Review Article
Does Application of Brassinosteroids mitigate the Temperature Stress in Plants?
Professor, Department of Botany, Dean, Faculty of Science and Computer Science, Telangana University, Nizamabad, Telangana, India
*Corresponding author: B Vidya Vardhini, Professor, Department of Botany, Telangana University, Nizamabad, Telangana, India, E-mail: drvidyavardhini@rediffmail.com
Received: February 17, 2019 Accepted: March 25, 2019 Published: April 5, 2019
Citation: Vardhini BV. Does Application of Brassinosteroids mitigate the Temperature Stress in Plants? Int J Earth Sci Geol. 2019; 1(2): 59-65. doi: 10.18689/ijeg-1000107
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The temperature across the globe is constantly changing for the worse of the biotic beings viz., the flora and fauna. Rapid temperature changes are the result of continuous interference of human beings as a bane post the rapid industrialization and urbanization. The ever increasing and decreasing ranges of temperature in the environment are leading to the malfunctioning of various physiological and biochemical processes in plants thereby resulting in severe temperature stresses in terms of high temperature/heat stress as well as low temperature stress (chilling and freezing stress). Brassinosteroids (BRs) are a novel group of plant growth regulators (PGRʼs) with significant growth promoting activity. BRs were initially extensively studied for their profound growth promoting physiological responses viz., growth and yield, seed germination, photosynthesis, senescence, photomorphogenesis, flowering etc. BRs have been further explored for stress-protective properties in plants against a number of abiotic stresses like heat, chilling, freezing, drought, flooding, oxidative, salt, allelochemicals, radiation, light, wind, heavy metals stresses etc,. and can be aptly stated that BRs induce plant tolerance to a wide spectrum of stresses. The present review is a study on the role of BRs in mitigating the effect of temperature stress in plants viz., high temperature/heat stress as well as low temperature stress (chilling and freezing stress).
Keywords: Brassinosteroids; Chilling stress; Freezing stress; High temperature stress; Low Temperature stress.
Introduction
Brassinosteroids (BRs) are a novel type of polyhydroxy steroidal phytohormones that are capable of emphatically exhibiting pronounced growth-promoting influence [1,2]. The discovery of this new group of PGRs (plant growth regulators) way back in the early 70ʼs [3-5] followed by the research work in the late 70ʼs by Grove et al. [6] led to the recognition of BRs as a potential 6th group of PGRs. BRs are usually classified as C27, C28 or C29 BRs according to the number of carbons in their structure and brassinolide (BL), 28-homobrassinolide (28-HomoBL) and 24-epibrassinolide (24-EpiBL) are the three potential BRs of the present world of research and development [7] are represented in figure 1.
BRs was first studied as the regular PGRs capable of modulating a wide range of physiological functions like source/sink relationships, seed germination, photosynthesis, senescence, photomorphogenesis, flowering and responses to different abiotic and biotic stresses [8]. The research on BRs exhibited their ability in overcoming various abiotic stresses like high temperature [9], low temperature in terms of chilling [10,11] as well as freezing [12], salt [13,14], light [15], water in terms of drought [16,17] as well as flooding [18], heavy metals [19-21], osmotic [22], herbicide [23], pesticide [24], inorganic pollutants [25,26] as well as organic pollutants [27,28] stresses. Further, BRs were also capable of overcoming different biotic stresses caused by viruses [29,30], nematodes [31,32], fungi [33], insects [34], bacteria [35] etc. The recent studies on BRs also revealed their ability in overcoming certain unique stresses like newly reclaimed sandy soil stress [36], shade stress [37], preservative stress [38], petroleum polluted soil stress [39] etc. The present review focuses on the ability of BRs in mitigating temperature stress in different plants viz., high temperature/heat stress as well as low temperature stress (chilling and freezing stress).
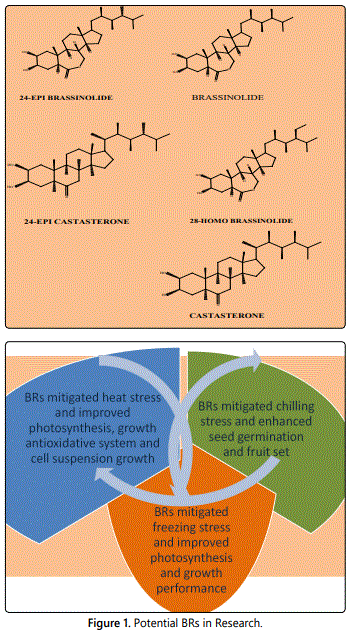
Temperature causes a lot of physiological changes in plants especially in the disruption of the enzymes which are basically proteins capable of solarization as well as crystallization due to high or low temperature. This in turn affects the major metabolic processes like photosynthesis and respiration. Application of PGRs especially BRs mitigated the different stresses in plants caused by different temperature regimes. The recent changes in the environment across the globe due to various reasons especially global warming is posing a severe threat to the plants due to fluctuations of temperatures. Hence the present review article is to show the role of BRs as effective PGRs in mitigating various temperature stresses in different plants and their potentiality in combating the negative effect of temperature stresses in plants.
BRs in mitigating different Temperature Stresses in Plants
BRs are known to mitigate various stresses including temperature stress which includes high temperature/heat stress and low temperature stress (chilling and freezing). Application of 24-EpiBL at 10-11, 10-9 and 10-7 M to three Brassica species (B. carinata, B. juncea and B. napus) under high as well as low temperature (4, 14, 34 and 44°C) for 5 hours mitigated high and low temperature stresses in all the three Brassica species by decreasing the lipid peroxidation in terms of MDA (malondialdehyde) content and accumulation of the osmolyte, proline [40]. Chen et al. [11] reported that cold-induced oxidative stress in grapevine seedlings was mitigated by foliar treatment of 24-epiBL by regulating the ascorbate-glutathione cycle. Further, Zhao et al. [41] also reported that application of 24-epiBL mitigated a combination of drought and heat stress in Triticum aestivum L. seedlings by increasing the rate of photosynthesis and Rubisco activase gene expression.
