Review Article
Organic and Organic-Inorganic Solar Cells: From Bulk Heterojunction to Perovskite Solar Cells
1Faculty of Sciences, Industrial University of Santander, Bucaramanga, Colombia
2Department of Chemistry, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
*Corresponding author: Carlos A Echeverry, Postdoctoral Associate, University of Santander, Bucaramanga, Colombia E-mail: caechego@uis.edu.co
Edison Castro, Postdoctoral Associate, University of Pittsburgh, Pittsburgh, Pennsylvania, USA, E-mail: eac106@pitt.edu
Received: October 1, 2018 Accepted: October 22, 2018 Published: November 27, 2018
Citation: Echeverry CA, Castro E. Organic and Organic-Inorganic Solar Cells: From Bulk Heterojunction to Perovskite Solar Cells. Int J Chem Res. 2018; 1(1): 1-8. doi: 10.18689/ijcr-1000101
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Due to the negative impact on the environmental using fossil fuels, photovoltaic (PV) technology has become a new alternative for renewable energy in the last two decades. Although inorganic solar cells have dominated in the PV field, metal-free organic compounds have introduced a new challenge because of their great properties that include: i) high light-harvesting ranges, ii) optical- and electrochemical properties can be easily tuned by decorating their structure through a several synthetic approaches, and iii) their technology has high flexibility and low manufacturing cost. In this short review, we depicted our work in the synthesis of organic compounds and their applications in the PV field, specifically in bulk heterojunction solar cells (BHJSCs), dye-sensitized solar cells (DSSCs) and organic-inorganic perovskites solar cells (O-IPSCs).
Keywords: Fossil fuels; Photo excitation; Greenhouse gases; Photovoltaic.
Introduction
Global warming, as a direct consequence of the emission of greenhouse gases, is one of the greatest threats to the health of humans and ecosystems in our planet, being one of the most important difficulties to overcome by the scientific community. Looking for new alternatives (environmentally friendly, price-effective and efficient) researchers are devoting great attention into the PV technology. The sun continuously releases a tremendous amount of energy in the form of electromagnetic radiation and this almost limitless source of energy is available at no cost [1]. PV solar cells (PVSCs) [2] can convert sunlight into storable energy. One may think that this is a new technology, but it has been there for almost 2 centuries. The PV effect was first observed by Becquerel AE in 1839 [3]. PV technology is based on the creation of an electron-hole pair upon photon absorption in cells composed of two different layers (p-type and n-type) of semiconductor materials. In these cells, absorption of a photon by the n-type material provides enough energy to move an electron from one layer to the other, leading to the generation of electrical power [4].
Currently, inorganic solar cells based on materials such as silicon, cadmium telluride, or copper indium germanium selenide exhibit relatively high solar energy conversion efficiencies above 20%, and dominate commercially available PV technologies [2,5,6]. However, inorganic solar cells present some limitations such as rigidity, weight and high manufacturing costs compared to those based on organic-inorganic materials, so many alternative materials are under investigation. In this regard, organic and hybrid inorganic-organic solar cells (OSCs and O-ISCs) are promising sources of renewable energy.
This short review will focus on the application of different synthetic materials developed by our groups and their applications in PV devices, specifically in the field of BHJSCs, DSSCs and perovskite solar cells (PSCs). We will survey the relevant aspects on energy requirements, design, synthesis, electrochemical and optoelectronic characterization of the new materials for each application.
Organic Photovoltaics (OPVs)
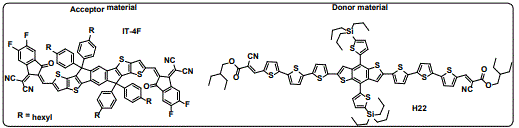
Currently, OPVs are based on two types of electron donor materials: polymers and small molecules [7,8]. Organic solar cells based on BHJ configurations have attracted considerable interest due to their easy fabrication, relatively low cost and flexibility [9-11]. In BHJ configurations, electron-donating conjugated compounds and electron-accepting (fullerene or non-fullerene) derivatives are mixed together to form a continuous interpenetrating micro-heterogeneous network with a large interfacial area for efficient exciton dissociation [12]. Over the last few years, a significant increase in the power conversion efficiencies (PCEs), exceeding 13% [13,14], has been achieved by devices based on polymer donors. On the other hand, small molecule donor based OPVs (SM-OPVs) have achieved PCEs above10% [15]. Small-molecule donors offer several advantages over polymeric materials, such as easy purification, well-defined molar masses and molecular structures, high solubility and good batch-to-batch reproducibility [8]. Comprehensive reviews based on small molecules for BHJSCs have been reported by Hou [16] and Mishra [8]. The chemical structures of the compounds that have been used to achieve the highest PCE values are shown in figure 1.
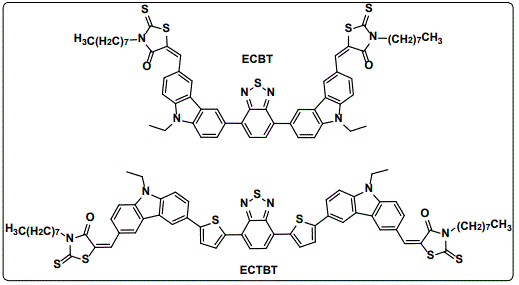
We have contributed in this type of solar cells by designing and synthesizing new small molecules, which were used as the electron donor materials in BHJSCs. Our strategy was to use donor-acceptor-donor (D-A-D) backbone structures ECBT and ECTBT [17] (Figure 2).
