Research Article
A Cardiovascular Magnetic Resonance Study on the short and long-term effects of Coronary Artery Bypass Graft Surgery on the Right Ventricular Systolic Function
1Department of Cardiology, VU University Medical Center, Amsterdam, the Netherlands
2Institute for Cardiovascular Research VU University ICaR-VU, Amsterdam, the Netherlands
3Department of Cardiovascular Sciences, East Carolina University, Greenville, NC, USA
*Corresponding author: Constantin B Marcu, Department of Cardiovascular Sciences, East Carolina University, 115 Heart Drive, Greenville, NC, USA 27834, E-mail: marcuc@ecu.edu
Received: March 11, 2017 Accepted: March 24, 2017 Published: March 30, 2017
Citation: Becker MAJ, Robbers LFHJ, Brouwer WP, et al. A Cardiovascular Magnetic Resonance Study on the short and longterm effects of Coronary Artery Bypass Graft Surgery on the Right Ventricular Systolic Function. Madridge J Cardiol. 2017: 2(1): 14-20. doi: 10.18689/mjc-1000105
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Purpose: Right ventricular (RV) systolic function is an important prognostic factor in various cardiovascular diseases. Echocardiography, by measuring the tricuspid annular plane systolic excursion (TAPSE), often demonstrates an apparent decreased RV function after coronary artery bypass grafting (CABG). Cine cardiovascular magnetic resonance imaging (CMR) with steady state free precession (SSFP) is considered the gold standard for evaluation of RV systolic function. We used this technique to evaluate RV systolic function at baseline (T0), short term (T1, 4±1 months), mid-term (T2, 7±1 months) and long-term (T3, 32±11 months) follow-up after CABG. Also, we assessed if grafting of the right coronary artery (RCA) had any influence on the postoperative RV function.
Methods: Thirty-four patients (29 men; mean age 63±9 years) had CABG and underwent serial CMR examinations. Dedicated software (Qmass, Medis, Leiden, the Netherlands) was used for quantification of RV ejection fraction (RVEF), on a stack of short-axis cine SSFP images. TAPSE was measured on a four chamber cine image.
Results: During all follow-up time points, RVEF remained unchanged (57±14%, 59±14%, and 58±12% respectively, all p-values: 1.0), compared to baseline measurement (61±11%). TAPSE, however, showed a significant decrease after CABG at all time points (17±5mm vs. 10±4mm, vs. 9±3mm and vs. 10±3mm respectively; all p-values <0.001). No differences in RV systolic function were found between patients with or without revascularization of the RCA.
Conclusion: Our CMR study showed a preserved RV systolic function after CABG at short-, mid- and long-term follow-up while TAPSE showed a persistent decrease after surgery, thereby underestimating true systolic function of the RV. Revascularization of the RCA had no influence on the postoperative RV systolic function.
Keywords: Right ventricular function; TAPSE; Cardiovascular Magnetic Resonance Imaging; Coronary Artery Bypass Graft.
Introduction
The right ventricular (RV) systolic function is an important prognostic variable in a wide range of cardiovascular diseases [1-5]. A frequently observed phenomenon after coronary artery bypass graft (CABG) surgery is an apparent decrease in RV systolic function, when the tricuspid annular plane systolic excursion (TAPSE) is measured on Echocardiography [6-9]. However, cardiovascular magnetic resonance imaging (CMR) is considered the gold standard for quantitative non-invasive assessment of ventricular function [10-16]. Currently, only limited data are available on the assessment of RV systolic function after CABG with CMR. Furthermore, several studies reported conflicting findings about the change in RV systolic function after CABG. Due to these ambiguous results, the effects of CABG on long-term RV systolic function are still unclear. The aim of this study was to investigate the long-term effects of CABG on the RV systolic function, using CMR to asses TAPSE and RV volumes and function [1,10,12,15]. Secondly, we assessed whether grafting of the right coronary artery (RCA) had any influence on the postoperative RV systolic function.