BRs in mitigating Heat/High Temperature Stress in Plants
Cukor et al. [42] reported that application of BRs positively regulated the seed germination of Scots pine cultivated under standard and heat stress conditions. BRs were reported to play a positive role in mitigating the high temperature or heat stress in plants [43,44]. Wilen et al. [45] in the early 90ʼs studied that supplementation of 24-EpiBL markedly enhanced the tolerance to high temperature stress in brome grass cell suspension cultures by enhanced accumulation of ABA-inducible heat stable proteins. Hayat et al. [46] observed that treatment of 28-homoBL to Vigna radiata c.v. T-44 plants mitigated the stress generated by high temperature by improved membrane stability index (MSI), leaf water potential (ψ), increased activities of antioxidative enzymes as well as proline levels. Cao and Zhao [47] studied that foliar application of 0.005 mg/L of BR to two varieties of Indica rice (Oryza sativa L.) seedlings viz., Xieqingzao B (heat-sensitive) and 082 (heat-tolerant) mitigated high temperature stress by enhanced activities of peroxidase (POD), super oxide dismutase (SOD) isozymes expression levels and reduced MDA levels and leakage of leaf electrolytes. BRs were found to enhance the rate of photosynthesis by increasing the CO2 fixation and the antioxidative system activities in tomato plants by mitigating high temperature stress [48]. Mazorra et al. [49] studied that the pre -incubation with 24-epiBL or MH5 (polyhydroxylated spirostanic analogue of BR) for around 24 hours mitigated heat stress in tomato leaf discs by enhanced the activities of catalase (CAT), peroxidase (POD) and super oxide dismutase (SOD). Further, a study on mitochondrial small heat shock proteins (MT-sHSPs) of tomato showed the leaves did not preferentially accumulate in 24-epiBL treated plants at 25°C but accumulated at 38°C [50]. Further, Mazorra et al. [51] also reported that application of EpiBL induced tolerance to heat shock (HS) in tomato seedlings [BR-deficient mutant (extreme dwarf d(x)), a partially BR-insensitive mutant curl3(-abs) allele (curl3 altered brassinolide sensitivity) and a line over expressing the dwarf, BR-biosynthesis gene (35SD)] by reduced ion leakage, lipid peroxidation and increased antioxidative systems.
Homo BL was found to mitigate the negativity of heat stress in growth of apical meristems of banana shoots cultured in vitro conditions [52]. Janeczko et al. [53] studied that application of 24-epiBL mitigated heat stress and improved the physiological functions of barley. Dhaubhadel et al. [54] observed that application of 24-epiBL resulted in enhanced basic thermo tolerance of tomato seedlings which might have been due to the protection of the translational machinery as well as heat-shock protein synthesis by BR-application [55]. BRs mitigated heat-induced inhibition of photosynthetic capability by enhanced carboxylation efficiency as well as antioxidative enzyme system in Lycopersicon esculentum [48]. Foliar treatment of 24-epiBL mitigated the ill effects of high-temperature-induced inhibition of photosynthesis in two cultivars of melon (Cucumis melo L.) seedlings [56]. A preliminary laboratory research established that the tomato leaf ultra structure was less affected in Bio Bras 6-treated leaves subjected to high temperature stress [57]. Further, Sam et al. [58] observed that a BR-analogue (Bio Brass-6) mitigated the negative effect of high temperature stress (40°C for 1.5 h) on leaf ultra structure of tomato plants and improved the internal membrane system of chloroplasts and mitochondria. Even, Niu et al. [59] also observed that foliar treatment of BRs to (Trin.) Tzvelev grown under high temperatures mitigated the stress and improved the morphological and physiological traits of Leymus chinensis.
Krishna et al. [60] observed that supplementation of 24-epiBL resulted in enhanced basic thermotolerance of tomato seedlings. BRs mitigated the high-temperature injury in Ficus concinna seedlings by enhanced antioxidative defense mechanism and improved glyoxalase systems [61]. BRs were also found to improve the rate of photosynthesis, lipid peroxidation, and rice seed set under high temperature stress [62,63]. 24-EpiBL supplemented tomato pollen showed higher in vitro pollen germination and increased tube growth subjected to high temperature stress [64]. Further, exogenously treated BRs enhanced the development of heat-stressed rice pollens [65]. Recently, Liu et al. [66] reported that BRs improved the lipid productivity and enhanced the stress tolerance of Chlorella cells subjected to high temperature.
BRs in mitigating Low Temperature in Plants
Plants are prone to low temperature stress during winter or autumn. Low temperature stress includes chilling stress as well as freezing stress. The research study conducted by Janeczko et al. [67] showed that BR infiltration prior to cold treatment reduced the ion leakage in rape plants and stated that BRs are potential mitigators of low temperature stress.
