1Indian Institute of Technology Guwahati, India
2Assam Don Bosco University, India
Tetrahydrothiopyrans and its derivatives are important structural moiety of many bioactive naturally occurring molecules. They are found in many petroleum products and also play a key role in the biological activities of a number of pharmaceutical agents. Synthesis of these tetrahydrothiopyran moieties have always been an exciting challenge for the synthetic organic chemists. Hence an efficient, metal free, methodology for the diasterio selective synthesis of tetrahydrothiopyrans using Prins enol-ether cyclization reaction from enol ethers mediated by trimethylsilyltrifluoromethanesulfonate (TMSOTf ) in good yields under mild reaction conditions has being developed.
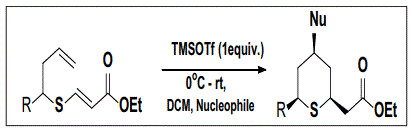
The reaction with TMSOTf in Toulene/CH2Cl2 (1:1) solvent system produced 2,4,6-trisubstituted tetrahydrothiopyrans in 75% yield with dr 88:12. The structure of the compounds were confirmed by 1H, 13C NMR, IR and mass spectrometry. The diastereomeric ratio was determined from the 1H NMR of crude reaction mixture. The reaction was also screened with various Lewis and Brϕnsted acids with an aim to synthesize tetrahydrothiopyran ring system.
Biography:
Manash Jyoti Deka has completed his PhD at the age of 30 years from IIT Guwahati. He has published more than 10 papers in reputed journals. He is currently working as an Assistant Professor in the Department of Chemistry, Assam Don Bosco University.
Institute of Chemistry of New Materials of National Academy of Sciences of Belarus, Belarus
Magnetic nanoparticles (NP) of iron oxides and their composites with hydroxyapatite (HA) are used for the targeted delivery of biologically active compounds (BAS) and drugs and have a significant advantage over traditional methods of therapy, since the active substances immobilized on the nanocarrier are protected from chemical, enzymatic and immune degradation on the way to the target of therapy. A simple one-step method for the synthesis of magnetic nanocarriers of bioactive compounds (BAС)-new amides and benzohydrazides of the 2-arylaminopyrimidine series, containing fragments of the structure of known antitumor drugs, as well as functional NH2 groups providing chemosorption of BAС nanoparticles, was developed. A distinctive feature of the preparation of composites is the presence of 2-arylaminopyrimidine derivatives in the nanocarrier forming medium, including nanosized hydroxyapatite and magnetite - (HAP)FexOy or ((HAP)FexOy)PAD (PAD - polyaldehydedextran) in the covalent immobilization method. The composition, morphology and magnetic characteristics of synthesized nanocomposites were studied. Composites ((HAP) FexOy)BAS with an adsorbed biologically active substance based on 2-arylaminopyrimidinamide derivatives are bioactive nanosystems ready for in vivo applications, for example, for the treatment of bone diseases. To check the effectiveness of a such system using, the kinetics of the BAC release was studied in the work, on the basis of which it was shown that the biologically active compound is released in two stages within 5 hours: 80% of the active substance is released in the first 2 hours and then the remainder is released in the next 3 hours substances.
Biography:
Zhanna Ihnatovich, PhD in Chemistry, acting head of the laboratory of organic composite materials at the Institute of Chemistry of New Materials of the National Academy of Sciences of Belarus.
Research Areas: Development of the methods of synthesis of new amides of 2-arylaminopyrimidine series for the production of biologically active substances, potential inhibitors of tyrosine kinases, in order to create on their basis multifunctional nanocarriers of bioactive compounds for the targeted delivery. Zhanna Ihnatovich participated in the development and implementation of pilot-industrial technology for the synthesis of the pharmaceutical substance of the antitumor drug Imatinib.
