Review Article
A Review of Chemical Activating Agent on the Properties of Activated Carbon
Faculty of Health and Life Sciences, INTI International University, Malaysia
*Corresponding author: S.M. Ho, Faculty of Health and Life Sciences INTI International University Putra Nilai, 71800, Negeri Sembilan Malaysia, E-mail: soonmin.ho@newinti.edu.my
Received: April 18, 2022 Accepted: May 09, 2022 Published: May 18, 2022
Citation:: Ho SM. A Review of Chemical Activating Agent on the Properties of Activated Carbon. Int J Chem Res. 2022; S1(1): 1-13.doi: 10.18689/ijcr-s1-001
Copyright: © 2022 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Activated carbon has received great attention because of excellent properties including high surface area, high porosity and ability of tunable surface to various functional groups. Preparation of activated carbon by using various precursors was reported. In this work, the influence of activating agent on the properties of activated carbon prepared by using conventional and microwave heating processes was reported. The choice of activating agent (acidic, basic and neutral activating agent) is very important to improve surface area, porous structures and adsorptivity. Research findings showed that each activating agent reacted uniquely with raw materials. Selection of activating agent strongly depended on the target adsorbent, safe and readily available in the market. The reviewed literature indicated that the textural properties, the percentage of yield, iodine test and methylene blue numbers were affected by the concentration of activating agent, activation time, activation temperature and impregnation ratio. The adsorption studies were carried out by using various models such as Langmuir (monolayer coverage), Freundlich (beyond one layer) and Temkin model. Generally, high coefficient of correlation (R2 value) indicated that the model is a good fit for the data. The thermodynamic parameters (entropy, enthalpy and free energy) of adsorbate adsorbed onto activated carbon were investigated also.
Keywords: Activated Carbon; Carbonization; Chemical Activation; Surface Area; Adsorption; Porosity Structure.
Introduction
Activated carbon is defined as carbonaceous solid derived from various precursors through thermal processes[1]. The obtained activated carbon showed well-developed pores, excellent surface area and high adsorptive capacity[2]. These carbons could be used in different applications including chemical industry, air purification, wastewater treatment[3], pharmacy, food industry, automobile industry, drinking water treatment, indoor air decontamination, and gas purification. Classification of activated carbon could be divided into powdered, granular[4] and extruded form. While, the porous structures[5] were categorized as microporous (pore diameters of less than 2 nm), mesoporous (pore diameters between 2nm to 50 nm) and macro porous (pore diameters of more than 50 nm). On the other hand, based on the activated carbon market analysis report[6], the global market size will be increased from US $8.23 billion in 2021 to US $11.79 billion in 2026. Improved drinking water treatment, international rules monitoring the discharge of mercury, and rising automobile ownership rates are boosting the expansion of activated carbon business[7].
Several observations can be seen in the carbonization process including decomposition of organic material, carbon content is increased, impurities are removed and pore structures are created after released of volatile elements[8]. The temperature could be increased to speed up the reaction[9]. If too high temperature (more than 1000°C), resulted the ash which can close the pores. In the chemical activation process, the precursor is treated with acidic, salt and basic solutions[10]. Then, heated to specific temperature in activation kiln. Finally, washed with water to remove impurity from the carbon[11]. The main role of activating agent is the degradation of the cellulosic material, and pore structure was produced[12]. The surface area and pore size distribution strongly depended on the degree of impregnation. It could be defined as the mass of chemical and also the mass of precursors[13]. Other preparation variables such as pyrolysis temperature and the soaking time also affect the properties of activated carbon[14].
This work reviews the production of activated carbon via chemical activation process. Several types of activating agents (acidic activation, neutral activation, basic activation) were used to develop surface area, create porosity structure and enhance the adsorptivity. The objective of this work is to report the textural characteristics, surface morphology and surface chemistry of the obtained activated carbon by using various tools.
Literature Survey
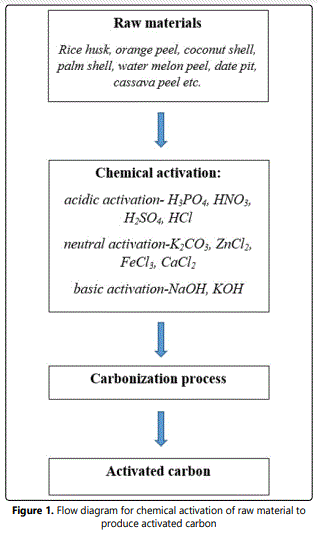
Activated carbon is considered as carbonaceous materials and became very important research area because of excellent properties. The activated carbon showed non-toxicity, higher surface area, high porosity structure and ability of tunable surface to various functional groups. The preparation of activated carbon from different precursors has both economic and environmental effects. Generally, it will convert unwanted, low-value agricultural wastes into very useful high-value adsorbent under chemical activation process. The activation and carbonization process are very important during the synthesis of activated carbon. The flow diagram for these processes as shown in figure 1. The carbonization temperature should be within the range of 200 to 1100°C. The carbon will change to ash if more than certain carbonization temperature. Chemical activation process has many advantages such as produce high yield, low energy cost and simple recovery process of activating agents if compared to physical activation. The properties of obtained materials were studied by using different techniques such as scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), x-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), and differential thermal analysis (DTA) technique.
