Research Article
Effect of Clodronate Administration on the Structure of the Primary Spongiosa derived from the Growth Cartilage in Growing Rats
1Pediatric Nephrology, Department of Pediatrics, Hospital Universitario Central de Asturias (HUCA), Oviedo, Asturias, Spain
2Division of Pediatrics, Department of Medicine, Faculty of Medicine, University of Oviedo, CP 33006 Oviedo, Asturias, Spain
3Fundacion para la Investigación Sanitaria del Principado de Asturias (FINBA), Oviedo, Spain
4Department of Developmental Biology, Harvard School of Dental Medicine, Harvard University, Boston, Massachusetts, USA
5Department of Morphology and Cellular Biology, Faculty of Medicine, University of Oviedo, Oviedo, Asturias, Spain
*Corresponding author: José M López, Professor, Department of Morphology and Cell Biology, School of Medicine, University of Oviedo, Spain, Tel: +34985103064, E-mail: jmlopez@uniovi.es
Received: October 21, 2019 Accepted: November 05, 2019 Published: November 13, 2019
Citation: Gil-Peña H, Fernández-Iglesias A, Fuente R, Alonso-Duran L, Santos F, López JM. Effect of Clodronate Administration on the Structure of the Primary Spongiosa derived from the Growth Cartilage in Growing Rats. Int J Biochem Pharmacol. 2019; 2(1): 27-35. doi: 10.18689/ijbp-1000106
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The effect of the inhibition of the resorptive activity of osteoclastic cells induced by bisphosphonate treatment on the primary spongiosa derived from the calcified cartilage of the growth plate was studied. We focused our attention on the primary spongiosa because it is the initial trabecular bone network that is first formed directly from growth plate mineralized cartilaginous septa. The study was carried out in male Sprague-Dawley rats at the age of 35 days, coinciding with the prepubertal growth spurt, a stage characterized by the highest values for growth rate. Animals were classified in two groups, controls and rats treated with clodronate 60 mg/kg/day. Body weights and tibial length were measured. The rate of longitudinal bone growth was obtained by calceine labelling and the height of the growth plate cartilage was measured. Histochemical analysis included Alcian blue staining, detection of tartrate-resistant acid phosphatise (TRAP) activity, von Kossa staining for mineralization and immunolocalization of proliferating cells. Microscopic examination revealed numerous tartrate-resistant acid phosphatase (TRAP)-positive cells at the chondroosseous junction and associated with subchondral trabeculae in control rat and that clodronate treatment induced a marked reduction of these cells. Clodronate-treated rats presented thinner subchondral trabeculae that were more irregularly oriented and decreased cell proliferation in the primary spongiosa. Results obtained showed that changes induced by clodronate treatment has little effect on the activity of the growth plate cartilage, without a significant effect on longitudinal bone growth even at doses much higher than those used in clinical practice.
Keywords: Growth Plate; Primary Spongiosa; Chondrocytes; Clodronate.
Introduction
The longitudinal growth of bones is the result of the action of the growth plate cartilage, a layer of cartilage located between the epiphysis and metaphysis of long bones. Growth plate activity leads to a continual generation of cartilage that acts as a scaffold for differentiation and growth of the osseous tissue [1,2]. A specific feature of chondrogenesis in the growth plate is that chondrocytes become aligned into longitudinal columns. At the uppermost position, on the zone adjacent to the epiphysis, chondrocytes are irregularly scattered in the cartilage matrix. This layer is narrow and irregularly contoured and is referred to as the resting zone. Chondrocytes of the resting zone are rounded, small and exhibit a low proliferative rate. Subsequently, chondrocytes become flattened, enter the cell cycle and undergo active cell division in a manner that daughter chondrocytes from a cell division lie one above the other and this lead to the formation of columns parallel to the long axis of the bone. This layer is referred to as the proliferating zone and actively contributes to elongation of the columns and of the whole growth plate. After a number of cell divisions, chondrocytes exit the cell cycle and begin to increase in cell volume up to ten times, a process that also contributes to the elongation of the columns and of the whole growth plate. Chondrocytes of this hypertrophic zone undergo terminal differentiation that involves secretion of signaling molecules influencing the behavior of endothelial cells, osteoclasts and osteoblasts. Finally, hypertrophic chondrocytes are replaced by blood vessels and osteoblasts, which then form osseous trabeculae at the metaphyseal end. Thus, cartilage increase is balanced with a coordinated process of cartilage resorption and replacement by cancellous bone at the diaphyseal side. As a result, the width of the growth plate remains basically constant while the amount of metaphyseal osseous tissue is continuously increasing [3].