Dye-Sensitized Solar Cells
The concept of treating semiconductors with dyes was introduced in late 1960s mainly due to their spectra responses, which were mostly in the ultraviolet region resulting in low conversion efficiencies [18-21]. Although, these cells appeared as promising renewable energy resource, it was not considered until 1991 when O’regan B and co-workers reported the first efficient DSSC device that was fabricated by using a ruthenium-based dye anchored to the nanoporous TiO2 surface, which showed a high PCE ranging from 7.1 to 7.9% [22]. Hereafter, the initial synthesis of new dyes for DSSC applications were based on ruthenium with different ligands (i.e. N3, N719 and C101 dyes, see figure 3), followed by the use of metal-free dyes [23-25]. The record efficiency for DSSC of 14.3% was reported in 2015 using organ silicon dye and a co-sensitizer based on triphenylamine with carboxylic units as anchor group [26].
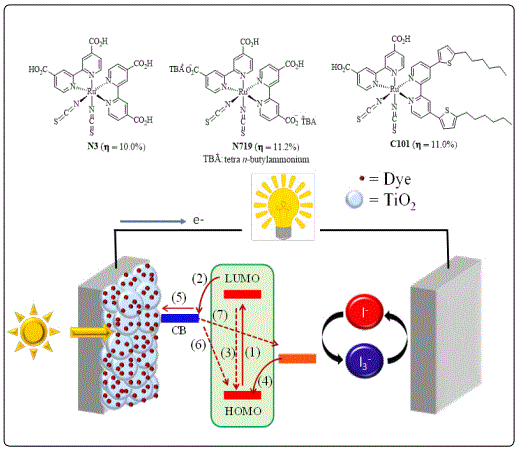
In a typical DSSC device, light is absorbed by the dye molecules, which are anchored to the TiO2 exciting electrons from the higher occupied molecular orbital (HOMO) to lower unoccupied molecular orbital (LUMO) (see figure 3, step 1). The photo excitation of the dye molecules results in an excited singlet state called exciton in which the electrons are linked to their corresponding holes by electrostatic Coulomb force, [27]. The electrons from the excited state of the dye are then injected into the conduction band of the TiO2 (E=~-3.90 eV vs vacuum) (step 2), this process competes kinetically with the state excited decay, which has a negative impact on the overall conversion efficiencies (step 3). The dye is restored to its ground state by an oxidation-reduction process with the electrolyte redox couple (step 4), which is also reduced by electron transfer form platinum counter electrode (step 5). The process of regeneration of the dye is competing with the electronic recombination between the semiconductor and the dye (step 6) as well as between the semiconductor and the redox couple system (step 7) [28].
The organic dyes that are one of the most important components of these cells, should meet certain requirements for an efficient electron transfer to the conduction band of the TiO2 and hence result in efficient power conversion. These requirements are namely: i) dye should be a stable electron donor compound with a broad absorption spectrum absorbing light in the visible range; ii) the oxidation potential of the donor moiety should be lower than the I-/I3 redox couple, ensuring an efficient dye regeneration as well as a stabilized charge-separated state; iii) there must be a strong interaction between TiO2 and the LUMO level localized near the anchoring group of the dye, ensuring good electronic coupling [29,30].
The interest on metal-free organic sensitizers has grown in recent years, as they offer several advantages over other sensitizers. Among these advantages are the higher molar absorption coefficients due to intramolecular π-π* transitions, easy modification due to relatively short synthetic routes and tunable electrochemical properties [31]. Different organic materials have showed great properties for DSSC applications, and taking a look at their structures, generally they are based on a donor moiety linked covalently to an acceptor moiety [32-34]. Several synthetic strategies have been studied to improve the optoelectronic properties of organic dyes, which can be summarized in: i) the use of bulky co-donor systems to block the approach of the electrolyte redox couple to the semiconductor surface avoiding the electronic recombinant processes [35]; ii) the development of novel anchoring units with better optical- and electrochemical properties as well as higher stability in comparison with the conventional carboxylic-based acceptor [36,37] and iii) the improvement of the light harvesting in the red region of the visible spectrum through the π-conjugation increase [38].
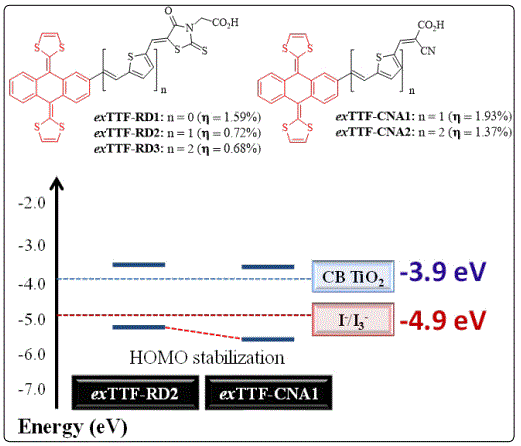
Following these strategies, we studied the changes in the optical and electrochemical properties upon the incorporation of different highly conjugated donor and co-donor systems linked to anchoring units’ as cyanoacrylic acid, rhodanine-3-acetic acid and acceptor systems without carboxylic groups with strong absorption in the visible range. Among the highly conjugated donor, ex TTF is one of the most studied systems due to its wide absorption spectrum, low oxidation potential and easy functionalization [39]. In 2014, we reported the synthesis of exTTF-based dyes endowed with a rhodanine 3-acetic acid unit as the acceptors and their further DSSC application, finding that the driving force for dye regeneration was negatively affected, because the difference between the first oxidation potential (HOMO) of the dyes and the I-/I3 redox couple was very small, resulting in a low PCE ranging from 0.68-1.59% [40]. The replacement of the anchoring unit based on rhodanine 3-acetic acid by cyanoacrylic acid resulted in a HOMO stabilization, which increased the driving force for dye regeneration (see figure 4) [41].