Institute of Chemistry of New Materials of the National Academy of Sciences of Belarus, Belarus
Nanoparticles (NP) of various nature change the properties of polymeric materials: optical, heat and electrophysical. Polyaniline (PANI) is an electrically conductive polymer, has properties typical for semiconductors, is characterized by high thermal and chemical stability, which allows one to obtain materials with strictly specified properties and therefore, to expand the scope of their application in various optoelectronic devices. The influence of PANI, PANI composites with Fe2O3, CeO2, Ag, as well as Ag/CeO2 and CeO2/Ag NPs on the thermal and electrical conductivity of polyvinyl alcohol (PVA) films stained with Chicago Sky Blue 6B (CSB) dichroic commercial dye was studied. The anisotropy of the thermal conductivity of colored film samples containing PANI composites with Fe2O3, CeO2, Ag and the Ag/CeO2 and CeO2/Ag composites increases in comparison with the CSB-colored film in 1.1 - 2.2 times and the anisotropy of the electrical conductivity of the films with composites increases significantly (30 - 80 times) compared to the stained sample. Colored films containing PANI composites with Fe2O3, Ag and CeO2/Ag composite are characterized by a high polarizing ability (PS) of 90 - 99% in the expanded spectral compared to Chicago Sky Blue 6B (594 - 690 nm, PS = 90-97%) in the range 561 - 698 nm and films with the addition of the PANI/Fe2O3 composite are resistant to UV light (the rate of decolorization of a colored sample with a composite is reduced by 45.4%).
The thermal and electrical conductivity of colored films can be improved by introducing PANI composites with Fe2O3, CeO2, Ag as well as Ag/CeO2 and CeO2/Ag which is important when film materials are used in devices operated under the influence of heat and electricity.
Biography:
Liudmila Filippovich, PhD in Chemistry, senior researcher at the Institute of Chemistry of New Materials of the National Academy of Sciences of Belarus.
Research Directions: Development of polymer multifunctional polarizing materials with the addition of organic dyes and metal nanoparticles, the study of their optical, thermophysical, electrically conductive and other properties. L. Filippovich participated in the development of a pilot industrial regulation for the manufacture of a film polarizer for near UV, visible and near infrared spectral regions.
1Institute of Bioorganic Chemistry, The NAS of Belarus
2Institute of Chemistry of New Materials of the National Academy of Sciences of Belarus, Belarus
Discovery of the nature of inhibiting cancer processes by small organic molecules has changed the principles of the development of drug compounds for antitumor therapy. Recent achievements in this area are associated with the design of small-molecule protein kinase inhibitors, organic compounds exhibiting directed pathogenetic action. 2-Arylaminopyrimidine derivatives are the most commonly used templates for the development of these molecules.
In this study, the methodology of the design of chimeric molecules and directed organic synthesis of potential anti-cancer compounds with multikinase profile were developed based on the derivatives of 2-arylaminopyrimidine and substituted aryl carboxylic acids containing as substituents pharmacophore fragments of piperazine, morpholine, isoxazole, isothiazole followed by in silico evaluation of their inhibitory activity against native and mutant Bcr-Abl tyrosine kinase. In doing so, the following studies were performed: i) computer-aided design of potential kinase inhibitor candidates consistent with the above methodology; ii) molecular docking of these compounds with the enzyme active site; iii) refinement of the ligand-binding poses by the PM7 semi-empirical quantum chemical method; iv) prediction of the interaction modes dominating the binding; v) calculation of the values of binding free energy and dissociation constant for the PM7-based ligand/Bcr-Abl complexes; and vi) selection of molecules most promising for synthesis and biochemical assays.
As a result, top ranked compounds that specifically and effectively interact with the active sites of the native and mutant Bcr-Abl tyrosine kinase and show the low values of binding free energy and dissociation constants were identified and synthesized.
Based on the data obtained, these compounds are suggested to present good scaffolds for the development of novel potent anti-tumor drugs.
Biography:
Elena Koroleva, Doctor of chemical sciences, principal researcher of the Institute of New Materials Chemistry, National Academy of Sciences of Belarus.
Field of scientific interests: chemistry of heterocyclic compounds, fine organic synthesis, bioorganic chemistry.
Institute of Chemistry of New Materials of the National Academy of Sciences of Belarus, Belarus
Electrically conductive polymers, which include polyaniline (PANI), represent a new class of synthetic materials that combine the chemical and mechanical properties of polymers with the conductive properties of metals and semiconductors. Since the 60yrs of the XX century, conductive polymers have become the object of close attention of researchers due to the possession of special properties caused with the presence in their structure of a conjugated bond system. Polyaniline and its composites with metal nanoparticles are increasingly used in such areas as medicine, electronic and optical devices, biosensors. We have developed an effective method for matrix polymerization of aniline using polyvinylpyrrolidone (PVP) of various molecular weights, carboxymethyl cellulose and dodecylbenzenesulfonic acid as steric stabilizers. Methods for the synthesis of polyaniline with NPs of silver, nickel, cobalt, copper, iron oxides, cerium dioxide, composites PANI/Ag/CeO2, PANI/Fe3O4/CeO2, Ag/CeO2 and CeO2/Ag using the methods of ultrasonic and hydrothermal synthesis are proposed. The dimensional parameters of composites, using the methods of dynamic light scattering, optical, scanning and transmission electron microscopy, are determined. The sizes of agglomerates of nanoparticles ranged from 10 to 100 nm and the sizes of their PANI composites ranged from 1 to 28 μm, depending on their nature. Composites were used for the subsequent production of polyvinyl alcohol films on their basis and the study of their physicochemical characteristics.