BRs in mitigating Chilling/Cold stress in Plants
BL mitigated the ill effect of chilling stress and increased the growth of cucumber [68] and maize [69] seedlings. The supplementation of BL markedly enhanced the seed germination and seedling growth of rice subjected to low-temperature stress [70]. The treatment of BL exhibited enhanced lamina joint-cell elongation under low-temperature stress in rice [71]. He et al. [72] stated that BL enhanced the growth of maize subjected to chilling stress. Xi et al. [73] observed that supplementation of 24-epiBL resulted in enhanced antioxidative defense mechanisms as well as modulated osmoregulatory systems in young grapevines (V. vinifera L.) grown under chilling stress. Liu et al. [74] studied that treatment of 24-EpiBL to Chorispora bungeana cell suspension cultures exposed to 4 and 0° C for 5 days of chilling stress showed mitigation of oxidative damage due to over production of ROS (reactive oxygen species) by increased antioxidative defense mechanism viz., enhancement in the activities of antioxidative components like APX (ascorbate peroxidase), CAT (catalase), POD, SOD, ASA (ascorbic acid) and decreased contents of GSH (reduced glutathione). Kumar et al. [75] reported that foliar application of 24-epiBL to Brassica juncea L. seedlings grown under 4°C of chilling stress exhibited reduced H2O2 concentration by enhanced antioxidant defense system (enhanced activities of various antioxidative enzymes viz., CAT, APX and SOD). Hu et al. [76] studied that foliar supplementation of 24-epiBL mitigated the 12/8°C chilling-induced inhibition of photosynthetic capacity of cucumber (Cucumis sativus L) plants by not only decreasing the production of ROS accumulation, but also enhancing the activities of SOD, APX; decreasing H2O2 and MDA. Further, Hu et al. [77] also studied that cucumber plants pretreated with 24–epiBL as well as 0.3 and 1.0 mmol·L-1 chlorpyrifos mitigated the phytotoxicity as well as chilling stress by enhancing the oxidative stress and regulating antioxidative enzymes (APX, GR [glutathione reductase], CAT and GPX).
Earlier research indicated by Ohshiro et al. [78] showed that 24-epiBL capably regenerated the bulbets of Lilium japonicam by breaking the dormancy. BL effectively mitigated the ill effects of chilling stress in tomato [54] and increased the growth of cucumber seedlings [79]. Fariduddin et al. [80] observed that 10−8, or 10−6 M 28-homoBL mitigated chilling stress (10/8°C, 5/3°C) by enhanced growth, photosynthesis, activities of antioxidant enzymes like CAT, POD, SOD and the osmolyte, proline in cucumber (Cucumis sativus L.) subjected to stress. Jiang et al. [81] studied that BRs were capable of protecting the photosynthetic apparatus from cold-induced damage in Cucumis sativus plants by enhancing the activities of Calvin cycle enzymes and enhancing the antioxidative system which in turn resulted in mitigation of the photo oxidative stress during the process of recovery from chilling injury. Wang et al. [82] reported that BRs ( 5, 10 and 15 µM) efficiently decreased the chilling injury of pepper fruit during 18-day storage at 3°C by decreasing the electrolyte leakage, MDA content and enhancing antioxidative enzyme activities (CAT, POD, APX and GR). Aghdam et al. [83] studied that application of 0, 3 and 6 µM BRs to tomato fruits stored at 1°C for 21 days decreased the chilling injury, electrolyte leakage, MDA content while increased proline levels, total phenols, phenylalanine ammonia-lyase (PAL) activity and maintained the membrane integrity.
Anwar et al. [84] observed that 24-EpiBL mitigated the endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings. BR-supplemented tomato (Lycopersicon esculentum) plants grew better than control plants under low temperature conditions [85]. BL supplementation resulted in increased energy status and proline metabolism in bamboo shoots during postharvest stage under chilling stress [86]. Watanabe et al. [87] studied that foliar application of Ts303, a BL analogue before one week of flowering enhanced fruit set in 15 year old trees of Japanese persimmon and 12 year old grape vines. Exogenous application of BL mitigated chilling stress in Leymus chinensis (Trin.) Tzvel by modulating morphological, physiological and biochemical traits [88]. Dong et al. [89] studied that treatment of a BR-analogue (BR-TS303) increased the resistance of Arachis hypogaea plant grown under chilling stress. Foliar treatment of 24-epiBL resulted in enhanced photosynthesis, anti-oxidant defenses and protected eggplant (Solanum melongena L.) seedlings from chilling stress [90].
Seed supplementation with TNZ303 which is mixture of jasmonic acid and BR-derivatives mitigated the formation of deformed leaves in cucumber plants treated subjected to cold stress [91]. Treatment of 0.01% BL solution increased the yield as well as the resistance to autumn low-temperature damage in rice crop [10]. Even a proteomics study also revealed the mitigative ability of BRs subjected to chilling stress in mung bean epicotyls [92]. Aghdam & Mohammadkhani [93] observed that postharvest supplementation of BL resulted in enhanced chilling stress tolerance in tomato fruit where as Wu [94] observed that supplementation of BRs enhanced the chilling resistance in Dendrobium huoshanense.
Supplementation with BRs increased the winter survival of winter rye (Secale cereale L.) by increased photosynthetic capacity [95]. Further, Pociecha et al. [96] also studied that pre-treatment with 24-EpiBL modified the cold-induced photosynthetic acclimation mechanisms and PGR responses of perennial ryegrass in cultivar-dependent manner. 24-EpiBL enhanced plant tolerance to low temperature stress in Lycopersicon esculentum Mill [97] and also mitigated the chilling-induced oxidative stress in pepper by enhancing antioxidative systems as well as maintenance of photo system II [98]. Hirai et al. [99] reported that BL improved the ripening of rice plants subjected to low temperature condition. Recently, Tavallali [100] observed that vacuum infiltration of 24-epiBL significantly delayed the chlorophyll degradation and maintained the quality of lime fruit during cold storage, thus increasing its shelf life. Even, Xia et al. [101] observed that BR-mediated apoplastic H2O2-glutaredoxin 12/14 cascade that regulated the antioxidant capacity in response to chilling stress in tomato plants.