Acidic Activation Agent
Phosphoric acid: Removal of phenanthrene by using activated carbon[15] produced from Vitis vinifera leaf litter under activating agent H3PO4. Textural properties such as surface area (109 to 295 m2/g), micro pore area (82 to 174 m2/g), micro pore volume (0.03 to 0.07 cm3/g), total pore volume (0.06 to 0.185 cm3/g) and pore size (2.19 to 3.29 nm) were studied. The coefficient value was determined, and found to be 0.98 (Langmuir), 0.99 (Freundlich), 0.92 (Temkin), and 0.98 (Dubinin-Radushkevich) in different isotherms. The Cucumismelo was used to produce activated carbon under chemical activation[16] process. Specific surface area (208 to 475 m2/g) and the percentage of removal (22.58% to 95.26%) of methylene blue and acid orange 7 were reported. In the FTIR analysis, 1300-1000cm-1 attributed to phosphorus and phosphocarbon (P=O bond or O-C bond) in samples. Removal of methyl orange by using pomelo peel based activated carbon prepared under chemical activation[17]. The maximum adsorption of dyes could be observed at pH 3, and dosage of activated carbon is 1g/L. The removal of dye depicted multilayer adsorption process, followed Freundlich model, and supported pseudo-second order. The low-cost activated carbon was synthesized by using various precursors under chemical activation[18]. TSS (43-100%) and COD removal (48-99%) were reported in coconut shell, orange peel and banana peel based activated carbon, prepared under various size (75, 150 and 425 μm) and concentration (100, 200 and 500 mg/L). Based on the experimental results, orange peel based activated carbon showed more C-O and C=O groups, and more carbon & oxygen contents if compared to other activated carbons. The surface chemistry of olive stone based activated prepared via activating agent[19] was reported. Total pore volume (0.6 cm3/g) and BET surface area (1218 m2/g) were highlighted when the concentration of phosphoric acid was 80%. The grape seeds were used to produce activated carbon by chemical activation[20]. Experimental results displayed excellent mesopore volume development (0.24 cm3/g) and the highest surface area (1139 m2/g) with grape seed to phosphoric acid ratio of 1:3 at carbonization temperature of 500°C. The morphology studies revealed egg shell structure and granular morphology in sample. The Melia Azedarach waste sawdust derived phosphoric acid treated activated carbon was synthesized via chemical activation[21]. The obtained activated carbon indicated mesoporous development, average pore width of 20.33Å, and BET surface area of 293m2/g. Adsorption efficiency of 97.8% (reactive orange 16) was observed when the adsorbent dose=1g/L, and initial concentration =30 mg/L. The adsorption process was favorable, spontaneous, supported Langmuir model and confirmed the pseudo-second order model. Production of activated carbon by using orange peel via activation method[22]. Removal of methylene blue reached 277.7 mg/g with concentration of phosphoric acid =40%, activation time=12 hours, and carbonization temperature=500 °C. Pine strobus sawdust was employed to produce activated carbon via chemical activation technique[23]. Textural examination including yield (64.5%), moisture (6.87%), density (0.32 g/cm3), ash (22%), total pore volume (7.385e-1 cm3/g), average pore radius (1.49e+1 nm) and BET surface area (991 m2/g) was reported. Adsorption of methyl orange is complex process, and could be represented using pseudo-second order model. Corncob based activated carbon was synthesized by chemical activation via pyrolysis process[24]. The surface area (700 to 600 m2/g) and micropore volume (0.011 to 0.003 cm3/g) reduced with increasing activation temperature from 400 °C to 600 °C. The adsorption of methylene blue fitted well with Langmuir model (activation temperature=400 °C, 500 °C) and Freundlich isotherm (activation temperature=600 °C). The influence of concentration of phosphoric acid on the palm oil shell based activated carbon was reported[25]. Experimental findings showed the percentage of yield increased when the concentration of phosphoric acid was increased from 10% to 20%. However, reduced in the percentage of yield could be observed when further increase in concentration of phosphoric acid because of it releases the volatiles. The obtained activated carbon showed the highest adsorption for chromium but very poor for copper element. Overall, researcher has described that phosphoric acid could be used for breaking chemical bonds, protects the internal pore structure and caused development in porosity[26]. Researcher concluded that phosphoric acid can break down cellulose and hemicellulose (in biomass) to produce monomeric sugar. The H3PO4-treated activated carbon indicated multilayer filling in adsorption model, and mesoporous formation.
Nitric acid: Waste tea based activated carbon was prepared by microwave technique, and treated with nitric acid[27]. Nitric acid modification of carbons enhanced the oxygen and reduced the carbon content. More methylene blue and less phenol (due to absence of basic surface groups) could be removed in HNO3-treated activated carbon. Commercial coal based powder activated carbon was soaked into nitric acid solution[28]. The surface area (754.9 to 770 m2/g), total pore volume (0.429 to 0.461 cm3/g) and average pore size (2.2 to 2.4 nm) were reported for the carbon prepared under various concentrations of nitric acid (5%, 10%, 15%, 20%). Nitric acid could develop of pores, increase the contact area, and finally facilitate mass transfer during the adsorption process. There are very clear that increase in ratio of activating agent is proportional to the efficiency until the optimal condition is achieved, then created higher pore volume. Higher correlation coefficient (R2) value could be detected in pseudo-second order model (R2=0.992) if compared to pseudo-first order model (R2=0.9534). On the other hand, Langmuir model (R2=0.9874) was more appropriate for adsorption of nickel ions if compared to Freundlich model (R2=0.8698). Lapsi seed stone was employed for the synthesis of activated