Biography:
Novik Khrystsina, MSc in Chemistry, junior researcher, laboratory of optical multifunctional films at the Institute of Chemistry of New Materials of the National Academy of Sciences of Belarus.
Research Interests: Methods for producing of iron oxides, silver, cerium dioxide nanoparticles and their composites with bioactive compounds of the 2-arylaminopyrimidine series, production of polyaniline and its composites with metal nanoparticles.
Institute of Chemistry of New Materials of the National Academy of Sciences of Belarus, Belarus
Obtaining new compounds with pharmacological properties is possible by creating structures, which may include fragments of various biologically active compounds connected by such bonds as amide, sulfonamide or ester, which are easily broken down in the body by enzymes. Thus, these molecules can either exert an independent pharmacological effect after delivering them to the corresponding organs in the human body or enhance the effect of each other showing synergism.
The heterocyclic 2-aminopyrimidine system is one of important pharmacophores responsible for the biological properties of its derivatives. Derivatives were obtained on the basis of 2-aminopyrimidine, a wide spectrum of antitumor activity of which makes them an attractive object of organic synthesis and arouses interest in rationalizing the methods for their preparation. Amides and sulfonamides of oxydibenzoic acid exhibit various types of biological activity and used to treat malaria, leprosy, infections caused by mycobacteria, cognitive impairment, Alzheimerʼs disease, treat cancer, etc. We propose the methodology for the synthesis of sulfonamides based on derivatives of 2-arylaminopyrimidine and oxydibenzoic acid. This methodology includes the design of amide molecules using pharmacophore modeling, effective methods for the synthesis of key precursors and target compounds. New sulfonamides of 4-(4-carboxyphenyl)-3-sulfobenzoic acid containing fragments of heterocyclic amines (pyridine and pyrimidine) with preparative yields of 75-87% were synthesized. Experimental samples of the new sulfonamides of oxydibenzoic acid were synthesized for subsequent biological tests for antitumor activity on cell lines.
Biography:
Anastasia Yermolinskaya, Junior Researcher of the laboratory of organic composite materials at the Institute of Chemistry of New Materials of the National Academy of Sciences of Belarus.
Research Areas: Development of the methods of synthesis of new amides and sulfonamides of 2-arylaminopyrimidine series for the production of biologically active substances.
1Ecole Normale Supérieur Assia Djebar Constantine, Algeria
2Faculté de Médecine, Ville Universitaire Constantine 3, Algérie
3Ecole Normale Supérieure Assia Djebarde Constantine, Algérie
4Centre de Recherche en Bio Technologie, Algérie
5University Pole Ain El Bey Constantine, Algeria
6Institut Jean Lamour, Campus Artem, France
Azines, commonly known as bis Schiff bases, are a big class of compounds that undergo a wide variety of chemical and pharmacological processes and have been used as intermediates in heterocyclic synthesis. Literature survey revealed that azines were found to possess antimicrobial and anticancer activities. Although symmetrical azines are readily synthesized by the reaction of hydrazine with an excess of an aldehydes herein we report the preparation and the characterizations of two novel symmetrical azines and explore their biological activities for the first time. 1 H NMR, 13CNMR, UV-Vis, FT-IR Spectroscopy and DRX were used to characterize these azines structures. In addition, recent investigations described potential benefits of Schiff bases inreductive antioxidant capacity activities. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) evaluation inhibitory of these two new azines Schiff bases were measured and were compared to those of standard drugs.