BRs in mitigating Freezing/Frost Stress in Plants
Eremina et al. [102] reported that BRs participated in controlling the basic and acquired freezing tolerance of plants. Ma et al. [103] observed that foliar treatment of BRs (1 × 10−6 mol L−1) increased the growth and photosynthesis in terms of Stomatal conductance (Gs), intercellular CO2 concentration (Ci), transpiration rate (Tr) and photosynthetic saturated light intensity (LSP) in rapeseed (Brassica napus L.) subjected to freezing stress. Gallo et al. [12] observed that BRs mitigated the late frost stress in Fagus sylvatica L. plantation by improved growth performance and resistance.
Conclusion
The ability of BRs in mitigating different temperature stresses like heat, chilling as well as freezing is an established fact and the research of BRs as potential mitigators of various abiotic stresses especially temperature stress is gaining much importance in the current scenario of environmental stress research. Sadura and Janeczko [104] aptly stated that BRs are capable of inducing tolerance to high and low temperature in plants by modulating various physiological and molecular mechanisms. Further, Filek et al. [105] studied that BRs mitigated low temperature stress in winter wheat seedlings by regulating its membrane structure. Kaur et al. [9] observed that application of 28-homoBL regulated the antioxidant enzyme activities and gene expression in response to temperature-induced oxidative stress in Brassica juncea. Vardhini and Anjum [106] stated that BRs have the ability in overcoming various abiotic stresses in plants by positively modulating the antioxidative system of the plants. It is a well known fact that there is always a threat for the plants to face extreme heat or extreme cold temperatures due to the constant changes in the environmental conditions across the globe [107]. Hence, the present review article focuses on the role of BRs as potential PGRs that are capable of mitigating temperature stress (high temperature, low temperature and freezing) which is one of the main abiotic stresses that the plants are facing in the current scenario of ever changing temperature regimes.
References
- Vardhini BV. Modifications of morphological and anatomical characteristics of plants by application of brassinosteroids under various abiotic stress conditions - A review. Plant Gene. 2017; 11: 70-89. doi: 10.1016/j.plgene.2017.06.005
- Latha P, Vardhini BV. Effect of Homobrassinolide on the Growth of Mustard Crops Grown in Semi-arid Tropics of Nizamabad. International Journal of Current Research in Life Sciences. 2018; 7(6): 2320-2326.
- Mitchell JW, Mandava N, Worley JF, Plimmer JR, Smith MV. Brassins - a new family of plant hormones from rape pollen. Nature. 1970; 225: 1065-1066. doi: 10.1038/2251065a0
- Mitchell JW, Mandava NB, Worley JF, Drowne ME. Fatty hormones inpollen and immature seeds of bean. J Agric Food Chem. 1971; 19(2): 391-393. doi: 10.1021/jf60174a021
- Worley JF. Growth responses induced by brassins (fatty plant hormones) in bean plants. J Amer Soc. 1971; 96: 270-273.
- Grove MD, Spencer GF, Rohwededer WK, et al. Brassinolide, a plant growth promoting steroid isolated from Brassica napus pollen. Nature. 1979; 281: 216-217. doi: 10.1038/281216a0
- Latha P, Vardhini BV. Effect of homobrassinolide on bio-chemical activities and chlorophyll pigments of mustard plants grown in semi-arid tropics of Nizamabad. European Journal of Biomedical and Pharmaceutical Sciences. 2017; 4(8): 613-618.
- Rao SSR, Vardhini BV, Sujatha E, Anuradha S. Brassinosteroids-A new class of phytohormones. Curr Sci. 2002; 82(10): 1239-1245.
- Kaur H, Sirhindi G, Bhardwaj R, Alyemeni MN, Siddique KHM, Ahmad P. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in Brassica juncea. Scientific Reports. 2018; 8: 8735. doi: 10.1038/s41598-018-27032-w
- Wang XH, Shu C, Li HY, Hu XQ, Wang YX. Effects of 0.01% brassinolide solution application on yield of rice and its resistance to autumn lowtemperature damage. Jiangxi Nongye Xuebao (Acta Agriculturae Jiangxi). 2014; 26(5): 36-38.
- Chen ZY, Wang YT, Pan XB, Xi ZM. Amelioration of cold-induced oxidative stress by exogenous 24-epibrassinolide treatment in grapevine seedlings: toward regulating the ascorbate-glutathione cycle. Scientia Horticulturae. 2019; 244: 379-387. doi: 10.1016/j.scienta.2018.09.062
- Gallo J, Balas M, Linda R, Kunes I. Growth performance and resistance to ground late frosts of Fagus sylvatica L. plantation treated with a brassinosteroid compound. Journal of Forest Science. 2017; 63: 117-125. doi: 10.17221/67/2016-JFS
- Dong YJ, Wang WW, Hu GQ, et al. Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. Journal of Soil Science and Plant Nutrition. 2017; 17: 554-569.
- Hegazi AM, El-Shraiy AM, Ghoname AA. Mitigation of salt stress negative effects on sweet pepper using arbuscular mycorrhizal fungi (AMF), Bacillus megaterium and brassinosteroids (BRs). Gesunde Pflanzen. 2017; 69(2): 91-102. doi: 10.1007/s10343-017-0394-8.