An essential process for endochondral ossification is the controlled degradation of the cartilage to allow deposition of bone matrix by osteoblasts [4]. Multi-nucleated cells resembling osteoclast-like giant cells are present at the chondroosseous junction associated with the tips of in growing capillary sprouts. The invasion of the ossification front requires an extensive but selective degradation of cartilage matrix. Terminal transverse septum of growth plate cartilage are degraded to allow capillaries to bud into the lower hypertrophic zone but the vertical walls that divide the chondrocyte columns are preserved and form the longitudinal mineralized cartilage septa that comprise the substrate onto which osteoblasts deposit the bone of the primary spongiosa. Thus, an accurate matrix degradation is necessary for the formation of the primary bone trabeculae. Because of their evident role in cartilage resorption, multinucleated cells at chondroosseous junction are named for some authors chondroclasts although other consider them as osteoclasts since they express cellular markers of the osteoclast-like phenotype, like TRAP+, cathepsin K+, MMP9+, CD14-,CD51+, HLA-DR-, CD45+, and CD68+ [5].
The bisphosphonates are a class of chemicals that strongly inhibit osteoclast-mediated bone resorption and are effective for preventing and treating skeletal disorders associated with hyper resorption and bone loss. Overall, these drugs have been used for treatment of bone disorders in adults and have shown to have a strong safety and tolerability profile [6]. The use of bisphosphonate therapy in the pediatric population was introduced in 1998 to treat children with severe osteogenesis imperfecta and resulted in reduction in bone resorption, increase in bone density, and reduction in fracture incidence [7]. These drugs were subsequently considered for treatment of other pathologies like steroid-induced osteoporosis and hypercalciuria. As bone in children is a growing tissue their effect could be different from adults. Due to their inhibitory effect on osteoclast activity, these drugs could affect the function of chondroclasts at chondro-osseous junction and could interfere with cartilage resorption and the formation of the first trabecular structures at the primary spongiosa. In this way, it is known that treatment with bisphosphonates gives rise to transverse radiopaque lines in the metaphyseal or diaphyseal part of long bones that are considered as a trace of a temporary bone growth arrest [7]. Furthermore, it has been reported that some compounds closely related to clodronate are avidly taken up in the region of the growth plate where cartilage is being replaced by bone [8]. Thus, it could be considered that bisphosphonate treatment could affect normal growth plate function. However, most of the studies on these drugs have been performed on adults and their effect on growth plate activity are little known since most studies of the effects of bisphosphonates on long bones are limited to traditional radiography [9].
In the present work, we focused our attention on the primary spongiosa because it is the initial trabecular bone network that is first formed directly from growth plate cartilage. This first trabecular structure is composed of cartilage bars and woven bone and is subsequently resorbed and replaced by lamellar bone to produce the secondary spongiosa. We analyzed the effect of treatment with clodronate because it is one of the best known bisphosphonates that has been largely and effectively used for treating many osteometabolic disorders characterized by excessive resorption. Clodronate is metabolized by osteoclasts and induces the production of toxic analogs of adenosine triphosphate (ATP) that accumulate intracellularly in these cells, resulting in induction of osteoclast apoptosis [10,11]. On this basis, we hypothesize that inhibition of the resorptive activity of osteoclast-like giant cells induced by bisphosphonate treatment may affect the normal formation of the primary spongiosa derived from calcified cartilage.
Materials and Methods
The study was carried out in male Sprague-Dawley rats obtained from the animal facility building of the University of Oviedo. Rats of 22 days of age were housed in individual cages under controlled conditions of light (12 light/dark cycles) and temperature (21-23°C) with free access to ratsʼ standard diet and tap water. Procedures involving animals and their care were conducted according to Spanish law on the use of experimental animals, which acknowledges the European Directive 86/609. All experiments were performed in accordance with the European Community guidelines for the care and use of laboratory animals (no. 07430) and were approved by the institutional Ethical Committee of the University of Oviedo, Spain (BOPA 47).
After 3 days of adaptation, the animals were classified in two groups of six rats each for treatment with clodronate or vehicle. The dose of clodronate 60 mg/kg/day dissolved in sterile saline and vehicle was sterile saline. They were administered subcutaneously daily, treatment starting on the day 25 and continuing until day 35, when animals were sacrificed under lethal dose of Dolethal® anaesthesia. The dose of clodronate, 60 mg/kg/day, was chosen on the basis of that of humans, 23 mg/kg mg daily by oral administration. Since the absorption of clodronate by oral administration is about 2.5%, the effective dose is about 0.57 mg/day [12]. Thus, 60 mg/kg/day is about ten times greater than that used for humans. The age of the rats (35 days) was chosen to coincide with the prepubertal growth spurt, a stage characterized by the highest values for growth rate. All animals received intraperitoneal injections of calceine (Sigma, Saint Louis, MO, USA), 15 mg/kg body weight, and 5-bromo-2´-deoxyuridine (BrdU) (Sigma, Saint Louis, MO, USA), 100 mg/kg body weight, four days and one hour before sacrifice, respectively.