Biography:
Henia Bouzidi Mousser was a Lecturer since September 2004 and Research Director since September 2005. Professor since December 2010. Head of Department of Physics and Chemistry since April 2014 to September 2019 and Vice director in charge of post graduation and scientific research to date at the ENSADC (Algeria). Teachings : General Chemistry, Organic chemistry, Analytical Chemistry, Mineral Chemistry, Electrochemistry, Thermodynamics, Chemical kinetics, Surfaces Chemistry, Interfaces Physical Chemistry, Chemistry of surfactants. Several scientific and administrative responsibilities (project manager, thesis director, training manager, research laboratory director and department head). Author of over 20 Research articles and about 20 international conférences.
Adam Mickiewicz University, Poland
Carbon nitride is an organic polymer of the chemical formula of C3N4. Its structure closely resembles that of the layered structure of graphite, so it is called a graphitic carbon nitride and is denoted as g-C3N4. Due to the high nitrogen content, carbon nitride has high chemical and thermal resistance. Thanks to this, it is used as a support in catalysis. It is for this purpose that we used carbon nitride in our research.
Carbon nitride was doped by nickel and then was reduced at 525°C. Surprisingly, it was noted that the reduction at high temperature leads to a increase in the specific surface area of the catalyst, up to 380 m2g-1. Moreover, during the reduction process, significant amounts of gases (like NH3, CH4, HCN) are released. It indicates that the evolution of gases causes more porosity of the structure.
One of the problems of the 21st century is the growing pollution of the environment. One of them is the pollution of waters with organic compounds that can be removed in sorption processes. This is where the obtained material was used. However, for better results, the metal was washed out of the system. The developed specific surface of carbon nitride was used in sorption processes of organic dyes, like rhodamine B and methyl orange. For example, the maximum sorption od RhB is close to 200 mg g-1.
Biography:
Emilia Alwin is a PhD student in fourth year at the Faculty of Chemistry at Adam Mickiewicz University in Poznan. Emilia Alwin research focuses on heterogeneous catalysis using a carbon nitride as a support, which is doped with various noble and non-noble metals. The main research aspects relate to the use of these catalysts in the production of hydrogen, photocatalysis and sorption of organic dyes. The aim of the research is to further expand the possibilities of using modified carbon nitride structures.
Adam Mickiewicz University, Poland
The properties of silica, such as: low price, thermal stability, hardness, chemical resistance and nontoxicity make it one of the most frequently applied catalyst support. It has been shown that the activity of palladium catalysts for benzene, toluene and xylene hydrogenation is consistently higher with acidic supports than those with neutral silica [1,2]. For this reason modification of silica in order to generate the acidic properties seems to be especially interesting.
In this work, commercial silica (SiO2) was modified using aqueous solution of ammonium fluoride (SiO2-F) and ammonium chloride (SiO2-Cl). The resulting materials were used as supports for iridium active phase. The catalysts containing 1 wt.% of iridium supported on silica were prepared by conventional impregnation method using hexachloroiridic acid as metal precursor. The prepared catalysts and supports were characterized by N2 adsorption/desorption measurements and H2-TPR. The mean size of metal particles was determined by hydrogen chemisorption measurements. The acidity of modified SiO2 was estimated by means of temperature-programmed desorption of ammonia (NH3-TPD). The effect of acidity of supports on the activity of iridium catalysts for toluene hydrogenation was studied. The catalyst supported on silica modified using ammonium fluoride is the most active.
Biography:
Monika Kot is a PhD student in chemistry at Adam Mickiewicz University in Poznań (Poland). Her research topics focus on heterogeneous catalysis. She works on synthesis and characterization of iridium and nickel catalysts supported on silica supports with different acidity and structural properties for hydrogenation reactions.
Universidade de Santiago de Compostela, Spain
Biomass represents a major renewable carbon resource that can be used to produce fuels and bulk chemicals though manufacturing processes. 5-hydroxymethylfurfural (HMF) is one of the most promising biomass-based chemicals that has the potential to be converted to a variety of useful intermediates for polymers and many other fine chemicals. In this communication, we report the effective oxidation of HMF to 2,5-furandicarboxaldehyde (DFF) catalysed by two manganese(III)-Schiff base complexes in a pH 8.5, phosphate buffer at room temperature. DFF is a versatile precursor in the synthesis of functional polymers, pharmaceuticals, anti fungal agents and furan-urea resins.