carbon under chemical activation process[29]. Higher methylene blue number could be observed in carbon with a mixture of concentrated sulphuric acid and concentrated nitric acid (due to created more pores). The carob shell was used to prepare activated carbon through nitric acid activation[30]. The maximum removal of cadmium (59.25 mg/g) and cobalt (50.66 mg/g) was reported in the specific conditions such as impregnation ratio=0.1g/g, activation time=60 minutes, and temperature=500°C. Boehm titration of surface groups including carboxylic (0.455 meq/g), lactonic (0.499 meq/g), phenolic (0.508 meq/g), total acid (1.462 meq/g), total basic (0.443 meq/g) was highlighted. XRD studies showed two diffraction peaks attributed to (002) and (110) planes, reflected disordered graphitic structure. The adsorption capacity for cadmium and cobalt ions obeyed Langmuir model due to higher R2 value if compared to Freundlich isotherm. Oil palm shell was employed for the synthesis of activated carbon via chemical activation[31]. Three types of weight loss such as 30-160 °C (removal of gases and moisture), 160-450 °C (removal of lignin, hemicellulosic), 450-900°C (removal of cellulosic materials) could be detected in thermal analysis. Textural porous characteristics including BET surface area (325.4 m2/g), Langmuir surface area (491.6 m2/g), DR microspore volume (0.18 cc/g), total pore volume (0.19 cc/g), average pore diameter (11.45 Å), and DA pore diameter (8 Å) were reported. The nitric acid can remove some contaminants (Fe, Si, K and Al) attached to the surface of activated carbon. The activated carbon was produced by using African palm shell under chemical activation[32]. The BET surface area, and mircopore volume were 634 m2/g, and 0.25 cm3/g, respectively. Elemental analysis revealed 66.1% carbon, 31.4 % oxygen, 1.3 % hydrogen and 1.2% nitrogen. The obtained carbon has higher content of carboxylic (0.12 mmol/g), lactonic (0.21 mmol/g), and phenolic groups (0.35 mmol/g) because of nature of the surface is acidic (0.68 mmol/g) and the pHpzc of 6.2. The carbon dioxide adsorption is found to be 237 mg CO2/g carbon at 1 bar, 273 K. The almond shell was used to produce the activated carbon[33]. HNO3-treated activated carbon introduces oxygen surface complexes, affected surface area and the porosity of the carbons. FTIR studies revealed carboxyl group was fixed. The weed based activated carbon was proposed[34]. The kinetic data obeyed the pseudosecond order kinetic model and supported Langmuir isotherm model. The best removal of methylene blue was 161.29 mg/g in pH=7.4, temperature=50°C, agitation time=480 minutes, and carbon dosage 0.1g/50 mL. The bamboo based activated carbon[35] had the highest iodine number (1198 g of iodine/kg of carbon), indicating these carbons have many chemically active sites. Other characteristics such as bulk density (0.45 g/cm3), methylene blue adsorptive capacity (941.3 mg/g), pore volume (0.45 cm3) and ash content (2.76 %) were reported. HNO3-treated activated carbon can absorb the highest percentage of iron ions if compare to other NaOH-treated, H3PO4-treated, ZnCl2-treated carbons. On the other hand, these adsorbents can remove nickel, and zinc ions if compared to coconut and palm kernel shell based activated carbon due to cellulose nitrite produced during the activation process.
Sulfuric acid: The Scrap tire was used to produce activated carbon[36] under activation agent (fixed impregnation ratio of 1:1) at various carbonization temperatures (500, 600 and 700 °C). The surface area (1066.7 m2/g), iodine number (590.5 mg/g) and methylene blue adsorption (288.9 mg/g) were reported when the temperature was 500°C, for 60 minutes. Production of granular activated carbon by using coconut shell via chemical activation[37] was proposed. FTIR characterization revealed that C-O (1100 cm-1) and lactone (1410 cm-1) could be observed after acidic treatment. SEM characterization confirmed honeycomb shape and inconsistent pore structure by using 1M and 2M HCl, respectively. The surface area values were436, 525 and 372 m2/g in untreated carbon, 1M HCl-treated carbon and 2M HCl-treated carbon, respectively, indicating the destruction effect resulted from the surplus water vapor released through acid dehydration process[38]. Microporous volume (0.218 to 0.272 cm3/g) and total pore volume (0.229 to 0.291 cm3/g) were increased before and after chemical treatment. Adsorption data supported Langmuir model for all carbons, and the R2 values were more than 0.99. Coconut shell consisted of high cellulose (24.35%) and volatile (72%), could be used to prepare activated carbon[39]. Research findings showed iodine value increased with increasing activation temperature up to 600 °C, then dropped. The obtained carbon was able to absorb Cu2+, Mg2+, Al3+, Fe2+ and Ni2+ions, more effective than other activating agents such as NaOH and ZnCl2. The Euphorbia rigid a based activated carbon was produced to remove basic textile dye[40]. The surface area of these carbons was 741.2 m2/g, and the adsorption of dyes reached equilibrium within 1 hour. The experimental data supported Langmuir model (adsorption capacity=114.45 mg/g at 40 °C), pseudo-second order isotherm and intraparticle diffusion model. The activation energy was determined and found to be 55.5 kJ/mol. The activation of Catha edulis stem was carried out under sulfuric acid[41]. Proximate analyses of the obtained activated carbon such as moisture (4%), volatile matter (25%), ash content (18%) and fixed carbon (53%) were reported. Several peaks including 3300-3600 cm-1 (O-H group), 2944 cm-1(stretching C-H), 1500- 1600 cm-1 (carboxylate bond), 1425 cm-1 (C-C stretched aromatic) and 800 cm-1 (C=CH stretched) could be observed in FTIR studies. Production of activated carbon from sunflower oil cake by sulfuric acid activation was reported[42]. The adsorption data (methylene blue) fitted well with Langmuir model and pseudo-second-order model. Preparation of molasses based activated carbon by using chemical activation[43] was described. The obtained activated carbon showed highly