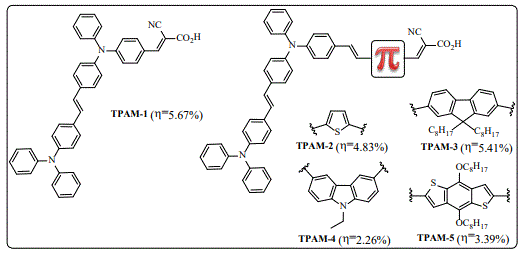
Considering the low oxidation potentials of the exTTF-based dyes that led to low conversion efficiencies in a typical DSSC device, even though such dyes showed a great absorption property in the visible range, the design of new efficient donors with co-donor systems for increasing molar absorptivity coefficients in the visible range was necessary. In 2015, we reported organic dyes based on two units of triphenylamine (TPA) as co-donor-donor (co-DD) with different π-spacers linked to cyanoacrylic acid as anchoring unit to the TiO2 (Figure 5) [42]. The devices fabricated with theses dyes showed good PCE values ranging from 2.26-5.67% whereas the ruthenium-(N719) based devices displayed a PCE value of 6.31%.
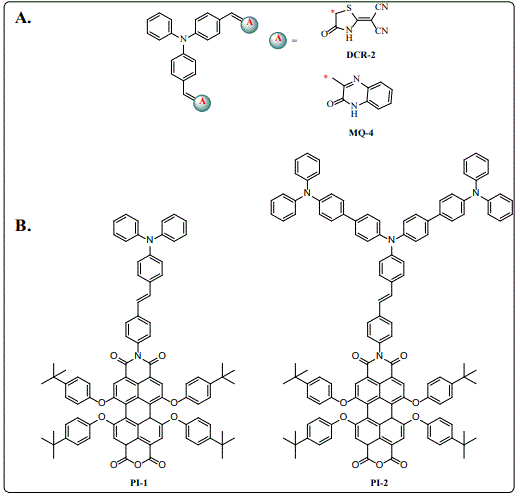
Different electron acceptors using a carboxylic acid (COOH) anchoring groups for binding to the TiO2 have been reported [43-46]. However, it was demonstrated that COOH dissociates on the TiO2 surface after long irradiation times, increasing the liability of these molecules [47]. Because of this, it is important the development of novel anchoring units that are able to bind strongly to the TiO2 [48-50]. Insuasty et al. [36] Reported the synthesis of push-pull systems covalently attached to the 2-(1,1-dicyanomethylene) rhodanine (DCRD) that can also work as an efficient anchoring unit to the TiO2 without the presence of the COOH group [37]. Echeverry et al. [51] developed triphenylamine-based dyes (DCR 1-5) using DCRD as efficient anchoring unit, which showed moderate conversion efficiency values. Among these dyes, a device made with DCR-2 (Figure 6a) exhibited the best PV performance with a short-circuit photocurrent density (Jsc) value of 7.76 mA/cm2, an open-circuit voltage (Voc) value of 0.621 V, and a fill factor (FF) of 0.682, corresponding to an overall PCE value of 3.78%. We [52] also reported the synthesis of different triphenylamine-based dyes linked to 3-methylquinoxaline-2(1H)one (MQ) as anchoring unit carboxylic acid-free, however the poor electron-acceptor character of MQ was reflected with low photoconversion efficiencies (Figure 6a).
Although, several organic dyes with promising results for DSSCs have been studied, there is still a need to design novel compounds with high light-harvesting abilities in the visible spectrum to improve the current densities of the devices [28]. The use of perylenetetracarboxylic dianhydride in optoelectronic devices has increased in the last decades [53], because of their strong absorption in the visible region with high molar extinction coefficients (ε=30,000-90,000 l mol-1 cm-1), high photo-stability and electron-drawing ability [54,55]. Echeverry et al. [56] reported the synthesis, structural and electronic properties of two novel dyes based on perylene imides PI-1 and PI-2 as well as their application in DSSCs (Figure 6b). DSSC devices based on PI-1 and PI-2 showed low conversion efficiencies of 1.0 and 1.3%, respectively. These low PCE values were associated with the weak driving force for the electron injection from the dyes to the conduction band of the TiO2. Although, the use of perylenebased dyes led to low efficiencies, these values were higher than 1.0%, very similar to other PCEs perylene-based DDSCs [57].
DSSCs continue being a promising energy resource for the future, due to their high PCE, low manufacturing cost and eco-friendly nature. Currently, the synthesis of new organic dyes and the manipulation of either photo anode or counter electrode continue being a hot topic, improving the efficiencies of these devices. We have used different strategies for improving the device PCEs, being the introduction of co-DD systems and DCRD as anchoring group the better ones.
Organic-Inorganic Hybrid Perovskite Solar Cells
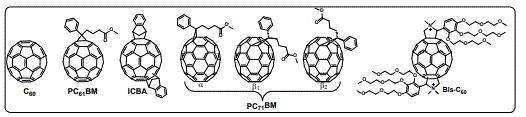
The unique properties of O-IPSCs make them promising candidates for developing next-generation PVs that would compete with silicon solar cells [58,59]. O-IPSCs have shown a remarkable PCE increase from 3.8% in 2009 to 23.3% in 2018 [60,61]. Among the different perovskite configurations [62,63], the inverted planar structure with a configuration: substrate transparent electrode (ITO or FTO)/hole transporting layer (HTL)/perovskite/electron transporting layer (ETL)/metal electrode has gained great attention due to its simple structure, low temperature processing and negligible hysteric behavior [64]. The most used ETL in this inverted configurations is the [6,6]-phenyl-C61 butyric acid methyl ester (PC61BM), besides PC61BM, other studies have shown that single or double fullerene layers (PC61BM/C60, ICBA/C60, C60/bis-C60), can also work as ETLs in O-IPSCs (Figure 7) [65-68]. Comprehensive reviews about fullerenes used in O-IPSCs has been reported by Castro et al. [63] and Gatti et al. [69]. However, the influence of the functional groups on the fullerene-based O-IPSCs is still being under investigation.