Methods
Study Population
The patients for this study were recruited from the CMR database of the cardiology department of the VU University Medical Center, Amsterdam, the Netherlands. CMR data of 34 patients who underwent CABG surgery between 2001 and 2004 were analysed. Exclusion criteria were contra-indications for CMR (e.g. cardiac pacemaker, ferromagnetic implants, claustrophobia, inability to stay in a supine position for 30-45 minutes) [12]. The study was approved by the local institutional review board and patients provided informed consent.
CMR
All CMR acquisitions were performed at baseline (preoperatively) (T0), at short term (T1, 4±1 months), mid-term (T2, 7±1 months), and long-term (T3, 32±11 months) follow-up after CABG on a 1.5 Tesla clinical MR system (Sonata, Siemens, Erlangen, Germany). Functional imaging was performed by using retrospectively ECG-gated steady-state free precession (SSFP) cine imaging with breath-holding. In each patient standard longaxis cine images were acquired. A total of 8-10 short-axis slices were obtained every 10mm, starting at the mitral valve annulus and covering the entire volume of both ventricles.
CMR analysis
Analysis of the CMR data for the assessment of RV volumes was performed with dedicated post processing software (Qmass, V2011, Medis, Leiden, the Netherlands) based on methods described before [12]. Endocardial borders of RV were outlined on short-axis cine images at end-diastole and end-systole. RV volumes and RVEF were measured and calculated using the disk-area summation method (modified Simpsonʼs rule) [17,18]. Trabeculae and papillary muscles were considered part of the blood pool volume. The fourchamber long-axis images were used to verify whether or not the basal short axis slice was part of the RV and included or excluded from the analysis.
In addition to the right ventricular volumes and function, the left ventricular ejection fraction was evaluated at T0 and T1 as well, to exclude LV dysfunction as a cause of eventual changes in RV function post CABG.
TAPSE was calculated on a four-chamber cine image using different software (Centricity Radiology RA600 v6.1, GE Healthcare, Buckinghamshire, United Kingdom). A fixed point on the thoracic wall, in line with the interventricular septum was chosen as the reference point. The distance between the reference point and the lateral side of the tricuspid annulus was measured during end-diastole (end-diastolic length [EDL] in mm) and end-systole (end-systolic length [ESL] in mm). TAPSE was defined as the difference between EDL and ESL [12] (figure 1).
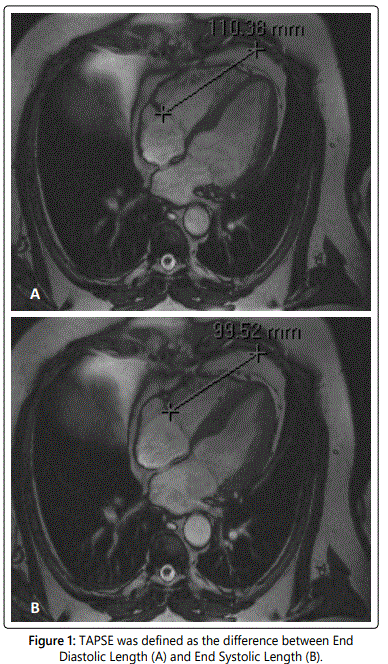
We divided our patients in two groups; one group with revascularization of the right coronary artery (RCA-group) and the other group with revascularization of coronary arteries other than RCA (non-RCA-group).
Statistical analysis
Statistical analysis was performed using SPSS 15.0 for Windows (SPSS Inc, Chicago, Illinois, USA). The repeated measurements during the follow-up period were initially compared using one-way ANOVA-analysis with Bonferroni correction. To compare the LVEF before and after CABG, a paired-samples t-test was used. Comparisons between the RCA- and non-RCA-group were made with the independentsamples t-test. The results are expressed as the mean and one standard deviation (mean±SD). A p-value of less than 0.05 was considered to be statistically significant.
