1University of Science and Technology of China, China
2Polymer Materials Research Department, Advanced Technologies and New Materials Research Institute (ATNMRI), City of Scientific Research and Technological Applications (SRTA-City), Egypt
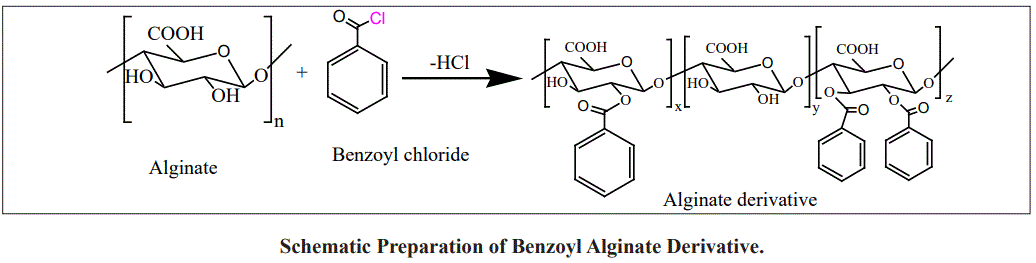
In this study, Functional substitution of alginate with phenolic groups was prepared via coupling its hydroxyl groups with benzoyl chloride. The changes in the chemical and physical properties of the new derivatives were investigated by Fourier transform infrared (FT-IR), H-Nuclear magnetic resonance (H-NMR), thermal gravimetric analysis (TGA) and scanning electron microscope (SEM). ABTS and DPPH methods examined the antioxidant properties of new alginate bases. The biocidal activity against four different bacterial strains, the biocidal activity against four different bacterial strains, S. haemolyticus MST1 (Gram-positive bacteria), P. aeruginosa MST2 and E. coli MST5 (Gram-negative bacteria) demonstrates slightly improve inhibition activity compare to alginate.
Biography:
Smaher M. Elbayomi completed her B.Sc. (with Distinction) in the Faculty of Science (Damietta) with Major in Chemistry (with Honors) in Mansoura University from September, 2006-May, 2009 and she completed her M.Sc. in the Faculty of Science with Major in Organic chemistry in Damietta University. Right now she is pursuing her PhD in the Faculty of Science and Technology of China, Faculty of Chemistry and Material Science Polymer Science and Engineering from September 2017 to till now. She worked as a demonstrator in the Department of Chemistry, Faculty of Science (Damietta) in Mansoura University Egypt, from December 26, 2009 – December 2013; as an Assistant Lecturer, in the Department of Chemistry, in the Faculty of Science in Damietta University Egypt, from December 2013 until September 2017; Lecture in Hefei Normal University China, from September 2018-2019 and as a Lecture in Huainan Normal University China, from September 2018-2019.
1University of Naples Federico II, Italy
2Department of Medical and Surgical Science and Neuroscience, University of Siena, Italy
3Department of Biochemistry, Medical University of Warsaw, Poland
4Department of Pharmacology and Pharmacodynamics, Medical University of Lublin, Poland
Serotonin, an important neurotransmitter in the SNC and SNP, has been implicated in numerous physiological and physiopathological processes. Serotonin receptors may be involved in the regulation of impulsivity and alcoholism, in the different phases of sleep, sexual behavior, appetite control, thermoregulation, cardiovascular function and recently it has been found to show growth-promoting activity and to be functionally related to oncogenes. One of the most studied chemical classes, already known for their high affinity toward these receptors, is the long-chain arylpiperazines (LCAPs). In continuation of our research program, we designed a new set of derivatives where the piperazine-N-alkyl moiety has been linked to an isonicotinic or picolinic fragment as terminal part of LCAPs. The isonicotinic or picolinic scaffold was linked via two and three methylene spacing units to the N-4-aryl-substituted piperazines obtaining a complete structure-affinity and structureselectivity relationship study. The multireceptor profile of new isonicotinic and picolinic derivatives towards 5-HT1A, 5-HT2A and 5-HT2C receptors were also evaluated in binding assays for dopaminergic and adrenergic receptors. We have disclosed interesting compounds as 5-HT1A, 5-HT2A and mixed 5-HT1A/5-HT2C ligands. Compounds showing better affinity and selectivity binding profile towards 5-HT2A receptors have been tested on rat ileus disclosing an inhibitory effect on serotonininducted contraction. These assays have allowed to verify the role that serotonin receptors can have in the gastrointestinal tract, with a potential therapeutic profile as spasmolytic agents. Finally, derivatives with a better affinity/selectivity profile towards 5-HT1A have been evaluated by in vivo assay, to determine their functional activity.
Biography:
Elisa Magli graduated summa cum laude in Pharmacy at the University of Naples Federico II discussing a thesis entitled “Synthesis and pharmacological evaluation of Nʼ-cyanoisonicotinamidinic derivatives as serotoninergic ligands”. She earned her PhD on serotoninergic ligands synthesis and evaluation of their antitumor activity in 2011. From 2015 she held the position of Researcher in Medicinal Chemistry at the University of Naples Federico II.
