Research Article
Serum Folate concentration and Coronary Heart Disease prevalence: Findings from the 1999-2012 National Health and Nutrition Examination Survey
Luohu Population & Family Planning Service Centre, China
*Corresponding author: Sifan Cao, Luohu Population & Family Planning Service Centre, Shenzhen, Peopleʼs Republic of China, E-mail: happy_csf@msn.com
Received: September 6, 2016 Accepted: September 19, 2016 Published: September 22, 2016
Citation: Cao S. Serum Folate concentration and Coronary Heart Disease prevalence: Findings from the 1999-2012 National Health and Nutrition Examination Survey. Madridge J Cardiol. 2016: 1(1): 1-4. doi: 10.18689/mjc-1000101
Copyright: © 2016 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective: Relationship between folate level and coronary heart disease (CHD) risk is still inconclusive. The current study is to explore the association between serum folate concentration and CHD prevalence.
Methods: A cross-sectional study was performed utilizing the 1999-2012 National Health and Nutrition Examination Survey (NHANES). Firstly, logistic regression analysis was applied to explore the association between folate status, as a category variable, and CHD prevalence, and odds ratios (ORs) along with 95% confidence intervals (CIs) were computed to measure the association strengths. Secondly, a combination of restricted cubic spline and logistic regression analysis was used to illustrate the non-linear association between folate level, as a continuous variable, and CHD prevalence, and OR curve was drawn to display the relationship.
Results: 33577 participants were included, with 1391 (4.14%) CHD patients. Persons in the highest tertile of serum folate concentration have elevated risk of CHD prevalence compared to those in the lowest tertile (adjusted OR: 1.20; 95% CI: 1.04-1.37, P=0.01). The OR curve also demonstrated a positive association between serum folate concentration and CHD prevalence.
Conclusions: The current study revealed that high serum folate concentrations are associated with an elevated CHD prevalence.
Abbreviations: CHD: Coronary Heart Disease; NHANES: National Health and Nutrition Examination Survey; NCHS: National Center for Health Statistics; BR: Bio-Rad; MA: Microbiologic Assay; LC-MS: Liquid Chromatography-Mass Spectrometry; MeFox: 4-α-hydroxy-5-Methyltetrahydrofolate; HDL: High-Density Lipoprotein; BP: Blood Pressure; HbA1c: Glycated Hemoglobin; FPG: Fasting Plasma Glucose; OR: Odds Ratio; CI: Confidence Interval; CVD: Cardiovascular Disease; 5-MTHF: 5-Methylenetetrahydrofolate.
Keywords: Folate; Coronary heart disease; Cross-sectional.
Introduction
Coronary heart disease (CHD) is still a significant contributor to mortality [1,2] and it has a prevalence of over 10% in the United States [3]. Previous studies have established several CHD risk factors, including age, gender, blood pressure, lipid profiles, diabetes, and smoking [4-6]. Recently, several investigations have shifted to the role of biomarkers on CHD risk [7-9]. A number of studies have focused on the association between folate, a popular biomarker, and CHD risk [10-15]. Nevertheless, the magnitude of the association is still unclear due to inconsistent findings. Therefore, the current cross-sectional study was conducted based on a large U.S. nationally representative sample, 1999-2012 National Health and Nutritional Examination Survey (NHANES), aiming to explore the relationship between serum folate concentration and CHD prevalence.
Methods
Study Population
Data were retrieved from the public available files of NHANES. NHANES was conducted by the National Center for Health Statistics (NCHS) and used a stratified, multistage probability design to obtain a national representative sample of the U.S. general population. This study was restricted to adults (≥ 20 years) who were included in NHANES during 1999 to 2012 (n=38024). Those with missing information on serum levels of folate and/or CHD status were excluded (n=4447). The NCHS Research Ethics Review Board reviewed and approved NHANES, and all participants provided written informed consent.
Serum Folate Concentrations
Three different laboratory methods were used to measure serum folate levels in the current study. The Bio-Rad (BR) radio assay, microbiologic assay (MA) and liquid chromatography–mass spectrometry (LC-MS) were used during 1999-2006, 2007-2010, and 2011-2012, respectively. As recommended by NHANES protocol, the BR and LC-MS values were converted to equivalent MA values using the following equation.
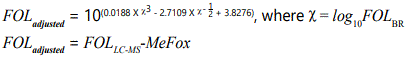
FOLBR and FOLLC-MS refer to the values obtained from BR and LC-MS assays, and FOLadjusted refers to the adjusted equivalent MA values. MeFox refers to the values of 4-α-hydroxy-5-methyltetrahydrofolate.