Results
Study population
In total, 34 patients (29 men and 5 women) underwent baseline CMR acquisitions. The mean age was 63±9 years. At T1 33 patients had a follow-up CMR, 32 patients had a follow-up at T2 and 27 had a follow-up CMR at T3. Co-morbidities of the population were systemic hypertension (n=11), chronic myocardial infarction (n=17), diabetes mellitus (n=5), hypercholesterolemia (n=7), arrhythmia (n=6) and valvular disease (n=6). Twenty-six patients had angina complaints of different severity (New York Heart Association class I [n=2], II [n=4] and III [n=20]). In 27 patients the RCA was involved in the revascularization and in 7 patients the RCA was not involved in the revascularization. The baseline and follow-up characteristics are shown in table 1.
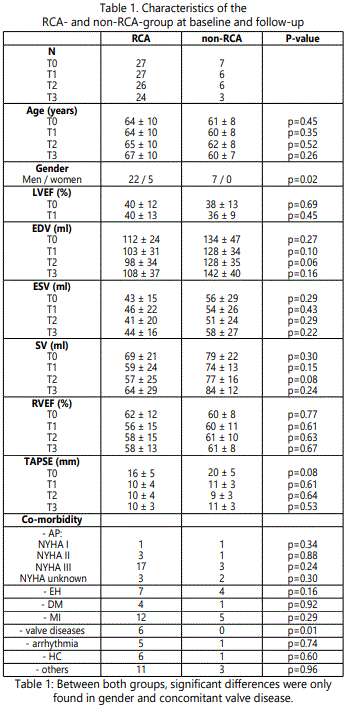
LVEF: left ventricular ejection fraction; EDV: end diastolic volume; ESV: end systolic volume; SV: stroke volume; RVEF: right ventricular ejection fraction; TAPSE: tricuspid annular plane systolic excursion; AP: angina pectoris; EH: essential hypertension; DM: diabetes mellitus; MI: myocardial infarction; HC: hypercholesterolemia.
CMR findings
The LVEF did not change significantly at T1 compared to T0 (40±12% at T0 to 39±12% at T1; p=0.71).
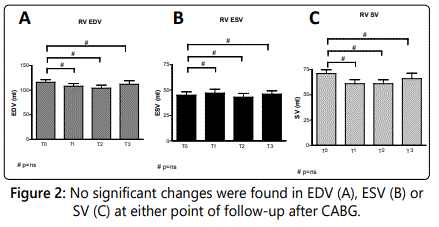
The end diastolic volume (EDV) showed no significant change at follow-up compared to baseline measurements (T0: 116±31ml vs. T1: 108±32ml, T2: 104±35ml, T3: 112±38ml. P-values are respectively 1.0, 0.89 and 1.0) (figure 2A).
The end systolic volume (ESV) did not change significantly after CABG (45±19ml at T0, 47±22ml at T1, 43±21ml at T2 and 46±17ml at T3. P-values =1.0) (figure 2B).
Stroke volume (SV) showed no significant change either after CABG (71±22ml at T0 to 61±23ml at T1, 61±25ml at T2 and 66±28ml at T3; p=0.63, p=0.60, p=1.0 respectively) (figure 2C).
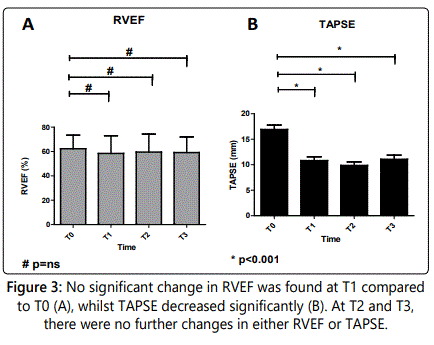
RVEF was not significantly changed at T1 compared to T0 (T0: 61±11% to T1: 57±14%, p=1.0). During the follow-up at T2 and T3, RVEF did not change either (59±14% and 58±12%, respectively. Both p-values=1.0) (figure 3A).
The TAPSE, however, was significantly decreased at T1 compared to T0 (17±5mm at T0 to 10±4mm at T1, p<0.001). During follow-up at T2 and T3, TAPSE remained significantly decreased in comparison to T0 values (at T2 9±3mm and at T3 10±3mm, both p-values <0.001). However, TAPSE showed no further decrease at T2 and T3 in comparison to T1 measurements (both p-values=1.0) (figure 3B).