Research Interest: Her activity is addressed to the design, synthesis and characterization of small molecules active on different pharmacological targets with particular attention to serotoninergic system.
1Warsaw University of Technology, Poland
2Institut für Organische Chemie und Makromolekulare Chemie, Heinrich-Heine-Universität Düsseldorf, Universitätsstraße, Germany
Fuorinated counterparts of hydrogenated molecules have been reportedly shown to have higher heat resistance as well as chemical stability. This work is a joint collaboration aimed at combiningideas of B. M. Schmidt group, which reported the application of fluorinated [4+4] molecular cages to effectively absorb gaseswith the works of A.Kasprzakgroup that gave birth to the synthesis of [3+2] ferrocene cages, which showed to be an effective catalysts of 1,1'-biphenyls, when combined with Pd. Together we contrived the idea to synthesize fluorinated analogues of [3+2] ferrocene cages and check if they bare the same characteristics as aforementioned cages, meaning that they might potentially act as a catalyst or a gas absorber. The imine formation reaction that has been employed for the creation of the discussed cages is very simple as it consists of just one step. It has been also concluded that the reaction itself doesnʼt require any sophisticated conditions, which further translates to trivialness of the synthesis of the materials that might possess a number of useful properties and applications.
Biography:
Maurycy Krzyżanowski currently in his sophomore year at Warsaw University of Technology, working in A. Kasprzak Functional Organic Compound Group. Recipient of the prestigious scholarship - ,,SzkołaOrłówʼʼ, financed by Ministry of Science and Higher Education within a funding from European Union.
1National Research-Development Institute for Chemistry and Petrochemistry (INCDCP- ICECHIM), Romania
2ECPM/ICPEES, University of Strasbourg, France
3University POLITEHNICA of Bucharest, Romania
The overall purpose of this study was to obtain polyester polyols from renewable resources in the attempt of partial or total replacement of polyols obtained from petroleum resources. There are numerous literature studies, in which commercial polyols (obtained from petroleum), necessary to obtain polyurethane foams were replaced with polyols synthesized from natural oils such as: castor oil, soybean oil, sunflower oil, rapeseed oil; herein olive oil was used. The obtained polyols were further used to obtain “green” flexible polyurethane foams. The synthesis process consisted of the following steps:
1. Epoxidation of olive oil. The chemical structure of olive oil was considered to be a triglyceride with three chains of oleic acid (oleic acid - approximately 83%).
2. Synthesis of the polyols from epoxidized olive oil by ring opening with: acetic acid, ethanol and diethyl amine. The best conversions were achieved for ethanol and acetic acid. The obtained polyols were checked for viscosity and characterized by NMR, FTIR, GPC, TGA and DSC.
3. Synthesis of polyurethane foams using commercial diisocyanate. Attempts to obtain flexible polyurethane foams with 100% oil-derived polyol have failed and therefore, partial replacement of commercial polyol was tested. As a result, formulation of PU foams containing 5, 10, 15, 25 and 35% oil-derived polyol were obtained and characterized by FTIR, TGA, DSC.
Acknowledgments
The work has been funded by the Operational Programme Human Capital of the Ministry of European Funds through the Financial Agreement 51668/09.07.2019, SMIS code 124705 and by a grant awarded by UEFISCDI in framework of the PCCDI competition, contract no. 70PCCDI/2018 SECURE-NET.
Biography:
Alina Elena Coman is a PhD student at University POLITEHNICA of Bucharest, Faculty of Applied Chemistry and Materials Science. During her studies she had published 3 articles as main author and 3 as co-author. She had participated in more than 10 international conferences and she is an author of 3 patent applications filed in 2019. In 2018 she was awarded with the 1st prize at the international conference “Priorities of Chemistry for a Sustainable Development PRIOCHEM - 14th Edition” for the best oral presentation. In 2019 she had attended an internship for 3 months at the University of Strasbourg, France, under the coordination of Prof. Luc Averous.
1University of Calcutta, India
2KPC Medical College & Hospital, India
Mthemoglobinemia is a hematological disorder in which there is an excess amount of hemoglobin containing iron in Fe3+ state, known as methemoglobin. Methemoglobin is more capable of binding to oxygen resulting in reduced oxygen release to tissues. Administration of low doses of nitrates over prolonged periods may lead to chronic methemoglobinemia [1]. Nowadays the therapeutic uses of natural products are well considered for the treatment of various diseases. Previous reports have shown that natural products like curcumin, vitamin E, vitamin C, etc., are capable to inhibit the nitrite induced methemoglobin formation [2]. Hence in this study we aimed to investigate the preventive role of polyphenols present in our diet, like caffeine and catechin hydrate which are commonly found in coffee and tea. Our study revealed that pretreatment with caffeine inhibited nitrite induced oxidation of hemoglobin to methemoglobin to some extent whereas itʼs one major metabolite, 1-methyluric acid, exhibited better efficiency at physiological concentration. On the contrary, catechin hydrate enhanced the rate of methemoglobin formation at higher concentration.