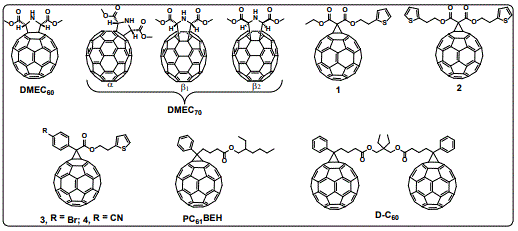
In a first attempt to understand the role of the fullerenes in O-IPSCs, we reported the synthesis, characterization and PV applications of DMEC60 and DMEC70 (Figure 8). It was found that inverted O-IPSCs based on DMEC60/70 as ETLs exhibited both higher PCE values and higher device-stability compared to devices based on the PC61/71BM, which was attributed to the efficient ability of these compounds to extract electrons from the perovskite layer, most-likely due to some specific interactions between the fullerenes’ addends (carbonyls and amino groups) and the perovskite crystals at the interfaces, as established by infrared (IR) spectroscopy [70,71].
A great effort to improve the Voc values has been devoted [72-74] and typically, fullerenes with lower LUMO energy exhibit higher Voc values. It has also been found that the energy disorder in the ETL influences the Voc values [75]. Taking this into consideration we investigated the effect of the C70 fullerene derivatives isomeric purity when used as ETLs in O-IPSCs (α-DMEC70, Figure 8) [71]. We found a considerable impact on the Voc values when using a pure isomer (α-DMEC70), and this remarkable effect can even reverse the expected results based solely on HOMO/LUMO energy level considerations [71,76].
Recently, we systematically studied the effect of new C60 fullerene derivatives functionalized with thiophene moieties as well as with electron donating or electron withdrawing groups bromine (Br) or cyano (CN) (1-4, respectively, figure 8), when used as the ETLs in O-IPSCs [77]. The photo conversion efficiencies of O-IPSCs based on these compounds were higher than those of devices based on PC61BM, which was associated with the better passivation ability, due to specific interactions between S...Pb atoms [77]. Important facts when synthesizing new ETLs are solubility and electron mobility, which need to be balanced. Unfortunately, in most cases solubility is accompanied by lower electron mobilities [78,79]. Keeping in mind these considerations, we synthesized PC61BEH (Figure 8) to be used as ETL in O-IPSCs, by changing the methyl group of the PC61BM for the 2-ethylhexyl group of PC61BEH, it was found that the solubility of PC61BEH in chlorobenzene was increased considerably (a factor of 3) without sacrificing the electron mobility nor the HOMO/LUMO energy levels, all in all, resulting in improved overall device performance [80].
For this technology be commercialized, the improvement of the long-term device stability needs to be addressed, one approach to overcome this issue is using hydrophobic compounds, which minimize the penetration of water into the perovskite layer in inverted O-IPSCs. In 2017, we reported for the first time, the use of a PC61BM-dimer (D-C60, figure 8), as an efficient ETL in inverted O-IPSCs, our idea was the use of a hydrophobic ETM to enhance the power endurance of the devices [81]. We not only improved the PCE value, but also the device stability of D-C60- based devices was substantially improved, as compared to devices based on PC61BM. This improved performance was attributed to synergetic effective perovskite film surface passivation and improved electron extraction, as determined by photoluminescence (PL) techniques and electrochemical impedance (EIS) [81].
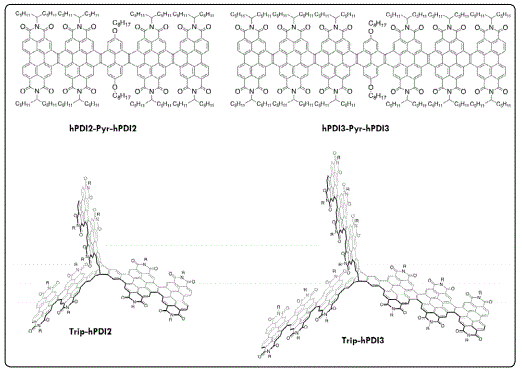
There are many hole transporting materials (HTMs) amenable for O-IPSCs fabrication [82]. However, the use of electron transporting materials (ETMs) has been limited mainly to fullerenes and their derivatives, and there was only few examples of efficient non-fullerene inverted O-IPSCs reported [83-86]. To further enhance O-IPSCs’ performance, we studied the effect of two atomically-precise edgefunctionalized graphene nanoribbons hPDI2-Pyr-hPDI2 and hPDI3-Pyr-hPDI3 (Figure 9), which outperformed PC61BM when used as ETLs in O-IPSCs. These new materials provided a facile fabrication process, high electron mobility, strong light absorption, and HOMO/LUMO levels that matched those of the lead-halide perovskite active layers [87-89].
We also studied the effect of three-dimensional graphene nanostructures (Trip-hPDI2 and Trip-hPDI3, (Figure 9) as the ETLs in O-IPSCs, which exhibited remarkable new properties, such as an enhancement in absorption, an ability to accept and delocalize 18 electrons into three-dimensions [90]. Devices based on largest of these compounds achieved a PCE value of 18.01%, which is one of the highest values for non-fullerene electron extracting layers in O-IPSCs [90].
Perspectives
Although a tremendous progress has been made toward increasing PCE values and device stability of OSCs and O-ISCs, there are still many barriers to further overcome to compete with the inorganic-based solar cells that currently lead the market.
To address one these barriers OSCs and O-ISCs are being studied under drastic conditions such as long-time lightsoaking conditions in the presence of air, and several research studies have been devoted to their stability under atmospheric conditions. To minimize fabrication costs, more amenable inexpensive organic materials are being studied to be used either as the electron acceptors, electron donors and ETMs or HTMs. This has called the attention of a large research community to design new efficient materials.
Conflict of Interest
There are no conflicts to declare.
Acknowledgments
The authors gratefully acknowledge financial support from COLCIENCIAS and the Universidad del Valle.