RCA vs. non-RCA
At baseline, the RCA and the non-RCA CABG groups were well matched for age, however, the non-RCA revascularized group did not contain any women (p=0.02). Concomitant valvular pathology (mitral valve regurgitation: n=5; tricuspid valve regurgitation: n=1) was significantly more seen in the RCA group than in the non-RCA-group (n=6 and n=0 respectively; p=0.01). At baseline, no significant differences in RV volumes, RVEF, LVEF and TAPSE were seen between the two groups (table 1).
In both the RCA- and the non-RCA-group, the LVEF showed no significant change at T1 compared to T0 (RCA: 40±12% at T0 to 40±13% at T1, p=0.98; non-RCA: 38±13% at T0 to 36±9% at T1, p=0.38). No significant difference was seen in LVEF between both groups at either time point (T0: p=0.69 and T1: p=0.45).
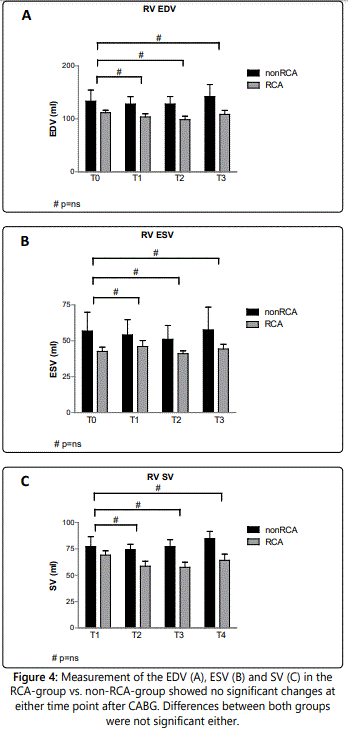
The differences in RV volumes between RCA- versus nonRCA-revascularization are outlined in figure 4 A-C. In both groups, we found no significant difference in EDV after CABG (RCA: 103±31ml at T1, 98±34ml at T2, 108±37ml at T3 compared to 112±24ml at T0, all p-values: 1.0; non-RCA: 128±34ml at T1, 128±35 at T2, 142±40ml at T3 compared to 134±47ml at T0, p=0.80 – 1.0) (figure 4A). Differences between both groups were not significant (p=0.06 – 0.27) (table 1).
The ESV showed no significant differences in follow-up measurements compared to T0 values (RCA: 46±22ml, 41±20ml, 44±16ml, respectively T1, T2, T3, in comparison to 43±15ml at baseline, all p-values: 1.0; non-RCA: at T1 54±26ml, T2 51±24ml, T3 58±27ml, compared to 56±29ml at T0, all p-values: 1.0) (figure 4B), nor between the RCA- and the non-RCA-group (p: 0.22 – 0.43) (table 1).
Evaluation of SV showed no significant differences either between follow-up measurements (RCA: 59±24 at T1, 57±25ml at T2 and 64±29 at T3, in comparison to 69±21ml at T0, p=0.56 – 1.0; non-RCA: 74±13ml, 77±16ml and 84±12ml at respectively T1, T2 and T3, compared to 79±22ml at T0, all p-values: 1.0) (figure 4C), nor between the two groups (p: 0.08 – 0.30) (table 1).
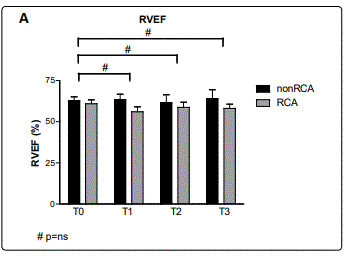
During follow-up measurements, RVEF was not significantly changed at T1 compared to T0 in either the RCAgroup (T0: 62±12% vs. T1: 56±15%, p=0.95) or the non-RCAgroup (T0: 60±8% vs. T1: 60±11%, p=1.0). RVEF remained unchanged during T2 (RCA: 58±15%, p=1.0; non-RCA: 61±10%, p=1.0) and T3 (RCA: 58±13%, p=1.0; non-RCA: 61±8%, p=1.0) in comparison to T0 values (figure 5A). Differences between the RCA and non-RCA group in RVEF were not significant at any time point (p: 0.61 – 0.77) (table 1).