- Kurepin LV, Joo SH, Kim SK, Pharis RP, Back TG. Interaction of brassinosteroids with light quality and plant hormones in regulating shoot growth of young sunflower and Arabidopsis seedlings. Journal of Plant Growth Regulation. 2012; 31(2): 156-164. doi: 10.1007/s00344-011-9227-7
- Gill MB, Cai K, Zhang G, Zeng F. Brassinolide alleviates the droughtinduced adverse effects in barley by modulation of enzymatic antioxidants and ultrastructure. Plant Growth Regul. 2017; 82(3): 447-455. doi: 10.1007/s10725-017-0271-6
- Li H, Mo Y, Cui Q, et al. Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and -susceptible watermelon genotypes. Plant Sci. 2019; 278: 32-43. doi: 10.1016/j.plantsci.2018.10.016
- Jakubowska D, Janicka M. The role of brassinosteroids in the regulation of the plasma membrane H+-ATPase and NADPH oxidase under cadmium stress. Plant Sci. 2017; 264: 37-47. doi: 10.1016/j.plantsci.2017.08.007
- Chandrakar V, Yadu B, Meena RK, Dubey A, Keshavkant S. Arsenic-induced genotoxic responses and their amelioration by diphenylene iodonium, 24-epibrassinolide and proline in Glycine max L. Plant Physiol Biochem. 2017; 112: 74-86.
- Dalyan E, Yuzbasioglu E, Akpinar I. Effect of 24-epibrassinolide on antioxidative defence system against lead-induced oxidative stress in the roots of Brassica juncea L. seedlings. Russ J Plant Physiol. 2018; 65(4): 570-578.
- Javadi A, Khomari S, Esmaeilpour B, Asghari A. Exogenous application of 24-epibrassinolide and nano-zinc oxide at flowering improves osmotic stress tolerance in harvested tomato seeds. Applied Ecology and Environmental Research. 2018; 16(4): 4401-4417.
- Sharma I, Bhardwaj R, Pati PK. Stress modulation response of 24-epibrassinolide against imidacloprid in an elite indica rice variety Pusa Basmati-1. Pestic Biochem Physiol. 2013; 105(2): 144-153. doi: 10.1016/j.pestbp.2013.01.004
- Shahzad B, Tanveer M, Zhao C. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotoxicol Environ Saf. 2018; 147: 935-944. doi: 10.1016/j.ecoenv.2017.09.066
- An YH, Zhou H, Yuan YH, et al. 24-Epibrassinolide-induced alterations in the root cell walls of Cucumis sativus L. under Ca(NO3)2 stress. Protoplasma. 2018; 255: 841-850.
- Nie WJ, Wang SS, Jing X, et al. Effects of exogenous 2,4-epibrassinolide on the growth and redox balance of cucumber seedlings under NaHCO3 stress. Ying Yong Sheng Tai Xue Bao. 2018; 29(3): 899-908. doi: 10.13287/j.1001-9332.201803.024
- Ahammed GJ, He BB, Qian XJ, et al. 24-Epibrassinolide alleviates organic pollutants-retarded root elongation by promoting redox homeostasis and secondary metabolism in Cucumis sativus L. Environ Pollut. 2017; 229: 922-931. doi: 10.1016/j.envpol.2017.07.076.
- Gupta P, Srivastava S, Seth CS. 24-Epibrassinolide and sodium nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil. 2017; 411: 483-498. doi: 10.1007/s11104-016-3043-6
- Bibi N, Ahmed IM, Fan K, et al. Role of brassinosteroids in alleviating toxininduced stress of Verticillium dahliae on cotton callus growth. Environ Sci Pollut Res Int. 2017; 24(13): 12281-12292. doi: 10.1007/s11356-017-8738-6
- Gunupuru LR, Perochon A, Doohan FM, Ali SS, Scofield SR. Virus-induced gene silencing (VIGS) for functional characterization of disease resistance genes in barley seedlings. Methods Mol Biol. 2019; 1900: 95-114. doi: 10.1007/978-1-4939-8944-7_7
- Jasrotia S, Ohri P. 24-Epibrassinolide reduces stress in nematode-infected tomato (Solanum lycopersicum L.) plants cultured in vitro. In Vitro Cell Dev Biol Plant. 2017; 53(6): 538-545. doi: 10.1007/s11627-017-9859-9
- Song LX, Xu XC, Wang FN, et al. Brassinosteroids act as a positive regulator for resistance against root-knot nematode involving respiratory burst oxidase homolog - dependent activation of MAPKs in tomato. Plant Cell Environ. 2018; 41(5): 1113-1125. doi: 10.1111/pce.12952
- Filek M, Sieprawska A, Oklestkova J, et al. 24-Epibrassinolide as a modifier of antioxidant activities and membrane properties of wheat cells in zearalenone stress conditions. J Plant Growth Regul. 2018; 37(4): 1085-1098. doi: 10.1007/s00344-018-9792-0
- Pan G, Liu Y, Ji L, et al. Brassinosteroids mediate susceptibility to brown planthopper by integrating with the salicylic acid and jasmonic acid pathways in rice. J Exp Bot. 2018; 69(18): 4433-4442. doi: 10.1093/jxb/ery223
- Yang AJ, Anjum SA, Wang L, et al. Effect of foliar application of brassinolide on photosynthesis and chlorophyll fluorescence traits of Leymus chinensis under varying levels of shade. Photosynthetica. 2018; 56(3): 873-883. doi: 10.1007/s11099-017-0742-z
- Hu CH, Guo J, Chen L, Li LP, Qiao L, He L. Influence of exogenous brassinolide on growth and resistance of maize seedling with preservative stress. Hunan Nongye Daxue Xuebao. 2014; 40: 113-116.