References
- Choubey PC, Oudhia A, Dewangan R. A review: Solar cell current scenario and future trends. Recent Res Sci Technol. 2012; 4(8): 99-101.
- McEvoy A, Castaner L, Markvart T. Solar Cells: Materials, Manufacture and Operation. 2nd edition. Oxford: Elsevier Ltd; 2012.
- Becquerel AE. Mémoire sur les effets électriques produits sous l’influence des rayons solaires. Comptes Rendus de l’Academie des Sciences. 1839; 9: 561-567.
- Srinivas B, Balaji S, Nagendra Babu M, Reddy YS. Review on Present and Advance Materials for Solar Cells. International Journal of Engineering Research-Online. 2015; 3: 178-182.
- Miles RW, Zoppi G, Forbes I. Inorganic photovoltaic cells. Mater Today. 2007; 10(11): 20-27. doi: 10.1016/S1369-7021(07)70275-4
- Bagher AM, Vahid MM, Mohsen M. Types of Solar Cells and Application. American Journal of Optics and Photonics. 2015; 3(5): 94-113. doi: 10.11648/j.ajop.20150305.17
- Thompson BC, Fréchet JMJ. Polymer–Fullerene Composite Solar Cells. Angew Chem Int Ed. 2008; 47(1): 58-77. doi: 10.1002/anie.200702506
- Mishra A, Bäuerle P. Small Molecule Organic Semiconductors on the Move: Promises for Future Solar Energy Technology. Angew Chem Int Ed. 2012; 51(9): 2020-2067. doi: 10.1002/anie.201102326
- Li G, Shrotriya V, Yao Y, Huang J, Yang Y. Manipulating regioregular poly(3-hexylthiophene) : [6,6]-phenyl-C61-butyric acid methyl ester blends-route towards high efficiency polymer solar cells. J Mater Chem. 2007; 17(30): 3126-3140.
- Brabec CJ, Sariciftci NS, Hummelen JC. Plastic Solar Cells. Adv Funct Mater. 2001; 11(1): 15-26.
- Loser S, Bruns CJ, Miyauchi H, et al. A NaphthodithiopheneDiketopyrrolopyrrole Donor Molecule for Efficient Solution-Processed Solar Cells. J Am Chem Soc. 2011; 133(21): 8142-8145. doi: 10.1021/ja202791n
- Lu L, Zheng T, Wu Q, Schneider AM, Zhao D, Yu L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem Rev. 2015; 115(23): 12666-12731. doi: 10.1021/acs.chemrev.5b00098
- Zhao W, Li S, Yao H, et al. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J Am Chem Soc. 2017; 139(21): 7148-7151. doi: 10.1021/jacs.7b02677
- Lee JM, Lim J, Lee N, et al. Synergistic concurrent enhancement of charge generation, dissociation, and transport in organic solar cells with plasmonic metal-carbon nanotube hybrids. Adv Mater. 2015; 27(9): 1519-1525. doi: 10.1002/adma.201404248
- Bin H, Yao J, Yang Y, et al. High-Efficiency All-Small-Molecule Organic Solar Cells Based on an Organic Molecule Donor with Alkylsilyl-Thienyl Conjugated Side Chains. Adv Mater. 2018; 30(27): 1706361. doi: 10.1002/adma.201706361
- Hou J, Inganäs O, Friend RH, Gao F. Organic solar cells based on nonfullerene acceptors. Nat Mater. 2018; 17(2): 119-128. doi: doi.org/10.1038/nmat5063
- Castro E, Cabrera-Espinoza A, Deemer E, Echegoyen L. Low-Energy-Gap Organic Based Acceptor–Donor–Acceptor π-Conjugated Small Molecules for Bulk-Heterojunction Organic Solar Cells. Eur J Org Chem. 2015(21): 4629-4634. doi: 10.1002/ejoc.201500552
- Hauffe K, Danzmann H, Pusch H, Range J, Volz H. New experiments on the sensitization of zinc oxide by means of the electrochemical cell technique. J Electrochem Soc. 1970; 117(8): 993-999. doi: 10.1149/1.2407745
- Gerischer HJP. Electrochemical techniques for the study of photosensitization. Photochem Photobiol. 1972; 16(4): 243-260. doi: 10.1111/j.1751-1097.1972.tb06296.x
- Tributsch H, Calvin M. Electrochemistry of excited molecules: photoelectrochemical reactions of chlorophylls. Photochem Photobiol. 1971; 14(2): 95-112. doi: 10.1111/j.1751-1097.1971.tb06156.x
- Tsubomura H, Matsumura M, Nomura Y, Amamiya T. Dye sensitized zinc oxide: aqueous electrolyte: platinum photocell. Nature. 1976; 261(5559): 402. doi: 10.1038/261402a0
- O’regan B, Grätzel M. A low-cost, high-efficiency solar cell based on dyesensitized colloidal TiO2 films. Nature. 1991; 353(6346): 737-740. doi: 10.1038/353737a0
- Nazeeruddin MK, Kay A, Rodicio I, et al. Conversion of light to electricity by cis-X2bis (2, 2′-bipyridyl-4, 4′-dicarboxylate) ruthenium (II) chargetransfer sensitizers (X= Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J Am Chem Soc. 1993; 115(14): 6382-6390. doi: 10.1021/ja00067a063