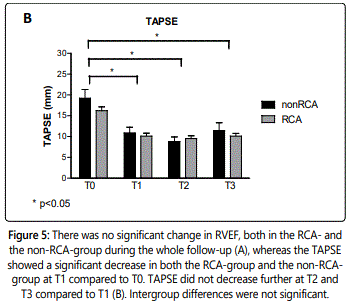
TAPSE decreased significantly in the RCA- as well as the non-RCA-group at T1 compared to T0 (RCA: 16±5mm at T0 to 10±4mm at T1, p<0.001; non-RCA: 20±5mm at T0 to 11±3mm at T1, p=0.003). TAPSE remained reduced, but did not decrease further at T2 and T3 compared to T1 in either the RCA- or the non-RCA-group (RCA: 10± 4mm at T2 and 10± 3mm at T3, both p-values: 1.0; non-RCA: 9±3mm at T2 and 11±3mm at T3, both p-values: 1.0) (figure 5B). Intergroup differences were not significant (p: 0.08 – 0.64) (table 1).
Discussion
This CMR study showed a significant decrease of TAPSE within 4 months after CABG which remained decreased during the whole long-term follow-up, even though the RVEF and the RV volumes remained unchanged after CABG. Whether the RCA was involved in the revascularization or not did not influence the outcome.
Prior studies have shown conflicting findings about the changes in RV parameters after CABG. An impairment of TAPSE was reported by some of them [6-8,11,19-21]. For example, Unsworth et al reported echocardiographic findings of a decreased longitudinal systolic right ventricular function after CABG, while the RVEF did not significantly change [9]. The impaired TAPSE together with the unchanged RVEF, as we also found in our study, suggests postoperative pericardial adhesion of the RV free wall [6,11,20-23]. Which can occur after partial removal of the parietal pericardium during open heart surgery [11]. Therefore RV systolic function visually seems to be impaired. It is believed that the paradoxical anterior motion of the ventricular septum during systole towards the RV, found after cardiac surgery, may be responsible for the preserved RVEF [6-8,11,13,19,23-25]. The effects of CABG on the RVEF are not entirely clear. While some studies reported an unchanged RVEF after CABG [9,26], others found a significant decrease in the RVEF [13,27-31]. Furthermore, the decreased RVEF found by Stein et al returned towards baseline values after manipulation with pharmacotherapy [31], and Joshi et al as well as Schirmer et al reported an increase in RVEF after CABG [32,33].
The controversies between the aforementioned studies may be caused by differences in techniques used to assess RV function. Due to its high accuracy and reproducibility, cardiovascular MRI has become the tool of choice for non-invasive assessment of cardiac function [1,10,16,34-36], with superior reproducibility in comparison to echocardiography [34]. In clinical practice however, 2D echocardiography still remains the primary imaging modality for assessing ventricular function. A simple and easily obtained echocardiographic parameter that reflects the function of the RV is the measurement of the TAPSE [10,12,26,37,38]. Longitudinal excursion (as reflected by TAPSE) plays a dominant role in RV systolic function [39]. Thus TAPSE is regarded as a validated indicator of right ventricular function, and has been correlated with RVEF assessed by CMR and radionuclide angiography [1,10,12,37].
In patients who underwent CABG, however, the longitudinal excursion of the RV might be reduced postoperatively and thus relying only on measuring the TAPSE may lead to an underestimation of the actual RV systolic function. Since RV function is a major determinant of quality of life in patients with cardiovascular diseases, measurement of TAPSE after CABG [26] should therefore be carefully interpreted.
Little is known about the long-term effect of CABG on the RV systolic function. The follow-up of the present study (T3: 32±11 months) is, to the best of our knowledge, the longest published so far. Previous studies with follow-up periods of 6-12 months showed conflicting results: some of them demonstrated no further changes [7,8], while others reported alterations in RV systolic function during the follow-up [6,33]. For example, Pegg et al reported a significant decreased RVEF after CABG, which recovered to preoperative levels in 6 months follow-up [13]. However, we could not find any further changes in the RV volumes and ejection fraction, nor a recovery of the decreased TAPSE during the whole follow-up after CABG.