microporous, and high surface area (1200 m2/g). The highest adsorption capacity of iodine (1430 mg/g) and methylene blue (435 mg/g) was highlighted. Biomass solid waste was used to produce activated carbon[44]. The adsorption data (methylene blue) fitted well with Langmuir model and pseudo-second-order isotherm. The adsorption capacity increased (126.9 to149.3 mg/g) with increasing the temperature (303 K to 323 K). Mango seed was used to prepare activated carbon[45]. The BET surface area of carbon range from 6 to 33 m2/g depending on the concentration of sulfuric acid. Researcher concluded that better results in amount of fixed carbon, the total acidity and amount of oxygenated surface group when the carbons were prepared by using the highest concentration of activating agent. Coconut based activated carbon was synthesized under chemical activation[46]. The percentage of yield is 30% with the BET surface area of 464 m2/g. Adsorption of malachite green dye followed Freundlich model. Carboxylic acid, alcohol and phenolic groups could be observed in the activated carbon. The Cocos nucifera shell was employed to prepare acidfunctional adsorbent under chemical activation[47]. The obtained carbon has high content of carbon (51.9%), oxygen (44.63%), low content of hydrogen (2.97%) and nitrogen (0.37%). XRD studies revealed amorphous structure and consisted of crystallographic planes of carbonaceous material. The adsorption capacity of methylene blue dye was 50.6 mg/g at 303 K, pH=8, and adsorbent dosage=0.1g/100mL. Ficus caricabast based activated carbon was produced via chemical activation[48]. Tempkin model, pseudo-second order model and Langmuir isotherm fitted well with adsorption data. Thermodynamic studies such as enthalpy (21.55 kJ/mol), entropy (76.2 J/mol.K) and free energy (-1.55 kJ/mol) were reported. Activated carbon was prepared from Parthenium hysterophorus through chemical activation[49]. The adsorption kinetic supported Freundlich model and Langmuir isotherm. Experimental findings showed the maximum methylene blue dye was removed within 60-90 minutes. Removal of malachite green on activated carbon produced from Parthenium hysterophorus Linn biomass[50]. The adsorption data were represented well by Langmuir model and pseudo-second order model. Experimental results revealed higher removal of dye could be reached by using lower concentration of malachite green and higher adsorbent dose. Removal of nickel by using Parthenium hysterophorus based activated carbon[51] was explained. The highest removal of nickel ions was observed within 240 minutes at pH 5. The monolayer adsorption capacity (17.24 mg/g) of nickel ions was detected by using Langmuir model. Several functional groups such as O-H, C-H, C=O, C-O were found in the activated carbon. The polyethylene terephthalate based activated carbon was prepared by using chemical activation[52]. The best conditions were described such as activation time=30 minutes, activation temperature=600 °C, and impregnation ratio=37.63%. The percentage of yield and surface area were 12.57% and 537 m2/g, respectively. FESEM studies confirmed that no pores could be observed on the surface of raw material, however, well-developed pores could be found in sulfuric acid treated activated carbon. Synthesis of bean husk based activated carbon under chemical activation[53] was proposed. The highest iodine number of sulfuric acid treated carbon was 1027 mg/g, indicating large micro pore and meso pore structure. The ash, bulk density and pH values were 3.5%, 0.4 g/mL, and 6.7, respectively. Periwinkle shell based activated carbon was produced under chemical activation[54]. The physio-chemical properties such as bulk density (1.38 gm/L), moisture (1.14%), pore volume (0.138L), porosity (2,76%), ash content (3%), iodine number (0.123 mg/g) and surface area (7.695 m2/g) were reported. Oil palm empty fruit bunch based activated carbon was produced by chemical activation[55]. The specific surface area (4.34 m2/g to 1.91 m2/g) reduced with increasing the concentration of sulfuric acid (2M to 3.5M). Proximate analysis of the obtained carbon such as moisture (2.44%), volatile (15.23%), fixed carbon (67.6%) and ash content (9.58%) was reported. FTIR spectra confirmed the presence of hydroxyl, carbonyl, carboxyl, and aromatic structure on the surface of activated carbon.
Hydrochloric acid: The main purpose to add hydrochloric acid as activating agent during the synthesis of activated carbon can remove lignin to enable symmetric pore creation. Acidic treatment of activated carbon resulted transform crystalline cellulose to amorphous. The Calophyllum inophyllum seed was used to prepare activated carbon via activation process[56]. The percentage of yield was found in the range of 12% to 57.92%. FTIR studies revealed that alkene, aromatic and ether could be seen in all carbons. The best conductivity was 6.97X10-9 S/cm by using 37%HCl based on Electrochemical Impedance Spectroscopy. The waste tyre was used to prepare activated carbon under chemical activation[57]. This activating agent can remove zinc, sodium, calcium from the tyre char, resulted in lower ash content. The obtained carbon indicated mesoporous with surface area of 960 m2 /g. The Redlich –Peterson model supported the adsorption of phenol onto adsorbent. Activated carbon prepared by using rice hulls and bean hulls via chemical activation was reported[58]. The best conditions to hydrolyze lignocellulosic material were 2% hydrochloric acid, 120 °C, and 90 minutes. The cellulose crystallinity index was increased from 0.32 to 0.46 (with and without acid treatment) and 0.43 to 0.61 in rice hull and bean hull, respectively. Nigerian coconut shell based activated carbon was produced to remove heavy metal[59]. The bulk density (0.68 g/cm3), ash content (10.23 g/cm3), moisture content (2.45%), porosity (10.1 %), volatility (23.43%) and char yield (66.25%) were studied. It is noted that the highest percentage of metal ions (Cu2+, Mg2+, Ni2+, Fe2+, Al3+) adsorbed in these carbons, due to the HCl-treated carbons created more reactive sites.