Oxidations of HMF to DFF were performed under mild conditions using an Iso-tech laboratory DC power supply. Sodium chloride acts as electrolyte in order to increase the conductivity of water and to reduce the power dissipation. The electro catalytic reaction proceeds through the oxidation of chloride into hypochlorite at alkaline pH in a chlorine-free medium. Four manganese(III)-Schiff base complexes were tested, two of them behave as efficient catalysts in the oxidation of DFF to HMF with conversion rates of 70-80 %. The two active catalysts have a short carbon chain between imine groups, a feature that leads to tetragonally elongated octahedral geometries. An axial water molecule in this class of distorted geometries is quite a labile ligand the loss of which would generate a vacant position in the coordination sphere to accommodate the substrate molecule. On the other hand the two manganese(III)-Schiff base with flexible chain between the imine groups gave only 11% conversion to DFF.
Biography:
Lara Rouco received her BSc degree at University of Santiago de Compostela (2016) and her MSc degree at University of Granada (2017). She is currently doing her PhD in biomimetic catalysts at the Department of Inorganic Chemistry, University de Santiago de Compostela. She has authored five scientific publications related to her area of expertise.
Universidade de Santiago de Compostela, Spain
Helicity can be found in a wide variety of natural and artificial structures. Helicoidal structures are considered to be promising architectures given their wide variety of properties. Thus, these chiral compounds have been exhaustively studied because of their involvement in different areas such as anion sensor, luminescence, magnetism, chirality, molecular machines, guest recognition and DNA binding. To prepare this type of metallo supramolecular structures, the ligands must be coordinated by wrapping around the metal ions. Particularly, helicates derived from Schiff bases are interesting due to the versatility and similarity of these ligands with other present in life.
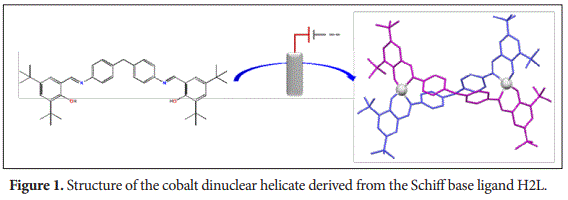
In this work, a novel neutral cobalt dinuclear helicate [Co2(L)2] 2CH3CN has been obtained by an electrochemical method (Figure 1). To achieve this, a dianionic and tetradentate ONNO Schiff base ligand H2L has been designed. This ligand possess two binding domains [NO] separated by a semi-flexible aromatic spacer. This helical complex has been fully characterized both in solution and solid state. The crystal structure consists of discrete dinuclear helical molecules, constructed from two bi deprotonated ligand units arranged around two Co2+ atoms.
Biography:
Sandra Fernández-Fariña received her BSc and MSc degree at University of Santiago de Compostela (2015 and 2016, respectively). She is currently doing her PhD in metallo supramolecular chemistry at the Department of Inorganic Chemistry, University of Santiago de Compostela. She has authored two scientific publications related to her area of expertise and 22 participations in national and international conferences.
Universidade de Santiago de Compostela, Spain
Metallosupramolecular Chemistry studies the reversible association of chemical systems by intermolecular bonds. Among the metallo supramolecular compounds, helicates stand out due to their applications in numerous areas such as chemistry of materials or molecular biology. These compounds are made up of organic ligands wrapped around a central axis defined by the metal centers. Bisthiosemicarbazone ligands are of great interest for the preparation of these helical compounds given their versatility as donor systems and their biological properties.
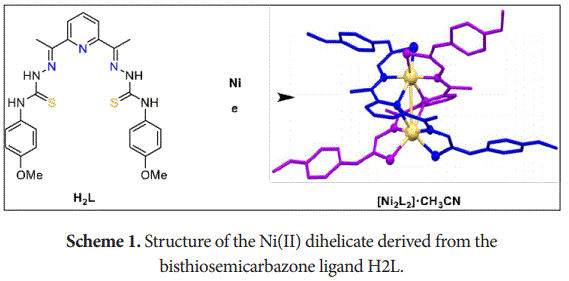
In this work, a pentadentate [N3S2] bisthiosemicarbazone Schiff base ligand H2L has been designed. This ligand turned out to be appropriate for obtaining neutral dihelicate compounds using an electrochemical procedure. The Ni(II) complex obtained shows a [Ni2L2] stoichiometry (Scheme 1). This dihelicate has been characterized by the habitual techniques in solution and solid state, including x-ray diffraction.
Biography:
Isabel Velo Heleno received her BSc and MSc degree at University of Santiago de Compostela (2018 and 2019, respectively). She is currently doing her PhD in Metallo supramolecular Chemistry at the Department of Inorganic Chemistry, University of Santiago de Compostela.