Various explanations of the reported changes in the function of the RV found after CABG have been suggested. Revascularization of a specific coronary artery, for example, was reported in some studies to be the cause of significant differences in RV function after CABG [9,27-32]. Nevertheless, in the present study, revascularization of the RCA did not have any influence on the outcome, which is in agreement with findings from other studies [7,8,13,20,31].
However, our study consisted of 34 patients only. Therefore, lack of significant differences may also be caused by this small sample size, in particular the non-RCA-group. Not every patient had follow-up CMR at all 3 time points. Six out of 7 patients of the non-RCA-group had a CMR at T2, and only 3 patients had CMR at T3, compared to respectively 26 and 24 patients in the RCA-group (table 1). Further assessment in larger cohorts is required to ascertain possible differences in RV volumes and function due to revascularization of the proximal RCA.
The first follow-up measurement was approximately 4 months after CABG, which is longer than the majority of studies. Previous studies reported RVEF changes within the first 6 hours to 6 days after CABG [13,27,29,40]. Since we determined the RV systolic function 4 months after CABG, transient changes immediately postoperatively have not been measured and are therefore unknown. Similarly, the interval between T2 (7±1 months) and T3 (32±11 months) was approximately two years. Transient changes in this interval could not be assessed as well.
Susceptibility artefacts caused by sternal wires after CABG may hamper accurate RV analysis. Previous studies reported good inter-observer variability when artefacts are absent [10,12,36]. In our study there were only minor susceptibility artefacts from sternal wires in the RV region which did not interfere with accurate measurement. Lastly, many variables may influence changes in RV function after CABG, such as the use of a cardiopulmonary bypass [9,13,19,29-33,41], varying surgical procedures [11,21,22,26] and the effects of the sternotomy itself [9,20]. Future studies are needed to assess all variables influencing RV functional changes after CABG.
Conclusion
The present study shows that CABG does not influence RV systolic function, even after long-term follow-up. In contrast to the RVEF, TAPSE showed a significant decrease after CABG, implying that TAPSE underestimates the actual RV systolic function after CABG. This suggests that TAPSE alone might be an unreliable parameter for determination of right ventricular systolic function after CABG.
Acknowledgements
We would like to thank the participating staff that was involved in acquiring the data and processing the results, but most of all the patients that participated in the trial. No grants were received for this study.
References
- Alpendurada4 F, Guha K, Sharma R, et al. Right ventricular dysfunction is a predictor of non-response and clinical outcome following cardiac resynchronization therapy. J Cardiovasc Magn Reson. 2011; 13: 68. doi: 10.1186/1532-429X-13-68
- Bleasdale RA, Frenneaux MP. Prognostic importance of right ventricular dysfunction. Heart. 2002; 88(4): 323-4.
- De Groote P, Millaire A, Foucher-Hossein C, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998; 32: 948-954. doi: 10.1016/S0735-1097(98)00337-4
- Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995; 25(5): 1143-1153. doi: 10.1016/0735-1097(94)00511-N
- Field ME, Solomon SD, Lewis EF, Kramer DB, Baughman KL, Stevenson LW, et al. Right ventricular dysfunction and adverse outcome in patients with advanced heart failure. J Card Fail. 2006; 12(8): 616-620. doi: 10.1016/j.cardfail.2006.06.472
- Alam M, Hedman A, Nordlander R, Samad B. Right ventricular function before and after an uncomplicated coronary artery bypass graft as assessed by pulsed wave Doppler tissue imaging of the tricuspid annulus. Am Heart J. 2003; 146(3): 520-526. doi: 10.1016/S0002-8703(03)00313-2
- Hedman A, Alam M, Zuber E, Nordlander R, Samad BA. Decreased right ventricular function after coronary artery bypass grafting and its relation to exercise capacity: a tricuspid annular motion-based study. J Am Soc Echocardiogr. 2004; 17(2): 126-131. doi: 10.1016/j.echo.2003.10.023
- Roshanali F, Yousefnia MA, Mandegar MH, Rayatzadeh H, Alinejad S. Decreased right ventricular function after coronary artery bypass grafting. Tex Heart Inst J. 2008; 35(3): 250-5.
