensitive biomarkers for early diagnosis is urgently needed [14]. Many alternative novel biomarkers have been investigated; however, their diagnostic values about early hepatocellular carcinomas remain controversial. Thus, it is necessary to identify new serologic biomarkers with both sufficient sensitivity and specificity to detect hepatocellular carcinomas at an early stage and/or with negative AFP [15]. Osteopontin is considered an important molecule in clinical research; as a target for better understanding of the pathophysiology, prognosis of many diseases including HCC and rational drug design [16].
Our research found no statistically significant difference in the mean values of ages or gender in all studied groups, and the mean value for ages in HCC group was near to those reported by El-Serag et al., (2013)who reported that, the mean value for age in HCC was 50 years [14] , and also near to Liu et al., (2014)who reported that, a mean age of incidence of HCC was 54 years [17]. On the other hand, the results obtained were different from that reported by Parikh and Hyman, (2007) who reported the mean age of presentation of HCC in Europe and the United States to be approximately 60 years; and that of patients in Asia and Africa to be between 20 and 50 years [18].
These variable age-specific patterns are likely related to differences in the dominant hepatitis virus in the population, the age at viral infection and the existence of other risk factors. Notably, while most HCV carriers become infected as adults, most HBV carriers become infected at very young ages. The peak onset age varies according to geographical areas where in China the mean age of diagnosis with HCC was 55–59 years and 63–65 years in Europe [14].
From the present study, it was clear that, HCC is more common in males than females. These results are nearly in accordance to those reported for Egyptian patients by Ashraf et al., (2014) as they reported that HCC patients were predominantly males. This may be explained by high incidence of carrier state of HBV in males [19].
Men are more likely to be affected than women with HCC. The reasons are not well understood, but several factors may explain this, males are more likely to be infected with HBV and HCV as well as, cigarette smoking and alcohol consumption are more common in men. Testosterone has been shown to correlate with HCC indicating a probable role for the sex hormones in the development of HCC [20]. Moreover, this augmented incidence may be due to existence of androgenic stimulation of transforming growth factor [21].
In this study, there was statistically significant increase in percentage of PVT in HCC group when compared to cirrhotic group, but there was no statistically significant difference in comparison between the same groups regarding as cites and Child-Pugh score.
The prevalence of PVT increases with the severity of cirrhosis, from 1%, in patients with compensated cirrhosis to 8-25% [22]. Many factors are involved: local (liver architectural changes, slowing of portal vein flow due to resistance increase in the cirrhotic liver, the presence of periportallymphangitis, systemic unbalanced hemostasis with a tendency to hypercoagulability, and other congenital and acquired factors [23].
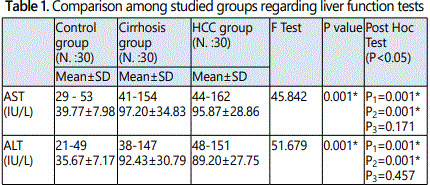
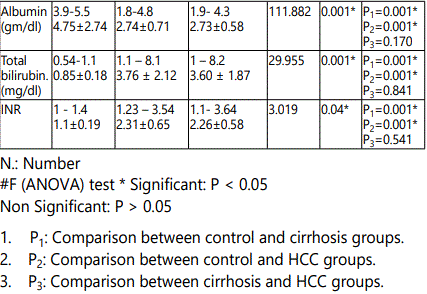
The present study showed a statistically significant increases in AST, ALT, serum bilirubin, INR and significant decrease in serum albumin observed in both HCC and cirrhotic group when compared to control group. In contrast, there were no statistically significant difference when comparing these laboratory investigations between cirrhotic group and HCC group.
These results agreed with Al-Jumaily & Khaleel (2012)who reported significant alteration in liver functions in the patient groups (cirrhotic and HCC groups) when compared to control subjects [24]. These data could be related to the fact that aminotransferases are typically elevated in all liver disorders, appearing to be more sensitive and specific tests for acute than chronic hepatocellular damage. Increased release, decreased clearance and/or impaired synthesis are all incriminated in their fluctuating levels [25].