Again, some selenium containing drugs are reported to exhibit potential anticancer effect. However, these anticancer drugs may exert adverse effects when used over a prolonged period. But little is known about the interaction of these selenium containing drugs with vital erythroite protein like hemoglobin. We found that selenium containing drugs like selenomethionine, selenocystine, methylseleninic acid and selenourea have toxic roles to induce methemoglobinemia. Hence our target is to find out whether antioxidants like vitamin C, caffeine and its metabolite can prevent the oxidation of hemoglobin induced by these selenium containing drugs. A comparative study of the antioxidants towards the prevention of methemoglobinemia has been performed using different spectroscopic techniques.
Biography:
Debashree Das has completed B.Sc and M.Sc in Chemistry in 2008 and 2010 respectively. She obtained her PhD from Saha Institute of Nuclear Physics (SINP) in 2017 under Prof. Abhijit Chakrabarti. Title of her thesis is “Spectrin and membrane interactions of heme and heme proteins”. After PhD she carried out her postdoctoral research funded by DST SERB in University of Hyderabad on “Ligand binding and chaperone like activities of seminal plasma proteins” for 2 years (2016 August-2018 August). Presently she is doing her second postdoctoral work funded by DSKPDF, UGC in University of Calcutta from August 2018.
1University of Calcutta, India
2University of Brighton, UK
3CMP Division, Saha Institute of Nuclear Physics, India
The article reports on the generation of Ru-morin nanocomposites using a simple methodology in presence and in absence of γ-irradiation. The nanocomposites were characterized using several techniques including, N2 adsorption-desorption, Brunauer−Emmett−Teller (BET) method, transmission electron microscopy (TEM), Fourier transform infra-red (FTIR) spectroscopy, powder X-ray diffractometric (XRD) technique, dynamic light scattering (DLS) and X-ray photoelectron spectroscopic (XPS) methods. The results revealed the production of comparatively smaller sized particles with smaller pores when prepared in presence of energetic γ-irradiation. The irradiated nanocomposite was found to be an eligible candidate for analytical sensing of Ce(IV), Ce(III) and Dy(III) out of a set of different lanthanoids. The lowest values of limit of detection (LOD) out of all the pH conditions for Ce(IV), Ce(III) and Dy(III) were 0.09 μM (at pH 12), 0.08 μM (at pH 12) and 5.37 μM (at pH 2) respectively using absorption spectroscopy. The LOD of Ce(IV) at pH 7 was found to be 0.35 μM by fluorescence spectroscopic method. It is established that the sensing is a pH dependent phenomenon which enables a selective and mutually exclusive sensing of these three lanthanoids individually.
Biography:
Pritam Singh received his Bachelors and Masters degree in Chemistry from University of Calcutta in the year of 2014 and 2016 respectively. Currently, he is working as a Ph. D scholar at University of Calcutta under UGC, NET-JRF scheme as senior research fellow. His main area of research is in the field of designing porous materials for the application in solving different industrial and environmental issues. He has four international publications in this field. He has also received a best poster presentation award in an International conference organized by the Indian Chemical Society.
Universidade de Santiago de Compostela, Spain
Decomposing water is the more direct way to produce hydrogen, which can be stored and utilized as a transportable fuel or converted into energy-rich organic molecules, to cope with the intermittent character of the solar radiation. The Oxygen Evolving Complex (OEC) is the native enzyme that catalyzes the oxidation of water in natural photosynthesis to release oxygen. This constitutes one of the half reactions of water splitting. The creation of biomimetic systems to reproduce the basic chemistry of this process gives us more insight into better understanding this crucial natural reaction which is responsible of the atmospheric oxygen that we breathe.
In this communication we report the ability of a number of manganese complexes to split water, that has been studied by means of water photolysis experiments. The synthetic models to be presented show different structural features: monomers, m-aquodimers, m-phenoxodimers, dimer-of-dimers and tetrameric complexes.
The discussion concerning the photolytic behaviour encompasses the advances made in the new insights on the structural features ascertained through the development of characterization techniques. Supramolecular interactions arise as a key factor to enhance the ability of these systems to split water. A dimer-of-dimers manganese complex, described in this work, appears as a precursor of an extremely active photolytic catalyst.
Biography:
Marcelino Maneiro received his Ph.D. degree in 1998 at University of Santiago de Compostela USC (Spain), working on the field of artificial photosynthesis. From 1998 to 2000, Maneiro was a Postdoctoral Fellow at Princeton University, USA; in 2004, he was a Visiting Researcher at the RCSI, Ireland. Since 2000, Maneiro has occupied different researcher and academic positions at USC and he became a Professor of inorganic chemistry in 2007. Maneiro has authored more than 60 scientific publications. His research topics focus on biomimetic catalysts studying their capacity to oxidize water or their antioxidant activity.