Body weights were measured in all rats on the day they were killed. Tibiae were immediately isolated after death, measured with a sliding mechanical caliper (accuracy 10 mm) and cut through the sagittal plane of the epiphysis into two equal-sized parts, obtaining four tibial halves from each animal. Two tibial halves were embedded in paraffin to obtain sections which were used for histochemical studies. One tibial half was processed for mineralization studies and the last tibial half was processed to obtain semithin sections. Tissues for histochemical studies were fixed by immersion in 4% paraformaldehyde at 4°C for 12 hours, rinsed in PBS, decalcified in 10% EDTA (pH 7.0) for 48 hours at 4°C, dehydrated through a graded ethanol series, cleared in xylene and embedded in paraffin. Sections were cut at a thickness of 5 µm and mounted on Super frost Plus slides (Menzel-Glaser). Serial sections were used for trichrome staining, detection of tartrate-resistant acid phosphatase (TRAP) activity and localization of proliferating cells. Weigertʼs hematoxylin/alcian blue/picrofuchsin was used to distinguish cartilage matrix (blue) from bone matrix (red). TRAP activity was used as a marker of the osteoclast lineage [13] and was determined by incubation with 50 mM sodium acetate (pH 5.2) containing 0.15% Napthol-AS-TR-phosphate, 50 mM sodium tartrate, and 0.1% Fast Red T.R. (Sigma). Proliferating cells were visualized immunocytochemically. Sections were placed in 2 N HCl for 30 min at 37°C for DNA denaturation, washed and incubated with an anti-BrdU monoclonal antibody (1:20; Sigma). Immunostaining was then performed using a mouse ExtrAvidinR peroxidase staining kit (Sigma). For studies of matrix mineralization, tissues were fixed in 4% paraformaldehyde in PBS for 5 hours at 4°C, dehydrated in acetone, embedded in Durkupan-ACM (Sigma) and sectioned at 2 µm on a ReicherUltracut E ultramicrotome. The von Kossa staining was used to detect mineralization by setting sections in 1% AgNO3 for 60 min at room temperature and fixed with 5% sodium hyposulfite. Semi thin sections were obtained from tissues fixed in 2% glutaraldehyde and 0.7% ruthenium hexamine trichloride (RHT) (Strem Chemicals) in 0.05 M cacodylate buffer, pH 7.4, for 3 hours at 4°C. They were then post fixed in 1% osmium tetroxide and 0.7% RHT in cacodylate buffer for 2 hours. After washing, they were dehydrated with acetone, embedded in Durkupan-ACM (Sigma), sectioned at 0.5 µm on a ReicherUltracut E ultramicrotome and stained with toluidine blue.
Determination of the rate of longitudinal bone growth was obtained by measuring the distance between the zone of vascular invasion in the growth plate and the proximal end point of the fluorescent label of calceine. Measurements of the distance between the zone of vascular invasion into the growth plate and the proximal end point of the calceine front were obtained at four randomly determined locations on each of the three sections per animal, and the mean of these measurements divided by 4, the number of days between calceine injection and killing, was considered the longitudinal bone growth per day in each animal. Likewise, the height of the growth plate was estimated by measuring its height at four randomly chosen locations on each section.
Standard bone histomorphometric analyses [14] were performed on von Kossa stained sections by using Bioquant Image Analysis software (BioquantOsteo, Image Analysis Corporation, Nashville, USA). Trabecular bone micro architecture was evaluated in the metaphysis in a region that began proximal to the growth plate and extending 500 µm downward through the metaphysis of the tibia, thereby comprising the primary spongiosa. Trabecular bone parameters included trabecular bone volume fraction (BV/TV, %), endochondral cartilaginous matrix volume (CgV/TV, %), trabecular thickness (Tb.Th, mm), trabecular number (Tb.N, mm−1), trabecular spacing (Tb.Sp, mm), TRAP+ osteoclast-like cell number (Oc.N, mm) and percent surface (Oc.S, %). Finally, the number of proliferating cells (BrdU-labeled cells) in the primary spongiosa was counted and the total number was referred to the bone area (N.Pc/B.Ar, mm2). Experimental values are given as means ± standard error of the mean (SEM). The comparisons between the clodronate treated and control groups were analyzed with t-tests. Results were considered statistically significant if a p value was <0.05. Statistical analyses were performed using SPSS version 24.0.
Results
Mean body mass was similar in both treated and control rats (Table 1). Likewise, there was no significant difference in mean tibial length between control and clodronate-treated rats (Table 1). Furthermore, the treatment had no effect on the overall structure of the growth plate, the chondrocytes being organized into cell stacks orientated parallel to the vertical axis of the tibia as in the control animals, and there was no significant alteration in growth plate height, or proliferating or hypertrophic zone heights (Table 1). The rate of longitudinal bone growth was slightly decreased in clodronate-treated rats compared with the control but such difference was not statistically significant (Table1).