CHD Prevalence
Participants were asked whether they had ever been told by a doctor or other health professional that they had CHD. Prevalence was calculated as the number of people who had CHD divided by the total number of participants.
Covariates
The 10-year CHD risk predictors identified in Framingham Heart Study were included as covariates and adjusted in multivariate analysis, which included age, gender, diabetes, smoking, total cholesterol, high-density lipoprotein (HDL), and blood pressure (BP) [16]. Age was categorized as 20-39 years, 40-59 years and 60 years or over. Diabetes was defined as any participant who had a glycated hemoglobin (HbA1c) level≥6.5%, a fasting plasma glucose (FPG) level ≥126 mg/dL, or had ever been told by a doctor or other health professional that he/she had diabetes [17]. Smoking status was determined by self-reported current smoking. Total cholesterol and HDL levels were categorized as ideal (total cholesterol: <200 mg/dL; HDL: ≥60 mg/dL), intermediate (total cholesterol: 200-239 mg/dL; HDL: 40-<60 mg/dL) and poor (total cholesterol: ≥240 mg/dL; HDL: <40 mg/dL) [18]. Blood pressure status was divided into normal (systolic BP<120 mm Hg and diastolic BP<80 mm Hg), prehypertension (systolic BP 120-139 mm Hg or diastolic BP 80-89 mm Hg) and hypertension (systolic BP≥140 mm Hg or diastolic BP ≥90 mm Hg) [19].
Statistical Analysis
Mean ± standard deviation and number (percentage) were used to display continuous variables and categorical variables, respectively.
Firstly, univariate and multivariate logistic regression analyses were applied and odds ratios (ORs) and 95% confidence intervals (CIs) were obtained to explore the relationships between serum folate concentration and CHD prevalence. Serum folate concentration was divided into tertiles and the first tertile was used as the reference group.
Secondly, restricted cubic spline in logistic regression analysis was used to develop OR curve and examine the possible nonlinear dose-response association between serum folate concentration and CHD prevalence. Folate status was treated as a continuous variable with four knots (5th, 35th, 65th and 95th) suggested by Harrell [20]. The covariates were also adjusted and the median value of serum folate concentration, 36 nmol/L, was chosen as the reference point [21].
All statistical tests were conducted with Stata software, version 14.1 (Stata Corporation, College Station, Texas). A twosided P value < 0.05 was considered statistically significant.
Results & Discussions
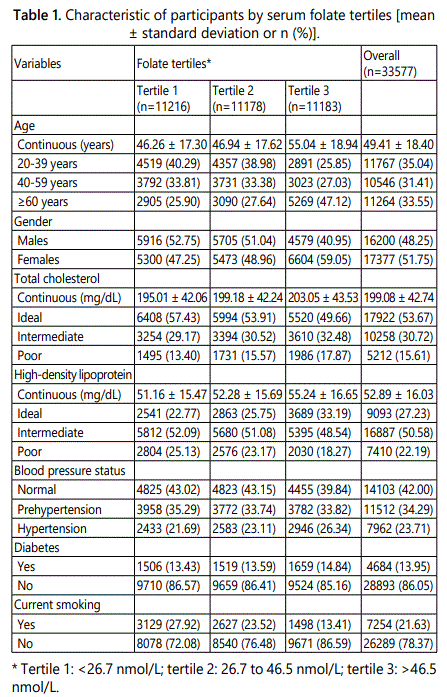
Finally, 33577 participants were included and 1391 (4.14%) self-reported CHD were documented. The characteristics of the subjects are presented in Table 1.
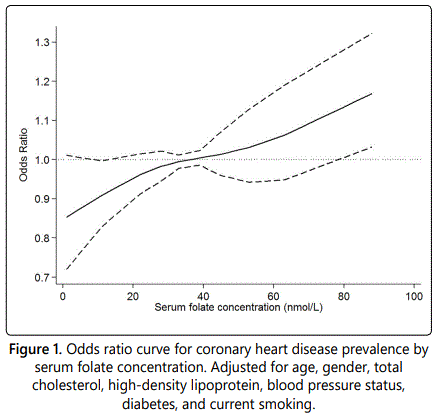
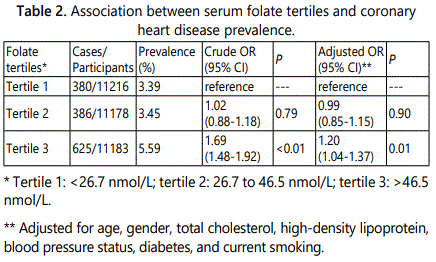
In the univariate analysis, those with a serum folate concentration in the third tertiles were linked to 69% greater risk of CHD compared to those from the reference group (OR: 1.69; 95% CI: 1.48-1.92, P<0.01). Adjustment of potential confounders did not alter the association (OR: 1.20; 95% CI: 1.04-1.37, P=0.01) (Table 2). The results of the association between folate status, as a continuous variable, and CHD prevalence are displayed in Figure 1. The risk of CHD was increased with the raise of serum folate levels, although the upward trend was slightly attenuated around 30 to 40 nmol/L. Thus, the findings of the nonlinear association were generally consistent with those in the tertiles analysis.