- Nazeeruddin MK, Zakeeruddin S, Humphry-Baker R, et al. Acid–Base equilibria of (2, 2 ′-Bipyridyl-4, 4 ′-dicarboxylic acid) ruthenium (II) complexes and the effect of protonation on charge-transfer sensitization of nanocrystalline titania. J Inorg Chem. 1999; 38(26): 6298-6305. doi: 10.1021/ic990916a
- Gao F, Wang Y, Shi D, et al. Enhance the optical absorptivity of nanocrystalline TiO2 film with high molar extinction coefficient ruthenium sensitizers for high performance dye-sensitized solar cells. J Am Chem Soc. 2008; 130(32): 10720-10728. doi: 10.1021/ja801942j
- Kakiage K, Aoyama Y, Yano T, Oya K, Fujisawa J, Hanaya M. Highlyefficient dye-sensitized solar cells with collaborative sensitization by silylanchor and carboxy-anchor dyes. Chem Commun (Camb). 2015; 51(88): 15894-15897. doi: 10.1039/c5cc06759f
- Clarke TM, Durrant JR. Charge photogeneration in organic solar cells. Chem Rev. 2010; 110(11): 6736-6767. doi: 10.1021/cr900271s
- Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H. Dye-sensitized solar cells. Chem Rev. 2010; 110(11): 6595-6663. doi: 10.1021/cr900356p
- Wiberg J, Marinado T, Hagberg DP, Sun L, Hagfeldt A, Albinsson B. Effect of anchoring group on electron injection and recombination dynamics in organic dye-sensitized solar cells. J Phys Chem C. 2009; 113(9): 3881-3886. doi: 10.1021/jp8101139
- Jose R, Kumar A, Thavasi V, Fujihara K, Uchida S, Ramakrishna S. Relationship between the molecular orbital structure of the dyes and photocurrent density in the dye-sensitized solar cells. Appl Phys Lett. 2008; 93(2): 023125. doi: 10.1063/1.2957988
- Mishra A, Fischer MK, Bauerle P. Metal-free organic dyes for dye-sensitized solar cells: from structure: property relationships to design rules. Angew Chem Int Ed. 2009; 48(14): 2474-2499. doi: 10.1002/anie.200804709
- Tigreros A, Dhas V, Ortiz A, et al. Influence of acetylene-linked π-spacers on triphenylamine–fluorene dye sensitized solar cells performance. Sol Energy Mater Sol Cells. 2014; 121: 61-68. doi: 10.1016/j.solmat.2013.10.035
- Pati PB, Yang W, Zade SS. New dyes for DSSC containing triphenylamine based extended donor: Synthesis, photophysical properties and device performance. J Spectrochimica Acta Part A: Mol Biomol Spec. 2017; 178: 106-113. doi: 10.1016/j.saa.2017.01.048
- Kumar R, Sudhakar V, Prakash K, Krishnamoorthy K, Sankar M. Tuning the Photovoltaic Performance of DSSCs by Appending Various Donor Groups on trans-Dimesityl Porphyrin Backbone. ACS Appl Energy Mater. 2018; 1(6): 2793-2801. doi: 10.1021/acsaem.8b00458
- Ning Z, Tian H. Triarylamine: a promising core unit for efficient photovoltaic materials. Chem Commun. 2009; 45(37): 5483-5495. doi: 10.1039/b908802d
- Insuasty A, Ortiz A, Tigreros A, Solarte E, Insuasty B, Martín N. 2-(1, 1-dicyanomethylene) rhodanine: A novel, efficient electron acceptor. Dyes Pigm. 2011; 88(3): 385-390. doi: 10.1016/j.dyepig.2010.08.011
- Mao J, He N, Ning Z, et al. Stable Dyes Containing Double Acceptors without COOH as Anchors for Highly Efficient Dye-Sensitized Solar Cells. Angew Chem Int Ed. 2012; 124(39): 10011-10014. doi: 10.1002/anie.201204948
- Sandoval-Torrientes R, Calbo J, García-Fresnadillo D, Santos J, Ortí E, Martín N. Rhodanine-based dyes absorbing in the entire visible spectrum. Org Chem Front. 2017; 4(6): 1024-1028.
- Wenger S, Bouit P-A, Chen Q, et al. Efficient electron transfer and sensitizer regeneration in stable π-extended tetrathiafulvalene-sensitized solar cells. J Am Chem Soc. 2010; 132(14): 5164-5169. doi: 10.1021/ja909291h
- Echeverry CA, Herranz MÁ, Ortiz A, Insuasty B, Martín N. Rhodanine-3-acetic acid and π-extended tetrathiafulvalene (exTTF) based systems for dye-sensitized solar cells. New J Chem. 2014; 38(12): 5801-5807. doi: 10.1039/c4nj01261e
- Echeverry CA, Cotta R, Castro E, Ortiz A, Echegoyen L, Insuasty B. New organic dyes with high IPCE values containing two triphenylamine units as co-donors for efficient dye-sensitized solar cells. RSC Adv. 2015; 5(75): 60823-60830. doi: 10.1039/C5RA07720F
- Su J-Y, Lo C-Y, Tsai C-H, et al. Indolo [2,3-b] carbazole Synthesized from a Double-Intramolecular Buchwald–Hartwig Reaction: Its Application for a Dianchor DSSC Organic Dye. Org Lett. 2014; 16(12): 3176-3179. doi: 10.1021/ol500663b
- Fujisawa J, Hanaya M. Light Harvesting and Direct Electron Injection by Interfacial Charge-Transfer Transitions between TiO2 and Carboxy-Anchor Dye LEG4 in Dye-Sensitized Solar Cells. J Phys Chem C. 2017; 122(1): 8-15. doi: 10.1021/acs.jpcc.7b04749