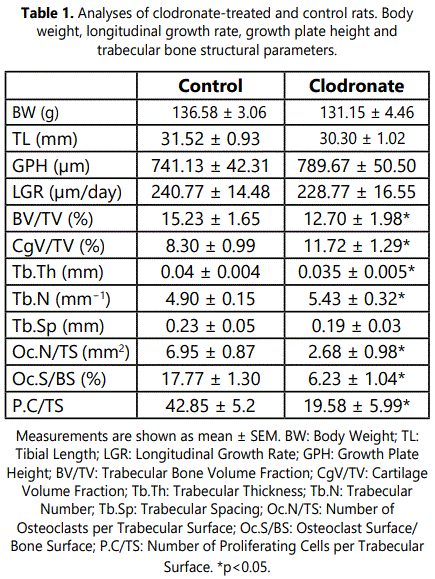
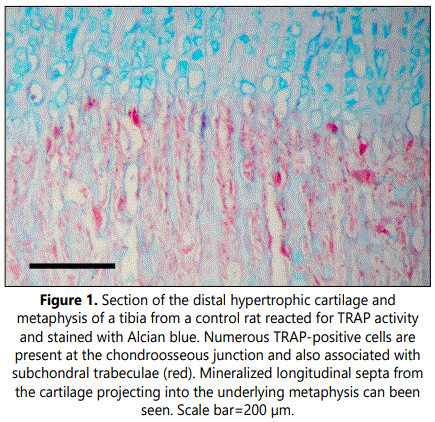
Microscopic examination of the zone immediately below the growth plate cartilage in tibial sections from control rats revealed numerous tartrate-resistant acid phosphatase (TRAP)-positive cells at the chondroosseous junction and also associated with subchondral trabeculae mainly oriented parallel to the long axes of the bone (Figure 1). Calcification was observed at the lowest layers of the hypertrophic zone in the longitudinal septa of cartilaginous matrix between columns of chondrocytes whereas little to no mineralization was found in the transverse septa between adjacent chondrocytic lacunae within a row. Transverse unmineralized septa of terminal hypertrophic chondrocytes were observed to be degraded and invading capillary vessels and mononuclear cells passed into opened hypertrophic chondrocytic lacunae. In control rats, a clear inter linkage between the mineralized longitudinal septa of the hypertrophic cartilage and the primary spongiosa was observed. Mineralized longitudinal septa projected into the underlying metaphysis and served as a scaffold upon which osteoblasts were able to spread and deposited mineralized osseous matrix. As a result, first trabecular bone structures containing cartilaginous matrix cores were found nearly the growth plate (Figure 1). Multinucleated large osteoclast-like cells were observed at the base of the growth plate close to endothelial cells and perivascular cells at the forefront of the advancing capillaries (Figure 2). These cells were not located just in the advancing capillary front but were closely behind it, appearing to be associated with calcified longitudinal septa and not with resorption of transverse to allow the advance of invasive capillaries (Figure 2).
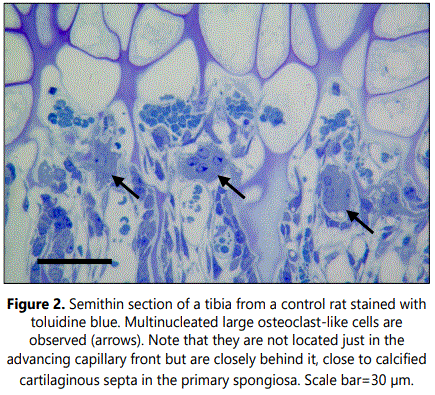
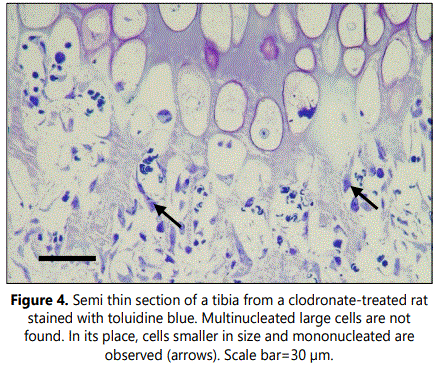
Histological analyses of TRAP staining in the primary spongiosa of tibial sections from clodronate treated rats revealed a noteworthy decrease when compared with control rats (Figure 3). Both osteoclast-like cell number and osteoclast surface were significantly decreased, with a 3-fold decrease of these cells in clodronate-treated rats (Table 1). Microscopic examination of semithin sections revealed that multinucleated large osteoclast-like cells disappeared in tibial sections from clodronate treated rats (Figure 4). In these samples, some TRAP-positive cells persisted but they were smaller in size and mono nucleated. Vascular invasion was found to be altered by clodronate treatment. Capillary network was less organized in clodronate treated rats, with metaphyseal blood vessels oriented rather randomly (Figure 4), in contrast to the orderly directional growth of blood vessels in control rats, where metaphyseal blood vessels showed a strictly delineated orientation at the osteochondral junction towards the longitudinal septae of the terminal row of hypertrophic chondrocytes (Figure 3). Vessels at the osteochondral junction of clodronate treated rats showed a more irregular profile and blood vessel density was reduced.