Liver function tests (LFT) are a helpful screening tool, which are an effective modality to detect hepatic dysfunction. Since the liver performs a variety of functions, so no single test is sufficient to provide complete estimate of function of liver. Often clinicians are faced with reports that not concordant with the clinical condition of the patient and they face difficulty in interpreting the LFT [26].
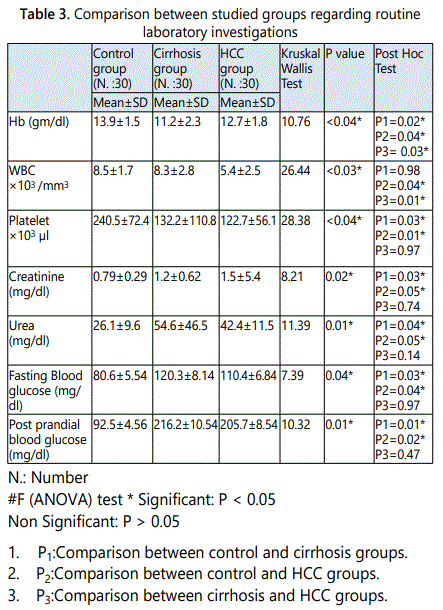
Our data of complete blood count agreed with Zakaria et al., (2004) who stated that the complete blood picture showed a significant anemia, thrombocytopenia and leucopenia in the cirrhosis and HCC groups compared to control [27]. El-Deeb et al., (2015) found that, cirrhotic and HCC patients showed a significant decrease in Hb concentration and platelet counts compared to healthy control, Hb concentration was significantly lower in cirrhotic cases than HCC patients, while no significant differences were recorded in platelet counts between the two patient groups. As regard WBC, there was a significant decrease in cirrhotic patients compared to control, while there was no statistically significant difference when comparing HCC with control group or cirrhotic patients studied groups [28].
In this research, there were statistically significant increases in the mean values of serum creatinine and urea in cirrhotic group and HCC group when compared to control group, in contrast there was no statistically significant difference in its mean value in cirrhotic group when compared to HCC group. Many changes in kidney function are strongly linked with increased mortality, extending to those with chronic liver disease. Chronic liver disease is associated with primary and secondary kidney disease and impacts markedly on survival [29].
This study showed that, there were statistically significant increases in the mean values of fasting and post prandial blood glucose levels in cirrhosis group as well as HCC group when compared to control group, but there was no statistically significant difference in its mean values in cirrhosis group when compared to HCC group.
In patients with cirrhosis, disorders of glucose metabolism range from glucose intolerance to overt diabetes [30]. Diabetes occuring as a complication of liver cirrhosis is known as hepatogenous diabetes (HD).Insulin resistance in muscular, hepatic and adipose tissues as well as hyperinsulinaemia seem to be pathophysiologic bases for HD. An impaired response of the B cells of pancreas and the hepatic insulin resistance are also contributing factors [31]. The causes of liver disease also appear to be an important risk factor for the development of diabetes in patients with cirrhosis. Actually, diabetes is more frequent in patients with hepatitis C-related cirrhosis or alcohol-related cirrhosis than in patients with biliary cirrhosis [30].
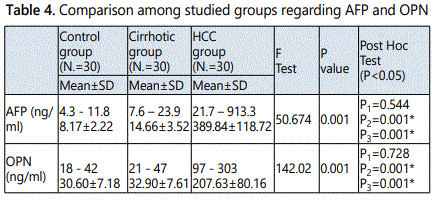
These results showed a statistically significant increase in the mean values of both serum AFP and OPN levels in HCC group when compared to both control group and cirrhosis group, in contrast there were no statistically significant difference in cirrhosis group when compared to control group. El Shafie et al., (2012) reported that the serum levels of AFP are significantly elevated in chronic liver diseases and more elevated in HCC cases [32]. However, Trevisani et al., (2001) reported that not all tumors secrete AFP, and serum concentrations are normal in up to 40 % of small HCC [33].