- Yang Z, Liu C, Li K, Cole JM, Shao C, Cao D. Rational Design of ithienopicenocarbazole-based Dyes and a Prediction of their EnergyConversion Efficiency Characteristics for Dye-Sensitized Solar Cells. ACS Appl Energy Mater. 2018; 1(4): 1435-1444. doi: 10.1021/acsaem.7b00154
- Xu M, Hu X, Zhang Y, Bao X, Pang A, Fang J-K. Novel Organic Dyes Featuring Spiro [dibenzo [3, 4: 6, 7] cyclohepta [1, 2-b] quinoxaline-10, 9′-fluorene](SDBQX) as a Rigid Moiety for Dye-Sensitized Solar Cells. ACS Appl Energy Mater. 2018; 1(5): 2200-2207. doi: 10.1021/acsaem.8b00261
- He H, Gurung A, Si L. 8-Hydroxylquinoline as a strong alternative anchoring group for porphyrin-sensitized solar cells. Chem Commun. 2012; 48(47): 5910-5912. doi: 10.1039/c2cc31440a
- Si L, He H, Zhu K. 8-Hydroxylquinoline-conjugated porphyrins as broadband light absorbers for dye-sensitized solar cells. New J Chem. 2014; 38(4): 1565-1572. doi: 10.1039/C3NJ01643A
- Higashino T, Fujimori Y, Sugiura K, Tsuji Y, Ito S, Imahori H. Tropolone as a High-Performance Robust Anchoring Group for Dye-Sensitized Solar Cells. Angew Chem Int Ed Engl. 2015; 54(31): 9052-9056. doi: 10.1002/anie.201502951
- Higashino T, Nimura S, Sugiura K, Kurumisawa Y, Tsuji Y, Imahori H. Photovoltaic properties and long-term durability of porphyrin-sensitized solar cells with silicon-based anchoring groups. ACS Omega. 2017; 2(10): 6958-6967. doi: 10.1021/acsomega.7b01290
- Echeverry CA, Insuasty A, Herranz MÁ, et al. Organic dyes containing 2-(1, 1-dicyanomethylene) rhodanine as an efficient electron acceptor and anchoring unit for dye-sensitized solar cells. Dyes Pigm. 2014; 107: 9-14. doi: 10.1016/j.dyepig.2014.03.010
- Caicedo M, Echeverry CA, Guimarães RR, Ortiz A, Araki K, Insuasty B. Microwave assisted synthesis of a series of charge-transfer photosensitizers having quinoxaline-2 (1H)-one as anchoring group onto TiO2 surface. J Mol Struct. 2017; 1133: 384-391. doi: 10.1016/j.molstruc.2016.12.021
- Li C, Wonneberger H. Perylene imides for organic photovoltaics: yesterday, today, and tomorrow. Adv Mater. 2012; 24(5): 613-636. doi: 10.1002/adma.201104447
- Edvinsson T, Li C, Pschirer N, et al. Intramolecular charge-transfer tuning of perylenes: spectroscopic features and performance in dye-sensitized solar cells. J Phys Chem C. 2007; 111(42): 15137-15140. doi: 10.1021/jp076447c
- Langhals H, Obermeier A, Floredo Y, Zanelli A, Flamigni L. Light-Driven Charge Separation in Isoxazolidine–Perylene Bisimide Dyads. Chemistry. 2009; 15(46): 12733-12744. doi: 10.1002/chem.200901839
- Echeverry CA, Cotta R, Insuasty A, et al. Synthesis of novel light harvesters based on perylene imides linked to triphenylamines for Dyes Sensitized Solar Cells. Dyes Pigm. 2018; 153: 182-188. doi: 10.1016/j.dyepig.2018.02.009
- Shibano Y, Umeyama T, Matano Y, Imahori H. Electron-donating perylene tetracarboxylic acids for dye-sensitized solar cells. Org Lett. 2007; 9(10): 1971-1974. doi: 10.1021/ol070556s
- Lee MM, Teuscher J, Miyasaka T, Murakami TN, Snaith HJ. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science. 2012; 338(6107): 643-647. doi: 10.1126/science.1228604
- Stranks SD, Eperon GE, Grancini G, et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science. 2013; 342(6156): 341-344. doi: 10.1126/science.1243982
- Kojima A, Teshima K, Shirai Y, Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J Am Chem Soc 2009; 131(17): 6050-6051. doi: 10.1021/ja809598r
- NREL. Best Research-Cell Efficiencies. Accessed July, 2018.
- Castro E, Murillo J, Fernandez-Delgado O, Echegoyen L. Progress in fullerene-based hybrid perovskite solar cells. J Mater Chem C. 2018; 6(11): 2635-2651. doi: 10.1039/c7tc04302c
- Correa-Baena J-P, Abate A, Saliba M, et al. The rapid evolution of highly efficient perovskite solar cells. Energy Environ Sci. 2017; 10(10): 710-727.
- Meng L, You J, Guo TF, Yang Y. Recent Advances in the Inverted Planar Structure of Perovskite Solar Cells. Acc Chem Res. 2016; 49(1): 155-165. doi: 10.1021/acs.accounts.5b00404
- Chiang C-H, Tseng Z-L, Wu C-G. Planar heterojunction perovskite/PC71BM solar cells with enhanced open-circuit voltage via a (2/1)-step spin-coating process. J Mater Chem A. 2014; 2(38): 15897-15903.