- Han YY, Gang H, Li KR, Zhang XX. Effects of brassinolide on photosynthetic parameters of Robinia pseudoacacia seedlings in petroleum polluted soil. Nature Environment and Pollution Technology. 2017; 16: 199-204.
- Pradhan SK, Gupta RC, Kumar M. Effect of 24-Epibrassinolide on Lipid Peroxidation and Proline in three Brassica species under temperature stress. J Stress Physiol Biochem. 2013; 9: 376-384.
- Zhao G, Xu H, Zhang P, Su X, Zhao H. Effects of 2,4-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul. 2017; 81(3): 377-384. doi: 10.1007/s10725-016-0214-7
- Cukor J, Rasakova NM, Linda R, Linhart L, Gutsch MR, Kunes I. Effects of brassinosteroid application on seed germination of scots pine under standard and heat stress conditions. Baltic Forestry. 2018; 24: 60-67.
- Confraria A, Desikan R, Santos I, Neill S. Brassinosteroids protect plants against heat stress. Comp Biochem Physiol A Mol Integr Physiol. 2007; 146(4): S279-S279. doi: 10.1016/j.cbpa.2007.01.635
- Zhou J, Wang J, Li X, et al. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J Exp Bot. 2014; 65(15): 4371-4383. doi: 10.1093/jxb/eru217.
- Wilen RW, Sacco M, Gusta LV, Krishna P. Effects of 24-epibrassinolide on freezing and thermotolerance of bromegrass (Bromus inermis) cell cultures. Physiol Plant. 1995; 95(2): 195-202. doi: 10.1111/j.1399-3054.1995.tb00827.x
- Hayat S, Hasan SA, Yusuf M, Hayat Q, Ahmad A. Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ Exp Bot. 2010; 69: 105-112.
- Cao YY, Zhao H. Protective roles of brassinolide in rice seedlings under heat stress. Zhongguo Shuidao Kexue (China Rice Science). 2007; 21(5): 525-529.
- Ogweno JO, Song XS, Shi K, et al. Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J Plant Growth Regul. 2008; 27(1): 49-57. doi: 10.1007/s00344-007-9030-7
- Mazorra LM, Nunez M, Hechavarria M, Coll F, Sanchez-Blanco MJ. Influence of brassinosteroids on antioxidant enzymes activity in tomato under different temperatures. Biol Plant. 2002; 45(4): 593-596. doi: 10.1023/A:1022390917656
- Singh I, Shono M. Physiological and molecular effects of 24-epibrassinolide, a brassinosteroid on thermotolerance of tomato. Plant Growth Regul. 2005; 47: 111-119. doi: 10.1007/s10725-005-3252-0
- Mazorra LM, Holton N, Bishop GJ, Nunez M. Heat shock response in tomato brassinosteroid mutants indicates that thermotolerance is independent of brassinosteroid homeostasis. Plant Physiol Biochem. 2011; 49(12): 1420-1428. doi: 10.1016/j.plaphy.2011.09.005
- Nasser AH. Effect of homobrassinolide on in vitro growth of apical meristems and heat tolerance of banana shoots. International Journal of Agriculture and Biology. 2004; 6(5): 771-775.
- Janeczko A, Oklestkova J, Pociecha E, Koscielniak J, Mirek M. Physiological effects and transport of 24-epibrassinolide in heat-stressed barley. Acta Physiol Plant. 2011; 33(4): 1249-1259. doi: 10.1007/s11738-010-0655-y
- Dhaubhadel S, Chaudhary S, Dobinson KF, Krishna P. Treatment with 24-epibrassinolide, a brassinosteroid, increases the basic thermotolerance of Brassica napus and tomato seedlings. Plant Mol Biol. 1999; 40(2): 333-342. doi: 10.1023/A:1006283015582
- Dhaubhadel S, Browning KS, Gallie DR, Krishna P. Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J. 2002; 29(6): 681-691.
- Zhang YP, Zhu XH, Ding HD, Yang SJ, Chen YY. Foliar application of 24-epibrassinolide alleviates high-temperature-induced inhibition of photosynthesis in seedlings of two melon cultivars. Photosynthetica. 2013; 51(3): 341-349. doi: 10.1007/s11099-013-0031-4
- Sam O, Nunez M, Ruiz-Sanchez MC. Effect of a brassinosteroid analogue and high temperature stress on leaf ultrastructure of Lycopersicon esculentum. Biol Plant. 2001; 44(2): 213-218. doi: 10.1023/A:1010291124178
- Niu JH, Anjum SA, Wang R, et al. Exogenous application of brassinolide can alter morphological and physiological traits of Leymus chinensis (Trin.) Tzvelev under room and high temperatures. Chilean J Agric Res. 2016; 76(1): 27-33. doi: 10.4067/S0718-58392016000100004
- Jin SH, Li XQ, Wang GG, Zhu XT. Brassinosteroids alleviate hightemperature injury in Ficus concinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. AoB Plants. 2015; 7: plv009. doi:10.1093/aobpla/plv009
- Thussagunpanit J, Jutamanee K, Kaveeta L, et al. Comparative effects of brassinosteroid and brassinosteroid mimic on improving photosynthesis, lipid peroxidation, and rice seed set under heat stress. J Plant Growth Regul. 2015; 34(2): 320-331. doi: 10.1007/s00344-014-9467-4
- Thussagunpanit J, Jutamanee K, Sonjaroon W, et al. Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynthetica. 2015; 53(2): 312-320. doi: 10.1007/s11099-015-0106-5
- Singh I, Shono M. Effect of 24-epibrassinolide on pollen viability during heat stress in tomato. Indian J Exp Biol. 2003; 41(2): 174-176.