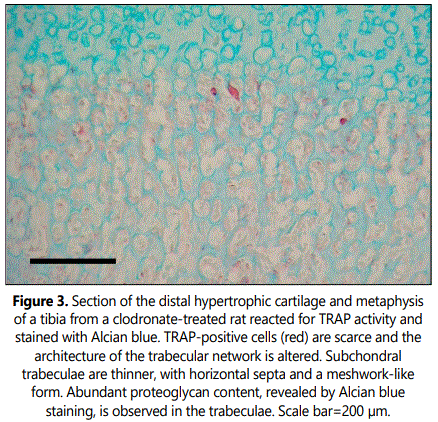
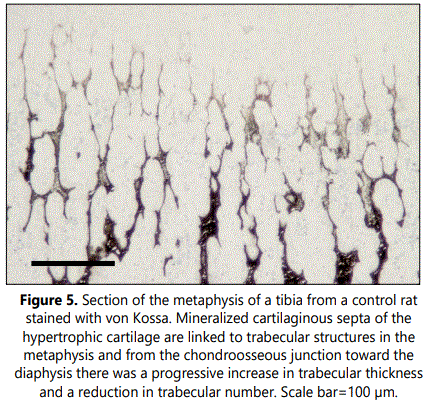
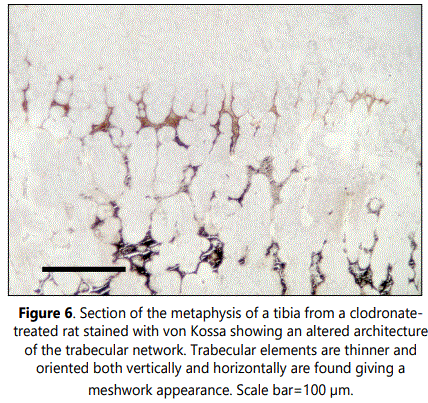
Subchondral trabecular structures in the metaphysis of control rats were linked to the longitudinal mineralized cartilaginous septa of the hypertrophic cartilage and were mainly oriented parallel to the long axes of the bone (Figures 1 and 5). From the chondroosseous junction toward the diaphysis there was a progressive increase in trabecular thickness and a reduction in trabecular number, indicating that both bone deposition and resorption occurred concomitantly. The architecture of the trabecular network of the metaphyseal region was found to be altered in clodronate-treated rats with modifications relative to the orientation, thickness and composition of the trabeculae (Figures 3 and 6). These animals presented thinner subchondral trabeculae that were preferentially arranged in the vertical direction but some horizontally oriented trabeculae were also present (Figure 6). Horizontally oriented trabeculae linked adjacent vertical trabeculae giving a meshwork appearance (Figures 3 and 6). Abundant proteoglycan content, revealed by Alcian blue staining, was found in subchondral trabeculae of clodronate-treated rats (Figure 2). Both horizontal trabeculae, resulting from unresorpted transversal septa of terminal hypertrophic chondrocytes, and remaining cartilaginous matrix cores indicated a decrease in cartilage resorption. The modification induced by clodronate treatment in the primary spongiosa was clearly demonstrated by the histomorphometric analyses. The analysis of trabecular parameters (Table 1) showed that treated rats had significantly lower values for trabecular bone volume fraction and trabecular thickness when compared with control rats. By contrast, the trabecular number did not change and this resulted in an increase in trabecular spacing in clodronate treated rats. Trabecular bone volume fraction and trabecular thickness decreased by 16% and 12.5%, respectively, in the clodronate group compared to controls. Likewise, cartilage volume fraction was a 41.2% higher in clodronate treated rats.
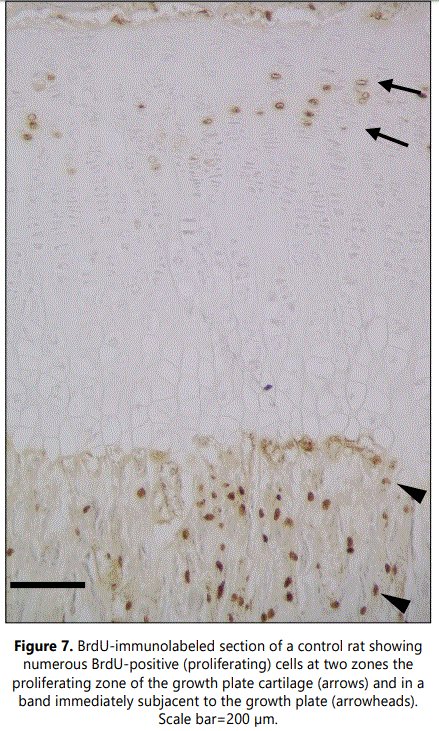
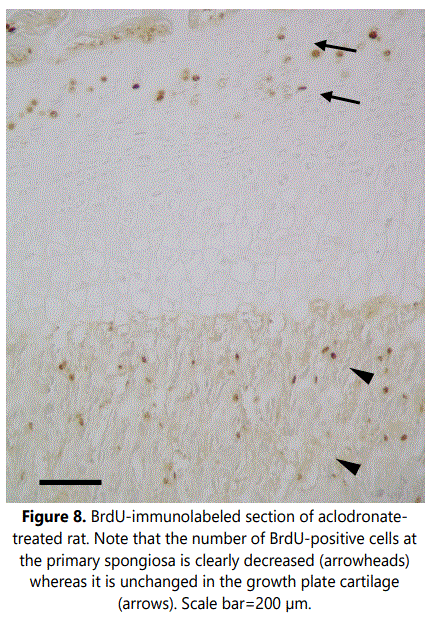
Examination of cell proliferation, assessed by BrdU incorporation, demonstrated two zones with high numbers of BrdUrd-positive cells (Figure 7). One corresponded to the region of the growth plate cartilage where chondrocytes undergo mitosis, the proliferating zone. The second was within the metaphyseal primary spongiosa, in a band immediately subjacent to the growth plate, and corresponded to osteoprogenitor cells capable of both proliferating and differentiating into bone-forming osteoblasts. Analysis of sections from clodronate treated rats revealed that the number of BrdUrd-positive chondrocytes in the growth plate cartilage did not change when compared with control rats. However, proliferation was found to be significantly decreased in the primary spongiosa of clodronate treated rats (Figure 8) (Table 1). Proliferating cells in the primary spongiosa included mesenchymal, osteoprogenitor, hematopoietic and vascular cells. Some of these cells migrate with the invading blood vessels into the hypertrophic cartilage and others generate osteoblasts that generate bone trabeculae at the primary spongiosa. Results obtained on the structure of growth plate and primary spongiosa showed that alteration was greater in the last than in the former, a fact suggesting that decrease of proliferating cells mainly affected to osteoprogenitor cells.