- Kim JH, Williams ST, Cho N, Chueh C-C, Jen AKY. Enhanced Environmental Stability of Planar Heterojunction Perovskite Solar Cells Based on BladeCoating. Adv Energy Mater. 2015; 5(4): 1401229-n/a. doi: 10.1002/aenm.201401229
- Liang P-W, Chueh C-C, Williams ST, Jen AKY. Roles of Fullerene-Based Interlayers in Enhancing the Performance of Organometal Perovskite Thin-Film Solar Cells. Adv Energy Mater. 2015; 5(10): 1402321. doi: 10.1002/aenm.201402321
- Azimi H, Ameri T, Zhang H, et al. A Universal Interface Layer Based on an Amine-Functionalized Fullerene Derivative with Dual Functionality for Efficient Solution Processed Organic and Perovskite Solar Cells. Adv Energy Mater. 2015; 5(8): 1401692. doi: 10.1002/aenm.201401692
- Gatti T, Menna E, Meneghetti M, Maggini M, Petrozza A, Lamberti F. The Renaissance of fullerenes with perovskite solar cells. Nano Energy. 2017; 41: 84-100. doi: 10.1016/j.nanoen.2017.09.016
- Tian C, Castro E, Wang T, Betancourt-Solis G, Rodriguez G, Echegoyen L. Improved Performance and Stability of Inverted Planar Perovskite Solar Cells Using Fulleropyrrolidine Layers. ACS Appl Mater Interfaces. 2016; 8(45): 31426-31432. doi: 10.1021/acsami.6b10668
- Castro E, Zavala G, Seetharaman S, D’Souza F, Echegoyen L. Impact of Fullerene Derivative Isomeric Purity on the Performance of Inverted Planar Perovskite Solar Cells. J Mater Chem A. 2017; 5(36): 19485-19490. doi: 10.1039/c7ta06338e
- Jeng J-Y, Chiang Y-F, Lee M-H, et al. CH3NH3PbI3 Perovskite/Fullerene Planar-Heterojunction Hybrid Solar Cells. Adv Mater. 2013; 25(27): 3727-3732. doi: 10.1002/adma.201301327
- Gil-Escrig L, Momblona C, Sessolo M, Bolink HJ. Fullerene imposed high open-circuit voltage in efficient perovskite based solar cells. J Mater Chem A. 2016; 4(10): 3667-3672. doi: 10.1039/C5TA10574A
- Xue Q, Bai Y, Liu M, et al. Dual Interfacial Modifications Enable High Performance Semitransparent Perovskite Solar Cells with Large Open Circuit Voltage and Fill Factor. Adv Energy Mater. 2017; 7(9): 1602333. doi: 10.1002/aenm.201602333
- Shao Y, Xiao Z, Bi C, Yuan Y, Huang J. Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat Commun. 2014; 5: 5784.
- Dai S-M, Zhang X, Chen W-Y, et al. Formulation engineering for optimizing ternary electron acceptors exemplified by isomeric PC71BM in planar perovskite solar cells. J Mater Chem A. 2016; 4(48): 18776-18782. doi: 10.1039/c6ta07750a
- Castro E, Fernandez-Delgado O, Arslan F, et al. New thiophene-based C60 fullerene derivatives as efficient electron transporting materials for perovskite solar cells. New J Chem. 2018; 42: 14551-14558.
- Zheng L, Zhou Q, Deng X, Yuan M, Yu G, Cao Y. Methanofullerenes Used as Electron Acceptors in Polymer Photovoltaic Devices. J Phys Chem B. 2004; 108(32): 11921-11926. doi: 10.1021/jp048890i
- Zhang Q-H, Hu W-D, Wang X-F, et al. Fullerene Multiadducts as Electron Collection Layers for Perovskite Solar Cells. Chem Lett. 2017; 46(1): 101-103. doi: 10.1246/cl.160881
- Tian C, Castro E, Betancourt-Solis G, et al. Fullerene derivative with a branched alkyl chain exhibits enhanced charge extraction and stability in inverted planar perovskite solar cells. New J Chem. 2018; 42(4): 2896-2902.
- Tian C, Kochiss K, Castro E, Betancourt-Solis G, Han H, Echegoyen L. A dimeric fullerene derivative for efficient inverted planar perovskite solar cells with improved stability. J Mater Chem A. 2017; 5(16): 7326-7332.
- Chueh C-C, Li C-Z, Jen AKY. Recent progress and perspective in solutionprocessed Interfacial materials for efficient and stable polymer and organometal perovskite solar cells. Energy Environ Sci. 2015; 8(4): 1160-1189.
- Zhao D, Zhu Z, Kuo M-Y, Chueh C-C, Jen AKY. Hexaazatrinaphthylene Derivatives: Efficient Electron-Transporting Materials with Tunable Energy Levels for Inverted Perovskite Solar Cells. Angew Chem Int Ed. 2016; 55(31): 8999-9003. doi: 10.1002/anie.201604399
- Sun C, Wu Z, Yip H-L, et al. Amino-Functionalized Conjugated Polymer as an Efficient Electron Transport Layer for High-Performance PlanarHeterojunction Perovskite Solar Cells. Adv Energy Mater. 2016; 6(5): 1501534-n/a. doi: 10.1002/aenm.201501534
- Zhu Z, Chueh C-C, Zhang G, Huang F, Yan H, Jen AKY. Improved AmbientStable Perovskite Solar Cells Enabled by a Hybrid Polymeric ElectronTransporting Layer. Chemsuschem. 2016; 9(18): 2586-2591. doi: 10.1002/cssc.201600921
- Gu P-Y, Wang N, Wang C, et al. Pushing up the efficiency of planar perovskite solar cells to 18.2% with organic small molecules as the electron transport layer. J Mater Chem A. 2017; 5(16): 7339-7344. doi: 10.1039/C7TA01764B
- Castro E, Sisto TJ, Romero EL, et al. Cove-Edge Nanoribbon Materials for Efficient Inverted Halide Perovskite Solar Cells. Angew Chem Int Ed. 2017; 56(46): 14648-14652. doi: 10.1002/ange.201706895
- Zhong Y, Trinh MT, Chen R, et al. Molecular helices as electron acceptors in high-performance bulk heterojunction solar cells. Nat Commun. 2015; 6: 8242. doi: 10.1038/ncomms9242
- Sisto TJ, Zhong Y, Zhang B, et al. Long, Atomically Precise Donor–Acceptor Cove-Edge Nanoribbons as Electron Acceptors. J Am Chem Soc. 2017; 139(16): 5648-5651. doi: 10.1021/jacs.6b13093
- Peurifoy SR, Castro E, Liu F, et al. Three-Dimensional Graphene Nanostructures. J Am Chem Soc. 2018; 140(30): 9341-9345. doi: 10.1021/jacs.8b04119