Basic Activation Agent
Sodium hydroxide: The doumpalm shell was used as starting material to produce activated carbon[60]. The charcoal was impregnated with different agents such as KOH, sodium hydroxide (NaOH) and ZnCl2 under impregnation ratio of 1:3. The highest surface area (SBET=226 m2/g) and highest porosity (average pore volume=0.096 cm3/g) could be observed in NaOH-AC, if compared to KOH-AC (SBET= 5.4 m2/g; average pore volume=0.068 cm3/g) and ZnCl2-AC (SBET= 0.84 m2/g; average pore volume=0.008 cm3/g). The removal of cadmium ion and lead ion was attained after 1 hour with capacities of 93.34% (NaOH-AC), 92.31% (ZnCl2-AC), and 71.62% (KOHAC) respectively. The best conditions for the removal of cadmium ions (NaOH-AC) at pH 5.5, adsorbent dose=1.5g/50mL, and initial cadmium concentration=40 mg/l. The algae meal was used as raw material for the preparation of activated carbon under KOH chemical activation[61]. The properties of algae meal including absence of heavy metal, low ash content, high carbon and high nitrogen. Results revealed that conventional activated carbons have higher surface area (2118 m2/g), higher pore volume (1.14 cm3/g), and better textural if compared to microwave activated carbon. The distiller’s grains were employed[62] for the synthesis of activated carbon under various concentrations of KOH. The pore structure was the most developed and achieved the highest yield (24.48%) when the concentration of KOH reached 50% if compared to the concentration KOH more than 50% (microporous changed to large pores) and less than 40% (low degree of activation). The best activation temperature (750°C) and activation time (120 minutes) also reported. The seed pods of Dialium guineense (agro-derived waste) was used to produce activated carbon by using activation agent[63]. Higher percentage of yield could be observed by using 1M NaOH if compared to 5M NaOH when the activation time was 90 minutes. The adsorption capacities of methylene blue of 9.6 mg/g and 9.5 mg/g could be detected by using 5M NaOH than other concentrations (1M and 3M). The mesoporous activated carbon was prepared by using cassava peel under activating agent[64]. The percentage of yield reduced when the carbonization temperature was increased from 480°C (yield=42%) to 780°C (yield=30%). Khat (Catha edulis) stem was employed for the production of activated carbon under activating agent[65]. Proximate analysis of obtained activated carbon including moisture content (5.38%), ash content (6.7%), volatile matter (4.25%) and fixed carbon (83.65%) was investigated. Isotherm parameters were calculated and higher coefficient value could be seen in Freundlich (R2 =0.98596) than Langmuir model (R2 =0.98172). Sugar cane bagasse based activated carbon was produced via activation process[66]. When the impregnation ratio was 3:1, specific surface area and mesopore volume were 1980 m2/g and 0.73 ml/g, respectively. The ESR studies confirmed the absence of oxygen, but presence of carbon centered stable radicals. The wood (Acacia auriculeaformis) was employed for the preparation of activated carbon under activation process[67]. The iodine number values are 304.57 mg/g, 190.35 mg/g and 380.71 mg/g when the activation agent was phosphoric acid, sodium hydroxide and sodium chloride, respectively. The highest surface area (561 m2/g) and highest pore volume (0.26 cm3/g) could be seen by using phosphoric acid. The chemical analysis showed the presence of lacton in the sample due to the sodium hydroxide (basic impregnating agent). Rice husk based activated carbon was synthesized via activation process under different activation temperatures[68]. BET surface area (93.89 to 429.8 m2/g), and total pore volume (0.12 to 0.29 cm3/g) increased, but average pore size reduced (5.03 to 2.69 nm) with increasing activation temperature from 650°C to 850°C. SEM studies confirmed developed pores in activated carbon prepared by using NaOH activation. The almond shell and anthracite were used to produce activated carbon under various activating agents[69]. Results indicated KOH and NaOH could be used for activating ordered precursor (anthracite) and less ordered lignocellulosic material (almond shell), respectively. The hydroxide activation developed porosity of obtained carbon if compared to physical activation, higher BET surface area could be detected by using KOH (2420 m2/g) and NaOH (2541 m2/g). Rice husk was used to produce activated carbon in the presence of activating agent[70]. Raman studies revealed that crystalline sizes (basal plane) were 3.27 and 3.46 nm in KOH-treated and NaOH treated activated carbon, respectively. The cyclic voltammetry and charge–discharge studies showed that KOH-treated activated carbon displayed better performance at low scan, while NaOH treated activated carbon indicated excellent at high scan rate and current density.
Potassium hydroxide: The properties of bamboo based activated carbons (treated with KOH) were studied[71]. The iodine value increased (726 to 1294 mg/g) initially, then dropped. Because of the pore could be widened, then burnt off with the increasing impregnation ratio. When the synthesis of activated carbon was incomplete (chemical activation), caused poor adsorption process due to incomplete removal of volatiles, partial elimination of the acidic functional groups. Adsorption of Cr(VI) ions followed pseudo-second-order model and Langmuir isotherm. The petroleum coke based activated carbon was synthesized using KOH activation[72]. The BET surface area (1780-1875 m2/g), micropore surface area (465-660 m2/g), mesopore surface area (726-795 m2/g) and total pore volume of pores (0.96-1.02 cm3/g) were reported. TEM studies revealed mircopores for the activated carbon prepared using KOH under high temperature. The flax residue was used to produce activated carbon under activation process[73]. XRD studies showed the obtained carbons were disordered and amorphous material. XPS investigations revealed the presence of carbon and oxygen in activated carbon. Coconut shell based activated carbon was produced under chemical activation[74]. Iodine value (266 to 500 mg iodine/g carbon), ash content (8 to 30%) and water content (10 to 15%) were highlighted. The microwave-induced KOH activation[75] was employed to synthesis coconut based activated carbon. Properties of activated carbon including volatile matter (28.46%), fixed carbon (69.49%), and ash (2.05%) were described. The activated carbon produced at radiation time of 20 minutes indicated surface area of 1768 m2/g with pore size of 2.7 nm. Chemical activation process showed the highest specific capacitance at low current density (156.3 F/g in 10 M KOH). The palm kernel shell was used to prepare activated carbon through chemical activation[76]. Several weight losses could be seen in thermogravimetry analysis including 50 -130°C (elimination of moisture), 240 to 360°C (decomposition lignocellulose biomass), and 360 to 450°C (lignin decomposition). The FTIR studies revealed that raw palm kernel shell illustrated more absorption peaks if compared to KOH-treated activated carbon. When