- Unsworth B, Casula RP, Kyriacou AA, et al. The right ventricular annular velocity reduction caused by coronary artery bypass graft surgery occurs at the moment of pericardial incision. Am Heart J. 2010; 159: 314-322. doi: 10.1016/j.ahj.2009.11.013
- Kjaergaard J, Petersen CL, Kjaer A, Schaadt BK, Oh JK, Hassager C. Evaluation of right ventricular volume and function by 2D and 3D echocardiography compared to MRI. Eur J Echocardiogr. 2006; 7(6): 430-438. doi: 10.1016/j.euje.2005.10.009
- Larrazet F, Czitrom D, Laborde F, Bouabdallah K, Folliguet T. Decreased right ventricular lateral wall velocities early after cardiac surgery. Echocardiography. 2011; 28(4): 438-441. doi: 10.1111/j.1540-8175.2010.01355.x
- Nijveldt R, Germans T, McCann GP, Beek AM, van Rossum AC. Semiquantitative assessment of right ventricular function in comparison to a 3D volumetric approach: a cardiovascular magnetic resonance study. Eur Radiol. 2008; 18(11): 2399-2405. doi: 10.1007/s00330-008-1017-7
- Pegg TJ, Selvanayagam JB, Karamitsos TD, et al. Effects of off-pump versus on-pump coronary artery bypass grafting on early and late right ventricular function. Circulation. 2008; 117(17): 2202-2210. doi: 10.1161/CIRCULATIONAHA.107.735621
- Pennell DJ. Cardiovascular magnetic resonance: twenty-first century solutions in cardiology. Clin Med (Lond). 2003; 3(3): 273-8.
- Hudsmith LE PS, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005; 7: 775-82.
- Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J. 2004; 25(21): 1940-1965. doi: 10.1016/j.ehj.2004.06.040
- Catalano O, Corsi C, Antonaci S, et al. Improved reproducibility of right ventricular volumes and function estimation from cardiac magnetic resonance images using level-set models. Magn Reson Med. 2007; 57: 600-605. doi: 10.1002/mrm.21157
- Corsi C LC, Catalano O et al. Improved quantification of left ventricular volumes and mass based on endocardial and epicardial surface detection from cardiac MR images using level set models. J Cardiovasc Magn Reson. 2005(7): 595-602.
- Michaux I, Filipovic M, Skarvan K, et al. A randomized comparison of right ventricular function after on-pump versus off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2011; 141(2): 361-367. doi: 10.1016/j.jtcvs.2010.02.023
- Mishra M, Swaminathan M, Malhotra R, Mishra A, Trehan N. Evaluation of Right Ventricular Function During CABG: Transesophageal Echocardiographic Assessment of Hepatic Venous Flow Versus Conventional Right Ventricular Performance Indices. Echocardiography. 1998; 15(1): 515-8. doi: 10.1111/j.1540-8175.1998.tb00577.x
- David JS, Tousignant CP, Bowry R. Tricuspid annular velocity in patients undergoing cardiac operation using transesophageal echocardiography. J Am Soc Echocardiogr. 2006; 19: 329-334. doi: 10.1016/j.echo.2005.09.013
- Wranne B, Pinto FJ, Hammarstrom E, St Goar FG, Puryear J, Popp RL. Abnormal right heart filling after cardiac surgery: time course and mechanisms. Br Heart J. 1991; 66(6): 435-42.
- Joshi SB, Salah AK, Mendoza DD, Goldstein SA, Fuisz AR, Lindsay J. Mechanism of paradoxical ventricular septal motion after coronary artery bypass grafting. Am J Cardiol. 2009; 103(2): 212-5. doi: 10.1016/j.amjcard.2008.08.067
- Santamore WP, DellʼItalia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. 1998; 40(4): 289-308.