- Fahad S, Hussain S, Saud S, et al. Exogenously applied plant growth regulators affect heat-stressed rice pollens. J Agro Crop Sci. 2016; 202(2): 139-150. doi: 10.1111/jac.12148
- Liu J, Qiu W, Xia D. Brassinosteroid improves lipid productivity and stress tolerance of Chlorella cells induced by high temperature. J Appl Phycol. 2018; 30(1): 253-260. doi: 10.1007/s10811-017-1223-2
- Janeczko A, Gullner G, Skoczowski A, Dubert F, Barna B. Effects of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. Biol Plant. 2007; 51(2): 355-358. doi: 10.1007/s10535-007-0072-2
- He RY, Wang GJ, Wang XS. Effects of brassinolide on growth and chilling resistance of maize seedlings. ACS Symposium Series. 1991; 474: 220-230. doi: 10.1021/bk-1991-0474.ch019
- Wu X, Lu XM. Effects of brassinolide on the antioxidant system and photosynthesis of cucumber seedlings under suboptimal temperature, light and salt environment. Ying Yong Sheng Tai Xue Bao. 2015; 26: 2751-2757.
- Fujii S, Saka H. Distribution of assimilates to each organ in rice plants exposed to a low temperature at the ripening stage, and the effect of brassinolide on the distribution. Plant Prod Sci. 2001; 4: 136-144. doi: 10.1626/pps.4.136
- Fujii S, Saka H. The promotive effect of brassinolide on lamina joint-cell elongation, germination and seedling growth under low-temperature stress in rice (Oryza sativa L.). Plant Prod Sci. 2001; 4(3): 210-214. doi: 10.1626/pps.4.210
- He RY, Wang GJ, Wang XS. Effects of brassinolide (BR) on the growth and chilling resistance of maize. ACS Symposium Series. 1991; 474: 220-230.
- Xi Z, Wang Z, Fang Y, et al. Effects of 24-epibrassinolide on antioxidation defense and osmoregulation systems of young grapevines (V. vinifera L.) under chilling stress. Plant Growth Regul. 2013; 71(1): 57-65. doi: 10.1007/s10725-013-9809-4
- Liu YJ, Zhao ZG, Si J, Di CX, Han J, An LZ. Brassinosteroids alleviate chilling-induced oxidative damage by enhancing antioxidant defense system in suspension cultured cells of Chorispora bungeana. Plant Growth Regul. 2009; 59(3): 207-214. doi: 10.1007/s10725-009-9405-9
- Kumar M, Sirhindi G, Bhardwaj R, Kumar S, Jain G. Effect of exogenous H2O2 on antioxidant enzymes of Brassica juncea L. seedlings in relation to 24-epibrassinolide under chilling stress. Indian J Biochem Biophys. 2010; 47(6): 378-382.
- Hu WH, Wu Y, Zeng JZ, He L, Zeng QM. Chill-induced inhibition of photosynthesis was alleviated by 24-epibrassinolide pretreatment in cucumber during chilling and subsequent recovery. Photosynthetica. 2010; 48(4): 537-544. doi: 10.1007/s11099-010-0071-y
- Hu X, Xin L, Sun L, Fu Z. Toxic effects of chlorpyrifos on cucumber and brassinosteroid-mediated responses under the conditions of chilling temperature and low light. Shengtai Duli Xuebao (Asian Journal of Ecotoxicology). 2013; 8: 513-520.
- Ohshiro T, Ohkawa M, Kitajima J, Mizuguchi S, Ikekawa T. Effects of cold temperature, 24-epibrassinolide and light on breaking dormancy of the regenerated bulblets of Lilium japonicum Thunb. Environment Control in Biology. 1997; 35(1): 29-34. doi: 10.2525/ecb1963.35.29
- Katsumi M. Physiological modes of brassinolide action in cucumber hypocotyl growth. ACS Symposium Series. 1991; 474: 246-254. doi: 10.1021/bk-1991-0474.ch021
- Fariduddin Q, Yusuf M, Chalkoo S, Hayat S, Ahmad A. 28-Homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. Photosynthetica. 2011; 49(1): 55-64. doi: 10.1007/s11099-011-0022-2
- Jiang YP, Huang LF, Cheng F, et al. Brassinosteroids accelerate recovery of photosynthetic apparatus from cold stress by balancing the electron partitioning, carboxylation and redox homeostasis in cucumber. Physiol Plant. 2013; 148(1): 133-145. doi: 10.1111/j.1399-3054.2012.01696.x
- Wang Q, Ding T, Gao L, Pang J, Yang N. Effect of brassinolide on chilling injury of green bell pepper in storage. Sci Hortic (Amsterdam). 2013; 144: 195-200. doi: 10.1016/j.scienta.2012.07.018
- Aghdam MS, Asghari M, Farmani B, Mohayeji M, Moradbeygi H. Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Sci Hortic (Amsterdam). 2012; 144: 116-120. doi: 10.1016/j.scienta.2012.07.008
- Anwar A, Bai L, Miao L, et al. 24-Epibrassinolide ameliorates endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings. Int J Mol Sci. 2018; 19(9): E2497. doi: 10.3390/ijms19092497
- Kamuro Y, Takatsuto S. Practical applications of brassinosteroids in agricultural fields. In: Sakurai A, Yokota T, Clouse SD (eds). Brassinosteroids – Steroidal Plant Hormones. Springer-Verlag, Tokyo. 1999: 223-241.