Results of OPN in this study agreed with some studies who reported that plasma levels of OPN were significantly higher in HCC plasma than in cirrhosis, chronic hepatitis C and healthy controls [7, 11, 36], suggesting the use of circulatory OPN as a complement diagnostic biomarker for AFP. In contrast to the current study, Zekri et al., (2011) reported that OPN levels in HCC patients were not significantly higher than that those with chronic liver diseases [37]. Also in another study, it was found that, the mean OPN level in HCC patients was not significantly different from HCV or other chronic liver disease patients, while it was significantly higher in these diseases than control group [38]. These contradictory results (which may be due to different sample sizes or due to population differences) directed our research to evaluate OPN in Egyptian patients.
The AFP which is the most widely used tumor marker for HCC has fallen short in accurate diagnose HCC, in discriminating between high and low risk individuals, and predicting high-risk histopathologic features such as microvascular invasion and tumor differentiation. Consequently, AFP has been excluded in some guidelines on HCC surveillance as well as in the diagnostic assessment of hepatic nodules found on surveillance imaging [34].
High ALT levels are associated with inflammation of the liver, and individuals who have high ALT levels are more likely to have damage to the liver that causing regeneration. This is a situation in which AFP is less specific for a diagnosis of HCC. Knowing a patient's ALT level can be useful because it helps the physician know whether an AFP measurement is more or less reliable. A very high AFP level of 400 or 500 ng/ml in the absence of other causes of elevation is strongly predictive of HCC [35].
When evaluating OPN and AFP in diagnosis of HCC, it was demonstrated that OPN at an optimal cut-off value 95 ng/ml had a better performance than AFP at a cut-off value 23 ng/ ml for HCC diagnosis. The sensitivity and specificity for HCC diagnosis were 61.54 and 82.35 vs. 46.15 and 88.24 with an area under the ROC curve of 0.676 vs. 0.600 for OPN vs. AFP, respectively.
This is in agreement with Shang et al., (2012) who found that OPN at an optimal cut-off of 91 ng/ml had a better performance than AFP at a cut-off of 20 ng/ml for early HCC diagnosis. The sensitivity and specificity for early HCC diagnosis were 74% and 66% vs. 53% and 93% with an area under the ROC curve of 0.76 vs. 0.71 for OPN vs. AFP, respectively [11].
In this study it was shown that, there was a statistically significant increase in the mean value of serum OPN in HCC patients with the tumour size >3cm (late stage of HCC) when compared to patients with the tumour size < 3cm (early stage of HCC). These data indicate that OPN levels increase in early stages of HCC and increase with the progress of the disease that indicated by increase in the tumour size.
OPN over expressing cells have reduced apoptotic cell death both in vivo and in vitro. OPN, acting through the RGD domain, and can promote NF-kB activation that protects cells from apoptosis. The predominant mechanism, by which OPN promotes tumor growth and metastasis through the RGD domain, is enhancement of the survival in the tumor microenvironment. Therefore, inhibitors directed against OPN may target multiple isoforms and inhibit cell survival mechanisms that involve the RGD domain, and NF-kB activation [39].
Early detection of HCC through surveillance methods has a major impact on patient outcomes, including increased survival. Efforts to identify HCC in individuals at risk at an early stage are critical for providing highly effective treatment, including primary curative hepatectomy, locoregional ablative therapy, or liver transplantation [40].
In a pilot study of 22 patients from the U.S. cohort who developed HCC during follow-up, osteopontin levels were shown to be elevated at least 1 year prior to diagnosis. Thus, measurement of osteopontin may represent a valuable new opportunity towards improved screening and early detection of HCC. The results of future validation studies and long-term follow-up will be awaited with great interest [41].
Conclusion
In the present study, we concluded that serum OPN has a better diagnostic performance than AFP for detection of HCC in Egyptian patients. so, it may be considered as a promising HCC biomarker for diagnosis, particularly for those with negative AFP.
Acknowledgements
References