The adverse heart health effects,caused by excess folate levels, were also observed in other studies, which are in agreement with the findings of this study. A recent study has found 31% and 51% increased risk of CHD among males and females with elevated folate levels (serum folate concentration ≥ 49.4 nmol/L) [22]. A large U.S. cohort observed a positive relationship between serum folate levels and cardiovascular disease (CVD) mortality among participants with diabetes [23]. The phenomenon can be explained by several possible mechanisms. One theory is that excess folate concentrations may lead to significant increase in the levels of non-bound folate, which will accelerate the degradation rate of folate [24]. Another is that high folate levels could reduce the levels of thymidylate and 5-methylenetetrahydrofolate (5-MTHF), thus impairing DNA integrity and affecting protein production [25]. However, some studies have displayed an inverse association between folate levels and CHD risk. A Canadian cohort has found that persons with lowest folate concentration have 69% additional risk of CHD mortality (rate ratio: 1.69; 95% CI: 1.10-2.61) in comparison to those with highest folate levels [10]. And a U.S. cohort has revealed who have folate level in the lowest tertile, compared with those in the highest tertile, have more than a 2-fold raised risk of dying from CVD. What is interesting, some studies have displayed the possible nonlinear association between folate and CHD. A U.S. study has reported a U-shaped relationship between serum folate concentration and circulatory disease incidence [26]. While, an Australian cohort has displayed a reversed U-shaped correlation pattern between folate level and CHD mortality [15]. Therefore, the true relationship of serum folate concentration and CHD risk is quite complicated and need further explorations.
The current study has several strengths. Firstly, it was based on a large national representative sample to assessing the relationship between folate level and CHD prevalence. Secondly, the variables that were recognized as CHD predictors were adjusted in the analysis, so as to eliminating their effects on CHD risk. There are some limitations to this study as well. One major concern is about the cross-sectional study design, which prevented the current study to demonstrate temporality between the folate level and CHD incidence and/or mortality. Another concern is the outcome and some covariates were self-reported and thus were not verified. Moreover, we do not take some diseases and conditions into consideration and they may have an influence on the folate-CHD association.
Conclusions
Using a large-scale U.S. national representative sample, the present study revealed that higher serum folate concentration was associated with significant higher CHD prevalence. The findings need to be confirmed by further investigations.
Acknowledgments
I gratefully acknowledge the staff at the National Center for Health Statistics who were responsible for planning and conducting the National Health and Nutrition Examination Survey.
Conflicts of Interest: The author reports no conflict of interest.
References
- Papandreou C, Tuomilehto H. Coronary heart disease mortality in relation to dietary, lifestyle and biochemical risk factors in the countries of the Seven Countries Study: a secondary dataset analysis. J Hum Nutr Diet. 2014; 27(2): 168-175. doi: 10.1111/jhn.12187
- Saito I, Yamagishi K, Kokubo Y, et al. Association between mortality and incidence rates of coronary heart disease and stroke: The Japan Public Health Center-based prospective (JPHC) study. Int J Cardiol. 2016; 222: 281-286. doi: 10.1016/j.ijcard.2016.07.222.
- Zhu KF, Wang YM, Zhu JZ, Zhou QY, Wang NF. National prevalence of coronary heart disease and its relationship with human development index: A systematic review. Eur J Prev Cardiol. 2016; 23(5): 530-543. doi: 10.1177/2047487315587402.
- Batty GD, Shipley M, Smith GD, Kivimaki M. Long term risk factors for coronary heart disease and stroke: influence of duration of follow-up over four decades of mortality surveillance. Eur J Prev Cardiol. 2015; 22(9): 1139-1145. doi: 10.1177/2047487314547659.
- Koopman C, Vaartjes I, Blokstra A, et al. Trends in risk factors for coronary heart disease in the Netherlands. BMC Public Health. 2016; 16(1): 835. doi: 10.1186/s12889-016-3526-7.