Discussion
In this study we focused our attention on the effect of the inhibition of the resorptive activity by clodronate treatment on primary spongiosa during the rapid growing period. We focused on the primary spongiosa because it directly derives from hypertrophic calcified cartilage and is the precursor of the trabecular bone. Our data showed that clodronate treatment has little effect on the activity of the growth plate cartilage but it affects at the level of formation of the primary spongiosa, without a significant effect on longitudinal bone growth even at doses much higher than those used in clinical practice. This result is in accordance with previous studies reporting that clodronate do not alter induces less changes on the growth plate structure than the nitrogen-containing bisphosphonates [9]. Nevertheless, a trend was seen towards a reduction in mean values for body weight, tibial length and longitudinal growth rate in animals treated with clodronate but this trend did not reach statistical significance. Likewise, although not statistically significant, there was a trend towards increased growth plate height, a fact suggesting a decreased growth plate cartilage resorption. Thus, further studies including a longer treatment period will clarify whether the modification on trabecular micro architecture at the primary spongiosa may have a long-term effect on bone health. In this way, it has been reported that all bisphosphonates are composed of an enzyme-resistant phosphorus-carbon-phosphorus (P-C-P) structure able to adhere strongly to hydroxyapatite crystals. They are embedded on the bone matrix and remain in the body for life [15,16]. Once embedded on the bone matrix, bisphosphonates affect osteoclasts by reducing their differentiation, recruitment, and activity [17,18]. It is considered that bisphosphonates are taken up by osteoclasts during bone resorption and, as a result, osteoclasts lose the ruffled border and their normal cytoskeleton structure [19]. On this basis, the safety of these compounds can be only be reliably assessed after years.
It has been reported that inhibition of osteoclastic activity reduces the activity of bone remodeling units, with an altered balance between bone formation and resorption in favor of the latter, leading to an increase of bone mass [20]. Our histological analysis revealed that clodronate treatment increases the density in mature trabeculae of the secondary spongiosa but decreases that of newly formed sub chondral trabeculae in the primary spongiosa. Thus, clodronate may have different effects in distinct areas of bone and this result is in good accordance with the mechanism of action of clodronate with reduced removal of the longitudinal cartilaginous septa from the resorptive zone and of cartilaginous matrix and trabeculae from the metaphyseal zone. In this way, it has been reported that clodronate has protective and anabolic effects on the cartilage and then it can be used to neutralize the process of cartilage degradation that occurs in the progression of rheumatoid arthritis [21,22]. Results obtained in the present study suggest that the first stage of the process of formation of a bone trabecular from longitudinal mineralized cartilaginous septa may have specific characteristics involving a major role of the osteoclastic activity. Fine trabecular structures of the primary spongiosa have to be modeled by osteoclastic resorption and osteoblast bone formation at independent sites to build the thicker trabecular structures of the secondary spongiosa. In this way, it is possible that osteoclasts may act in some way on the structure of longitudinal mineralized cartilaginous septa and make them available to serve as a scaffold for osteoblasts to spread and deposit mineralized osseous matrix. The location of multinucleated large osteoclast-like cells at the base of the growth plate behind the advancing capillary front, in close opposition to calcified cartilaginous longitudinal septa is in good accordance with this possibility.