the concentration of KOH increased, weaker bonds disappeared by thermal degradation. Development of banana peel based activated carbon under chemical activation for carbon dioxide removal was reported[77]. The obtained carbons produced the highest total pore volume (0.016 cm3/g), surface area (260 m2/g), and pore diameter (0.25 nm). In the adsorption test, these materials can remove up to 1.65% wt of carbon dioxide at 25 °C. Banana peel based activated carbon was fabricated via chemical activation process[78]. Large amount of KOH is needed to increase the percentage of yield (31.1%), then maximize the adsorption of copper ions (99.6%). Sea mango based activated carbon was synthesized through chemical activation[79]. The obtained carbons exhibited surface area of 451.87 m2/g, with developed new pores on the surface of adsorbent. Removal of basic red 46 (0.1g adsorbent, natural pH, 50 mg/L) achieved equilibrium in 30 minutes. Baobab fruit shell[80] was utilized to prepare activated carbon under various activating agents (ZnCl2, H3PO4, KOH). The H3PO4 - treated carbon showed the highest yield (34.396%), ash (17.7%), moisture (5.17%) and iodine number value (1248.35mg/g) if compared to other activating agents. SEM studies indicated porous (42.24 μm) structures in the KOHtreated carbons are greater than other agents, are very suitable to absorb methylene blue. Adsorption data supported Langmuir model (KOH-treated) and Freundlich model (H3PO4 - treated). The duckweeds plants were used to produce activated carbon through chemical activation[81]. The porosities are 22% and 38% in KOH-treated carbon and H3PO4 -treated carbon, respectively. Generally, KOH-treated carbon required 50-150 minutes to reach equilibrium adsorption (methylene blue). Based treatment of activated carbon caused the breakage of longer fibers and exfoliation[82]. The use of KOH resulted in improved porosity due to potassium interaction and the stretching of carbon layers[83]. KOH is more effective to create micro pores when the temperature above 800°C. When the temperature in the range of 230 to 650°C, the use of KOH can produce high char yield. The oxidation of biomass produces oxygen containing surface functional group may not be developed in this conditions[84].
Neutral Activation Agent
Potassium carbonate: Characterization of palm shell based activated carbon was highlighted[85]. Adsorption of carbon dioxide gas increased, but the percentage of yield reduced, with increasing the impregnation ratio and carbonization temperature. The highest surface area (1170 m2/g) was obtained when the activation duration=120 minutes, impregnation ratio=1, and carbonization temperature= 800 °C. On the other hand, the synthesis of large surface area (2000 m2 /g) and the highest total pore volume (1 cm3/g) corn cob based activated carbon was reported[86]. Experimental results confirmed that pore volume development strongly depended on the soaking time. Microwave heating technique was used to produce high surface area (1165 m2/g) rice husk based activated carbon[87]. The K2CO3 treated activated carbon indicated higher percentage of yield and better pore structure if compared to KOH treated activated carbon. Total pore volume (0.78 cm3/g) and monolayer adsorption capacity (441.52 mg/g) of methylene blue in K2CO3 treated activated carbon were reported. Researcher concluded that the activating agent showed excellent capacity in the microwave activation. Generally, biomass materials heat rapidly with higher thermal penetration as impregnated with chemical agents because of poor dielectric properties. Orange peel was used to produce activated carbon via chemical activation[88]. The BET surface area (1104.45 m2/g), total pore volume (0.615 m3/g), percentage of yield (80.99%) and monolayer adsorption capacity (382.75 mg/g) of methylene blue dye were pointed out. Sunflower seed oil was used to prepare activated carbon through microwave induced chemical activation[89]. The monolayer adsorption capacity of methylene blue (473.44 mg/g) and acid blue 15 (430.37 mg/g) were highlighted. Research findings revealed the BET surface area (1411.55 m2/g) and total pore volume (0.836 cm3/g) also. Mesoporous wood sawdust based activated carbon was produced via microwave heating[90]. The BET surface area (1496 m2/g), total pore volume (0.864 cm3/g), monolayer adsorption capacity of methylene blue (423.17 mg/g) and the percentage of yield (80.75%) were reported. The grape seed based activated carbon was mainly microporous[91]. The highest surface area (1238 m2/g) could be observed at 800°C, concentration of K2CO3 of 50 wt %. The percentage of yield reduced when the temperature and concentration of activating agent were increased. The microporous structure of bamboo based activated carbon[92] was produced by combined activation of H3PO4 and K2CO3. The highest surface area (2237 m2/g) and total pore volume (1.23 cm3/g) could be observed when the impregnation ratio =3:1 and temperature=750 °C. The adsorption of ciprofloxacin fitted well with Langmuir model and pseudo-second order model. Synthesis of cotton stalk based activated carbon by using microwave radiation[93] under chemical activation (to develop micro porosity and meso porosity structure)was proposed. The advantage of microwave heating technique could shorten the processing time if compare to conventional heating method. The adsorption data (methylene blue) followed the Langmuir isotherm. Eupatorium adenophorum was used to produce activated carbon via microwave heating method[94]. The activated carbon exhibited high surface area (2768 m2/g), high yield (32.88%), high iodine number (1696 mg/g), and high total volume (1.149 ml/g). SEM studies confirmed that activation process can remove surface impurities, which contribute to the pore formation. FTIR investigations revealed that several groups (OH stretching vibration, CH symmetric and asymmetric vibration, C=C symmetrical stretching) could be observed in the obtained carbon. The activated carbon with surface area of 1038 m2/g and pore volume of 0.49 cm3/g was produced by using sisal waste[95]. Adsorption kinetic (ibuprofen and paracetamol) fitted well with pseudo-second order model. Date stone was used to produce activated carbon through microwave technique[96]. Methylene blue uptake of 460 mg/g and the percentage of yield of 19.99% were highlighted at radiation power=660W, radiation time=8 minutes, and impregnation ratio=1.5 g/g. Total pore volume (0.656 m3/g) and surface area (1144.25 m2/g) were reported also. Langmuir model and pseudo-second order model showed well representation for adsorption data. The best conditions for the production of fungi residue based activated carbon[97] using microwave technique at radiation time=16 minutes, microwave power=520W, and K2CO3/edible fungi residue=0.8. The percentage of yield (23%), iodine number (732.74 mg/g) and total amount of methylene blue dye adsorption (172.43 mg/g) were reported. Langmuir isotherm supported adsorption process with monolayer adsorption capacity (19.6 mg/g) and the equilibrium adsorption constant (0.39 L/mg).