- Shah T, Palaskas N, Ahmed A. An Update on Gender Disparities in Coronary Heart Disease Care. Curr Atheroscler Rep. 2016; 18(5): 28. doi: 10.1007/s11883-016-0574-5.
- Carty CL, Kooperberg C, Liu J, et al. Leukocyte Telomere Length and Risks of Incident Coronary Heart Disease and Mortality in a Racially Diverse Population of Postmenopausal Women. Arterioscler Thromb Vasc Biol. 2015; 35(10): 2225-2231. doi: 10.1161/ATVBAHA.115.305838.
- Braga F, Pasqualetti S, Ferraro S, Panteghini M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta-analysis. Clin Chem Lab Med. 2016; 54(1): 7-15. doi: 10.1515/cclm-2015-0523.
- Salim A, Tai ES, Tan VY, et al. C-reactive protein and serum creatinine, but not haemoglobin A1c, are independent predictors of coronary heart disease risk in non-diabetic Chinese. Eur J Prev Cardiol. 2016; 23(12): 1339-1349. doi: 10.1177/2047487315626547.
- Morrison HI, Schaubel D, Desmeules M, Wigle DT. Serum folate and risk of fatal coronary heart disease. JAMA. 1996; 275(24): 1893-1896. doi: 10.1001/jama.1996.03530480035037.
- Folsom AR, Nieto FJ, McGovern PG, et al. Prospective study of coronary heart disease incidence in relation to fasting total homocysteine, related genetic polymorphisms, and B vitamins: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 1998; 98(3): 204-210. doi: http://dx.doi.org/10.1161/01.CIR.98.3.204.
- Giles WH, Kittner SJ, Croft JB, Anda RF, Casper ML, Ford ES. Serum folate and risk for coronary heart disease: results from a cohort of US adults. Ann Epidemiol. 1998; 8(8): 490-496. http://dx.doi.org/10.1016/S1047-2797(98)00027-1
- Loria CM, Ingram DD, Feldman JJ, Wright JD, Madans JH. Serum folate and cardiovascular disease mortality among US men and women. Arch Intern Med. 2000; 160(21): 3258-3262. doi: 10.1001/archinte.160.21.3258.
- Hung J, Beilby JP, Knuiman MW, Divitini M. Folate and vitamin B-12 and risk of fatal cardiovascular disease: cohort study from Busselton, Western Australia. BMJ. 2003; 326(7381): 131. doi: http://dx.doi.org/10.1136/bmj.326.7381.131
- Gopinath B, Flood VM, Rochtchina E, Thiagalingam A, Mitchell P. Serum homocysteine and folate but not vitamin B12 are predictors of CHD mortality in older adults. Eur J Prev Cardiol. 2012; 19(6): 1420-1429. doi: 10.1177/1741826711424568
- Wilson PW, DʼAgostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97(18): 1837-1847. doi: http://dx.doi.org/10.1161/01.CIR.97.18.1837
- Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. JAMA. 2015; 314(10): 1021-1029. doi: 10.1001/jama.2015.10029.
- National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002; 106(25): 3143-3421.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003; 42(6): 1206-1252. doi: 10.1161/01.HYP.0000107251.49515.c2
- OʼDonnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011; 306(20): 2229-2238. doi: 10.1001/jama.2011.1729.
- Vanderwall CM, Tangney CC, Kwasny MJ, Gustashaw KA. Examination of circulating folate levels as a reflection of folate intakes among older adult supplement users and nonusers in the National Health and Nutrition Examination Survey 2003-2004. J Acad Nutr Diet. 2012; 112(2): 285-290. doi: 10.1016/j.jada.2011.10.001.
- Afriyie-Gyawu E, Ifebi E, Ampofo-Yeboah A, Kyte B, Shrestha S, Zhang J. Serum folate levels and fatality among diabetic adults: A 15-y follow-up study of a national cohort. Nutrition. 2016; 32(4): 468-473. doi: 10.1016/j.nut.2015.10.021.
- Oleinik NV, Krupenko NI, Reuland SN, Krupenko SA. Leucovorin-induced resistance against FDH growth suppressor effects occurs through DHFR up-regulation. Biochem Pharmacol. 2006; 72(2): 256-266. doi: 10.1016/j.bcp.2006.04.005
- Sauer J, Mason JB, Choi SW. Too much folate: a risk factor for cancer and cardiovascular disease? Curr Opin Clin Nutr Metab Care. 2009; 12(1): 30-36. doi: 10.1097/MCO.0b013e32831cec62