- Liu Z, Li L, Luo Z, Zeng F, Jiang L, Tang K. Effect of brassinolide on energy status and proline metabolism in postharvest bamboo shoot during chilling stress. Postharvest Biol Technol. 2016; 111: 240-246. doi: 10.1016/j.postharvbio.2015.09.016
- Watanabe T, Noguchi T, Kuriyama H, Kadota M, Takatsuto S, Kamuro Y. Effects of brassinosteroid compound [TS303] on fruit-setting, fruit-growth taking roots and cold-resistance. Acta Hort. 1997; 463: 267-270. doi: 10.17660/ActaHortic.1998.463.32
- Wang R, Anjum SA, Niu J, et al. Exogenous application of brassinolide ameliorates chilling stress in Leymus chinensis (Trin.) Tzvel. by modulating morphological, physiological and biochemical traits. Bangladesh J Bot. 2016; 45: 143-150.
- Dong D, Li Y, Jiang L, Liang H, Huang J. Effects of long-lasting brassinosteroid TS303 and propyl dihydrojasmonate on enhancing peanut resistance to chilling. Guangxi Zhiwu. 2008; 28: 675-680.
- Wu XX, Ding HD, Chen JL, Zhu ZW, Zha DS. Exogenous spray application of 24-epibrassinolide induced changes in photosynthesis and antioxidant defences against chilling stress in eggplant (Solanum melongena L.) seedlings. J Hortic Sci Biotechnol. 2015; 90: 217-225. doi: 10.1080/14620316.2015.11513175
- Asao T, Tomita K, Taniguchi K, et al. Occurrence of deformed leaves in cucumber plants treated with cold water and its reduction in seedlings derived from TNZ303 (mixture of jasmonic acid and brassinosteroids derivative) treated seeds. Journal of the Japanese Society for Horticultural Science. 2002; 71(2): 297-299. doi: 10.2503/jjshs.71.297
- Huang B, Chu CH, Chen SL, Juan HF, Chen YM. A proteomics study of the mung bean epicotyl regulated by brassinosteroids under conditions of chilling stress. Cell Mol Biol Lett. 2006; 11(2): 264-278. doi: 10.2478/s11658-006-0021-7
- Aghdam MS, Mohammadkhani N. Enhancement of chilling stress tolerance of tomato fruit by postharvest brassinolide treatment. Food Bioproc Tech. 2014; 7(3): 909-914. doi: 10.1007/s11947-013-1165-x
- Wu J. Effects of brassinosteroids on chilling resistance of Dendrobium huoshanense. Xibei Zhiwu Xuebao. 2015; 35: 985-990.
- Pociecha E, Dziurka M, Oklestkova J, Janeczko A. Brassinosteroids increase winter survival of winter rye (Secale cereale L.) by affecting photosynthetic capacity and carbohydrate metabolism during the cold acclimation process. Plant Growth Regul. 2016; 80(2): 127-135.
- Pociecha E, Dziurka M, Waligorski P, Krepski T, Janeczko A. 24-Epibrassinolide pre-treatment modifies cold-induced photosynthetic acclimation mechanisms and phytohormone response of perennial ryegrass in cultivar-dependent manner. J Plant Growth Regul. 2017; 36(3): 618-628. doi: 10.1007/s00344-016-9662-6
- Cui LR, Zou ZR, Zhang J, Zhao YY, Yan F. 24-Epibrassinoslide enhances plant tolerance to stress from low temperatures and poor light intensities in tomato (Lycopersicon esculentum Mill.). Funct Integr Genomics. 2016; 16(1): 29-35. doi: 10.1007/s10142-015-0464-x
- Li J, Yang P, Gan Y, Yu J, Xie J. Brassinosteroid alleviates chilling-induced oxidative stress in pepper by enhancing antioxidation systems and maintenance of photosystem II. Acta Physiol Plant. 2015; 37: 1-11.
- Hirai K, Fujii S, Honjo K. Plant growth regulating action of brassinolide. 1. The effect of brassinolide on the ripening of rice plants under the low temperature condition. Jpn J Crop Sci. 1991; 60(1): 29-35. doi: 10.1626/jcs.60.29
- Tavallali V. Vacuum infiltration of 24-epibrassinolide delays chlorophyll degradation and maintains quality of lime during cold storage. Acta Sci Pol Hortorum Cultus. 2018; 17(1): 35-48. doi: 10.24326/asphc.2018.1.4
- Xia XJ, Fang PP, Guo X, et al. Brassinosteroid-mediated apoplastic H2O2-glutaredoxin 12/14 cascade regulates antioxidant capacity in response to chilling in tomato. Plant Cell Environ. 2018; 41(5): 1052-1064. doi: 10.1111/pce.13052
- Eremina M, Unterholzner SJ, Rathnayake AI, et al. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc Natl Acad Sci U S A. 2016; 113(40): E5982-E5991. doi: 10.1073/pnas.1611477113
- Sadura I, Janeczko A. Physiological and molecular mechanisms of brassinosteroid-induced tolerance to high and low temperature in plants. Biol Plant. 2018; 62(4): 601-616. doi: 10.1007/s10535-018-0805-4
- Filek M, Rudolphi-Skórska E, Sieprawska A, Kvasnica M, Janeczko A. Regulation of the membrane structure by brassinosteroids and progesterone in winter wheat seedlings exposed to low temperature. Steroids. 2017; 128: 37-45. doi: 10.1016/j.steroids.2017.10.002
- Vardhini BV, Anjum NA. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front Environ Sci. 2015; 2: 67. doi: 10.3389/fenvs.2014.0006
- Vardhini BV. An overview on the various physiological roles of brassinosteroids in the past decade-A Review. Asian Journal of Science and Technology. 2019; 10(1): 9320-9325