Zinc chloride: The zinc chloride is dehydrating agent, caused hydrolysis process[98] because of intermolecular exchange and molecular migration[99]. The chemical activation of rice husk char was performed by using zinc chloride[100]. Removal of methylene blue (149 – 264 mg/g) and the specific surface area (365 to 645 m2/g) increased when the temperature was increased from 600 to 800°C. SEM studies confirmed well developed micro pore structure. Zinc chloride was used as activating agent during the preparation of dried holdfast based activated carbon[101]. In the TG-DTA studies, decomposition of organic substance (alginic acid, cellulose and fucoidan) occurred after the evaporation of water at 100°C. The highest surface area achieved (1000 m2/g) when the zinc chloride more than 50% mass. The mechanisms of zinc chloride activation were discussed. Preparation of activated carbon through chemical activation enhanced the carbon content, and formed aromatic graphitic structure[102]. In the safflower seed press cake based activated carbon, the carbon content increased (49.5% to 76.3%) after carbonization and chemical activation process[103]. On the other hand, researchers found that percentage of yield reduced when high amount of zinc chloride was used because of removal of volatile matters via the collapsed of aromatic bond and aliphatic bonds (caused the loss in weight). Removal of methylene blue onto cashew nut based activated carbon was described[104]. The carbonization temperature of 500°C and impregnation ratio at 1.5:1 (zinc chloride/C) indicated the highest adsorption capacity for methylene blue (476 mg/g). The development of porosity strongly depended onto activation temperature and impregnation ratio. The new pores collapsed and became bigger pores at higher activation temperature. Experimental findings confirmed that the dye is attracted to the carbon surface via dipole-dipole interactions (nitrogen in methylene blue and phenolic of activated carbon). Palm oil mill waste based activated carbon was impregnated with zinc chloride, followed by carbonization process[105]. The obtained activated carbon showed specific surface area of 1058 m2/g, micro pore surface area of 721 m2/g, reached the highest methylene blue adsorption capacity (225.3 dye/g adsorbent). The pineapple waste was used to produce activated carbon through chemical activation process[106]. The adsorption data supported Langmuir model with correlation =0.969. The best impregnation ratio is 1:1, achieved the highest methylene blue removal capacity (288 mg/g). On the other hand, high surface area (2869 m2/g), mesopore volume (0.28 cm3/g) and micropore volume (1.69 cm3/g) could be observed for the Fox nutshell based activated carbon[107] using chemical activation (impregnation ratio is 2). Removal of methylene blue (249.88 to 968.7 mg/g) and phenol (19.84 to 75.37 mg/g) increased with increasing the initial concentration from 100 mg/L to 500 mg/L. Water melon rind was used to produce activated carbon under chemical activation[108]. The highest meso pore volume (1.4 cm3/g) could be observed in the adsorbent prepared with impregnation ratio of 3:1, carbonization temperature=600°C, and residence time =60 minutes. The maximum BET surface area was 1156 m2/g in the specific conditions such as impregnation ratio=2, carbonization temperature=700 °C, and residence time=1 hour. The chemical activation of watermelon peel based activated carbon was reported, then carbonized under different temperatures[109]. The percentage of yield (50 to 33%), and volatile content (34% to 20.4%) reduced, but ash content (18.6 to 28.6%) increased when the temperature was increased from 200 to 350°C. The activated carbon treated with 0.5M zinc chloride revealed the best removal of zinc (2.3%), copper (50.6%), iron (88%) and lead (100%). Characterization of grape stalk based activated carbon was reported[110]. BET surface area (1411 m2/g), iodine number (1760 mg/g), total pore volume (0.723 cm3/g) and the percentage of yield (26.48%) were highlighted under specific conditions such as carbonization time=2 hours, carbonization temperature=700°C, impregnation time=36 hours, and ZnCl2/grape stalk ratio (2:1). Sawdust was used to produce activated carbon[111]. The highest adsorption of iodine and methylene blue dye could be reached when impregnation ratio of 100% zinc chloride/sawdust, activation temperature=500°C, and carbonization time=60-90 minutes. Overall, researchers concluded that precursor (contained lignocellulose material) treated with ZnCl2 formed carbons showed excellent percentage of yield and well-developed porosity. The main disadvantage of this activating agent was toxic to aquatic organisms, further caused long-term adverse effects to the aquatic environment[112].