Results of our study show mild histological alterations in the capillary network at the osteochondral junction, an observation in accordance with the reported antiangiogenic properties of bisphosphonates [23,24]. Nevertheless, although vascular invasion is a crucial process in endochondral ossification, it has been reported that complete abolishment of osteoclast function by clodronate treatment at high dose or in osteoclast deficient (c-fos knockout) mice did not affect the process of angiogenesis and subsequent cartilage removal in endochondral ossification [25]. Nevertheless, capillaries are also present at bone remodeling sites and help further couple bone resorption and bone formation [26,27]. Then, histological alterations of the capillary network induced by decreased osteoclastic activity could have little effect on the vascular invasion of the growth plate cartilage but could have a more relevant role on the structure of the primary spongiosa. In this way, it is known that treatment of children with diverse bisphosphonates generates in X-ray images radiodense bands parallel to the growth plate that are named “growth arrest lines” or also Harris lines [7,28]. These transverse radiopaque lines are horizontal sclerotic lines formed in the metaphyseal zone of long bones as a consequence of a temporal disruption of the process of endochondral ossification [29]. These structures are traces of a temporary bone growth arrest in which the hypertrophic cartilage of the growth plate remains impenetrable for blood vessels and accompanying osteoblasts, which in turn cause mineralization along the horizontal chondrocyte layer at the end of the growth plate. As a result, a primary stratum horizontally to the horizontal axis of the growth plate is formed and prolonged periods of growth arrest result in the thickening of these horizontal structures. This suggests that modifications in the capillary network at the osteochondral junction of clodronate treated rats may relate to the changed primary spongiosa and to the generation of these bands of retained mineralized cartilage.
Two zones with high proliferative activity were found associated with the growth plate. One is the proliferating zone of the growth plate cartilage and the other is a zone of the metaphyseal primary spongiosa, immediately subjacent to the growth plate, where osteoprogenitor cells proliferate to subsequently differentiate into bone-forming osteoblasts. Results obtained in the present work showed that clodronate treatment does not alter the proliferation of chondrocytes but significantly decreases proliferation in the primary spongiosa. This reduction in cell proliferation may be a result of the reduction of osteoclastic activity because it has been demonstrated that growth factors like insulin-like growth factor type 1 (IGF-1) and transforming growth factor-beta (TGF-β) are released from the bone matrix due to osteoclast matrix resorption and such factors induce proliferation and differentiation of mesenchymal stem cells into osteoblasts [30-32]. Furthermore, osteoclasts may promote osteogenesis independent of resorptive activity since they produce secrete diverse signaling molecules, like platelet-derived growth factor, that induce recruitment of endothelial precursor cells and mesenchymal stem cells for angiogenesis and osteogenesis [33-35]. On this basis, the decreased proliferation activity in the primary spongiosa reported in the present work may be a consequence of a modification in cell signaling due to the alteration of osteoclatic number and/or activity.
The results of the present investigations are limited by the short duration of treatment, the inaccurate conversion of dose equivalents of oral with low percentage of absorption to subcutaneous administration and the potential pharmacodynamic differences between rodents and humans. Nevertheless, although results obtained on rats have translational limitations, they enable us to uncover clues about the effect of the clodronate disease on bone growth at the cellular level.
Conclusions
Present short-term study suggest that administration of high doses of clodronate have some effects on the structure of the primary spongiosa derived from calcified cartilage but they do not adversely affect the growth plate activity and skeletal growth. Nevertheless, although not statistically significant, a trend towards a reduction in mean values for body weight, tibial length and longitudinal growth rate was seen observed. Then longer-term studies would be required to assess if clodronate treatment could have a cumulative effect on growth plate activity and bone health.
Acknowledgments
This work was supported by Grants PI17/001745 and PI18/01757 (Ministerio de Ciencia, Innovación y Universidades, Proyectos de Investigaciónen Salud, Instituto de Salud Carlos III), European Regional Development Funds 2013-2016 (ERDF, Grupín 14-020), the Foundation of the University of Oviedo (FUO), Fondo Europeo de Desarrollo Regional (FEDER), and by the Foundation for Biomedical Research and Innovation of the Principado of Asturias (FINBA).
Competing Interests
The authors declare no competing interests.
References
- Samsa WE, Zhou X, Zhou G. Signaling pathways regulating cartilage growth plate formation and activity. Semin Cell Dev Biol. 2017; 62: 3-15. doi: 10.1016/j.semcdb.2016.07.008
- Mangiavini L, Merceron C, Schipani E. Analysis of Mouse Growth Plate Development. Curr Protoc Mouse Biol. 2016; 6(1): 67-130. doi: 10.1002/9780470942390.mo150094
- Melrose J, Shu C, Whitelock JM, Lord MS. The cartilage extracellular matrix as a transient developmental scaffold for growth plate maturation. Matrix Biol. 2016; 52-54: 363-383. doi: 10.1016/j.matbio.2016.01.008
- Álvarez J, Balbín M, Santos F, Fernández M, Ferrando S, López JM. Different bone growth rates are associatedwith changes in the expression pattern of types II and X collagens and collagenase 3 in proximal growth plates of the rat tibia. J Bone Miner Res. 2000; 15(1): 82-94. doi: 10.1359/jbmr.2000.15.1.82
- Knowles HJ, Moskovsky L, Thompson MS, et al. Chondroclasts are mature osteoclasts which are capable of cartilage matrix resorption. Virchows Arch. 2012; 461(2): 205-210. doi: 10.1007/s00428-012-1274-3
- Boonen S, Ferrari S, Miller PD, et al. Postmenopausal osteoporosis treatment with antiresorptives: effects of discontinuation or longterm continuation on bone turnover and fracture risk-a perspective. J Bone Miner Res. 2012; 27(5): 963-974. doi: 10.1002/jbmr.1570
- Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998; 339(14): 947-952. doi: 10.1056/NEJM199810013391402
- Heinrich SD, Gallagher D, Harris M, Nadell JM. Undiagnosed fractures in severely injured children and young adults. Identification with technetium imaging. J Bone Joint Surg Am. 1994; 76(4): 561-572. doi: 10.2106/00004623-199404000-00011
- Zhu ED, Louis L, Brooks DJ, Bouxsein ML, Demay MB. Effect of bisphosphonates on the rapidly growing male murine skeleton. Endocrinology. 2014; 155(4): 1188-1196. doi: 10.1210/en.2013-1993
- Lehenkari PP, Kellinsalmi M, Näpänkangas JP, et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002; 61(5): 1255-1262. doi: 10.1124/mol.61.5.1255
- Recenti R, Leone G, Simi L, et al. Clodronate acts on human osteoclastic cell proliferation, differentiation and function in a bioreversible manner. Clin Cases Miner Bone Metab. 2007; 4(2): 146-155.