- Toyoda T, Akasaka T, Watanabe N, et al. Evaluation of abnormal motion of interventricular septum after coronary artery bypass grafting operation: assessment by ultrasonic strain rate imaging. J Am Soc Echocardiogr. 2004; 17(7): 711-716. doi: 10.1016/j.echo.2004.03.033
- Tamborini G, Muratori M, Brusoni D, et al. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr. 2009; 10: 630-634. doi: 10.1093/ejechocard/jep015
- Bastien O, Durand PG, George M, Gurbala A, Estanove S. Evolution of right ventricular performance after CABG. Intensive Care Med. 1988; 14: 499-502. doi:10.1007/BF00256970
- Boldt J, Kling D, Thiel A, Scheld HH, Hempelmann G. Revascularization of the right coronary artery: influence on thermodilution right ventricular ejection fraction. J Cardiothorac Anesth. 1988; 2(2): 140-146. doi: 10.1016/0888-6296(88)90263-31. Alpendurada F GK, Sharma R et al. Right
- Durand M CO, Tessier Y et al. Right ventricular function after coronary surgery with or without bypass. J Card Surg. 2006; 21: 11-6. doi: 10.1111/j.1540-8191.2006.00161.x
- Kwak YL, Oh YJ, Jung SM, Yoo KJ, Lee JH, Hong YW. Change in right ventricular function during off-pump coronary artery bypass graft surgery. Eur J Cardiothorac Surg. 2004; 25(4): 572-7. doi: 10.1016/j.ejcts.2004.01.005
- Stein KL, Breisblatt W, Wolfe C, Gasior T, Hardesty R. Depression and recovery of right ventricular function after cardiopulmonary bypass. Crit Care Med. 1990; 18(11): 1197-200.
- Schirmer U CE, Lindner KH, Hemmer W, Georgieff M. Right ventricular function after coronary artery bypass grafting in patients with and without revascularization of the right coronary artery. J Cardiothorac Vasc Anesth. 1995; 9: 659-64. doi: 10.1016/S1053-0770(05)80226-5
- Joshi SB, Roswell RO, Salah AK, Zeman PR, Corso PJ, Lindsay J, et al. Right ventricular function after coronary artery bypass graft surgery--a magnetic resonance imaging study. Cardiovasc Revasc Med. 2010; 11(2): 98-100. doi: 10.1016/j.carrev.2009.04.002
- Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with twodimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002; 90(1): 29-34. doi.org/10.1016/S0002-9149(02)02381-0
- Longmore DB, Klipstein RH, Underwood SR, et al. Dimensional accuracy of magnetic resonance in studies of the heart. Lancet. 1985; 1(8442): 1360-2. doi: 10.1016/S0140-6736(85)91786-6
- Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004; 147(2): 218-23. doi: 10.1016/j.ahj.2003.10.005
- Kaul S TC, Hopkins JM, Shah PM. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J . 1984; 107: 526-31.
- Rudski LG, Lai WW, Afilalo J, Hua L, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010; 23(7): 685-713. doi : 10.1016/j.echo.2010.05.010
- Hammarstrom E, Wranne B, Pinto FJ, Puryear J, Popp RL. Tricuspid annular motion. J Am Soc Echocardiogr. 1991; 4(2): 131-9. doi: 10.1016/S0894-7317(14)80524-5
- Honkonen EL, Kaukinen L, Pehkonen EJ, Kaukinen S. Myocardial cooling and right ventricular function in patients with right coronary artery disease: antegrade vs. retrograde cardioplegia. Acta Anaesthesiol Scand. 1997; 41(2): 287-96. doi: 10.1111/j.1399-6576.1997.tb04681.x
- Michaux I, Filipovic M, Skarvan K, Schneiter S, Schumann R, Zerkowski HR, et al. Effects of on-pump versus off-pump coronary artery bypass graft surgery on right ventricular function. J Thorac Cardiovasc Surg. 2006; 131(6): 1281-8. doi: 10.1016/j.jtcvs.2006.01.035