Ferric chloride: The ferric chloride could be used as activation agent because it has many advantages including lower cost and more environmentally friendly. The cotton textiles were used to produce activated carbon via activation process[113]. The acidic surface groups could be detected by using FeCl3. The highest removal of chromium ions was 267.12 mg/g, obeyed Langmuir model and pseudo-second order isotherm. The cotton woven was employed for the preparation of activated carbon[114]. FeCl3 as activating agent could lower initial decomposition temperature to 135°C, further catalyzed decarboxylation and decarbonylation process, to form the microporous structures. Experimental results confirmed that longer activation time caused an increase the number of active sites in the obtained carbons. The cotton waste based activated carbon[115] showed higher BET surface area (504 m2/g) and could be used to remove methylene blue and eriochrome black T. The best experimental conditions were activation temperature=400°C, and activation time=60 minutes. Research findings showed the positively charged methylene blue could be easily attracted to the surface of FeCl3-derived activated carbon (the pHPZC lower than or around 5). The microporous date pit based activated carbon has been used to remove p-nitrophenol[116]. The iodine number (761.4 mg/g), mesopores volume (0.105 cm3/g), micro pores volume (0.468 cm3/g) and surface area (780 m2/g) were reported. The best adsorption capacity of p-nitrophenol of 184.86 mg/g, was well described by the Sips isotherm. The adsorption kinetic obeyed the pseudo-second-order model, however, intraparticle diffusion could not be considered as the rate limiting step. Date stones based activated carbon was produced via chemical activation[117]. The percentage of yield (47.08%) and methylene blue uptake (185.15 mg/g) were observed at activation time=1.25 hours, activation temperature=700 °C, and impregnation ratio (1.5). The surface area (780.06 m2/g) and iodine number (761.4 mg/g) were reported. The adsorption data supported Langmuir model. Tara gum based activated carbon was synthesized[118] (impregnation ratio of 2, temperature of 800°C), showed pore volume of 1 cm3/g, BET surface area of 1680 m2/g, the most developed micro pores structures (75%). Adsorption of antipyrine obeyed the Langmuir model. The free energy (-40.2 to -35.7 kJ/mol), enthalpy (-3 kJ/mol) and entropy (112 J/mol.K) were highlighted. The Lapsi seed stone was used to produce activated carbon via chemical activation process[119]. The obtained carbons have large adsorption capacity. The SEM studies revealed that FeCl3 - treated activated carbon showed lower adsorption capacity for methylene blue if compared to KOH-treated carbons. In other words, porosity development was still very poor if compared to basic activating agent. The coffee grounds were employed for the production of activated carbon[120]. Experimental results indicated that higher surface area could be obtained in FeCl3 - treated carbon (846 m2/g) than treatment with MgCl2 (123 m2/g). The electrochemical double layer capacitances of FeCl3 - treated carbon showed specific cell capacitance of 57 F/g. FeCl3 can boost the intermolecular interactions between activating agent and precursor (biomass). The most developed porosity could be observed in specific impregnation ratio (1.0 up to 4.0) and activation temperatures (700 to 900°C). The use of very high temperature caused lower porosity, an increase in the ash content.
Calcium chloride and magnesium chloride: The choice of the activating agent for the removal of anion is very important step to ensure better rates of removal. The chemical modification of the activated carbon by using CaCl2, caused the surface became positively charged and more prone to the adsorption process (reached efficiencies of more than 60%).
The Elaeis guineensis was used to produce granular type activated carbon[121] via chemical activation with MgCl2 and CaCl2. Surface area (10 to 501 m2/g) and pore volume (0.01 to 0.29 cm3/g) were reported. Enthalpies of immersion values were -7.4 and -42.7 J/g, -13.9 and -38.6 J/g, and -6.4 and -24.2 J/g, for water, benzene and cyclohexane, respectively. Removal of n-butylparaben from African palm shell based activated carbon[122] via chemical activation with CaCl2 and MgCl2. The pore volume (0.25 to 0.52 cm3/g) and surface area (608 to 1320 m2/g) were highlighted in these carbons. The adsorption of n-butylparaben varies between 0.74 to 1.38 mmol/g. Treatment with magnesium chloride and calcium chloride for the Elaeis guineensis based activated carbon showed mesoporous structures (entry of the adsorbate into the adsorbent)[123]. Higher surface area and higher pore volume could be obtained with reducing the concentration of activating agents. The pH at the point of zero charge (4.08 to 9.92), total acidity (23 and 262 μmol/g) and total basicity (123 and 1724 μmol/g) were reported.
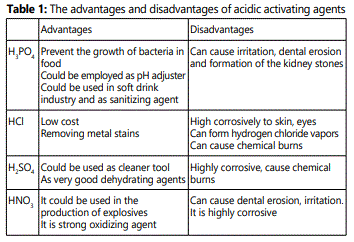
In this work, preparation of activated carbon via traditional activating agents such as strong base (NaOH, KOH), strong acids (HCl, HNO3, H2 SO4) and weak acid (H3PO4). These activating agents are highly corrosive chemicals. The handling of these chemicals needs stricter security measures, and increased the cost of the synthesis process. The advantages and disadvantages of acidic activating agents were highlighted in Table 1.
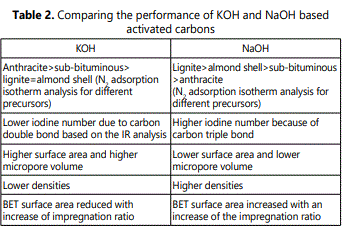
The sodium hydroxide and potassium hydroxide are very corrosive bases. The global production of sodium hydroxide higher than potassium hydroxide because of its lower cost[124]. Activated carbon produced by using these activating agents showed very low ash content, narrow porosity distribution and excellent adsorption capacity[125]. These alkaline activators promote the oxidation process, the obtained samples exhibited type II carbons. Table 2 showed the comparison of the performance of KOH and NaOH based activated carbons. Several products such as alkaline carbonate, hydrogen gas and alkaline metal were formed126 via hydroxide activation of carbon process as indicated in equation 1.
6MOH+2C→ 3H2+2M+2M2CO3,(where M:Na,K)
The major issue was the laboratory scale without any pilot study. In other words, more research should be carried out by using real textile wastewater. Also, multipollutant systems should be investigated to meet the needs of wastewater treatment. In future, other activating agents will be studied to observe the properties of activated carbon. The magnetic activated carbon will be investigated to remove dye, heavy metal, pesticide and other pollutants in wastewater.
Conclusion
In this review, various precursors were used to produce activated carbon under chemical activation process. Several types of activating agents such as acidic, neutral and basic activation were reportedhere. The activation conditions, impregnation ratio, impact on the surface area and the porosity structure were discussed. Characterization was done by using different tools and the adsorption mechanisms of activated carbon were studied via various isotherms.
Acknowledgement
The authors gratefully acknowledge the financial support provided by the INTI International University.
References