- Dimeglio LA, Ford L, McClintock C, Peacock M. A comparison of oral and intravenous bisphosphonate therapy for children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2005; 18(1): 43-53. doi: 10.1515/jpem.2005.18.1.43
- Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992; 13(1): 66-80. doi: 10.1210/edrv-13-1-66
- Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987; 2(6): 595-610. doi: 10.1002/jbmr.5650020617
- Adami S, Zamberlan N. Adverse effects of bisphosphonates. A comparative review. Drug Saf. 1996; 14(3): 158-170. doi: 10.2165/00002018-199614030-00003
- Fleisch H. Can bisphosphonates be given to patients with fractures? J Bone Miner Res. 2001; 16(3): 437-440. doi: 10.1359/jbmr.2001.16.3.437
- Salo J, Lehenkari P, Mulari M, Metsikkö K, Väänänen HK. Removal of osteoclast bone resorption products by transcytosis. Science. 1997; 276(5310): 270-273. doi: 10.1126/science.276.5310.270
- Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008; 19(6): 733-759. doi: 10.1007/s00198-007-0540-8
- Frediani B, Giusti A, Bianchi G, et al. Clodronate in the management of different musculoskeletal conditions. Minerva Med. 2018; 109(4): 300-325. doi: 10.23736/S0026-4806.18.05688-4
- Frediani B, Bertoldi I. Clodronate: new directions of use. Clin Cases Miner Bone Metab. 2015; 12(2): 97-108. doi: 10.11138/ccmbm/2015.12.2.097
- Wehling N, Palmer GD, Pilapil C, et al. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009; 60(3): 801-812. doi: 10.1002/art.24352
- Rosa RG, Collavino K, Lakhani A, et al. Clodronate exerts an anabolic effect on articular chondrocytes mediated through the purinergic receptor pathway. Osteoarthritis Cartilage. 2014; 22(9): 1327-1336. doi: 10.1016/j.joca.2014.07.009
- Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004; 114(5): 623-633. doi: 10.1172/JCI22087
- Kang JH, Choi NK, Kang SJ, et al. Alendronate affects cartilage resorption by regulating vascular endothelial growth factor expression in rats. Anat Rec (Hoboken). 2010; 293(5): 786-793. doi: 10.1002/ar.21092
- Deckers MM, Van Beek ER, Van Der Pluijm G, et al. Dissociation of angiogenesis and osteoclastogenesis during endochondral bone formation in neonatal mice. J Bone Miner Res. 2002; 17(6): 998-1007. doi: 10.1359/jbmr.2002.17.6.998
- Parfitt AM. The mechanism of coupling: a role for the vasculature. Bone. 2000; 26(4): 319-323. doi: 10.1016/S8756-3282(00)80937-0
- Chim SM, Tickner J, Chow ST, et al. Angiogenic factors in bone local environment. Cytokine Growth Factor Rev. 2013; 24(3): 297-310. doi: 10.1016/j.cytogfr.2013.03.008
- Nowak O, Piontek J. The frequency of appearance of transverse (Harris) lines in the tibia in relationship to age at death. Ann Hum Biol. 2002; 29(3): 314-325. doi: 10.1080/03014460110086105
- Scott AB, Hoppa RD. A re-evaluation of the impact of radiographic orientation on the identification and interpretation of Harris lines. Am J Phys Anthropol. 2015; 156(1): 141-147. doi: 10.1002/ajpa.22635
- Tang Y, Wu X, Lei W, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009; 15(7): 757-765. doi: 10.1038/nm.1979
- Xian L, Wu X, Pang L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012; 18(7): 1095-1101. doi: 10.1038/nm.2793
- Yang P, Lv S, Wang Y, et al. Preservation of type H vessels and osteoblasts by enhanced preosteoclast platelet-derived growth factor type BB attenuates glucocorticoid-induced osteoporosis in growing mice. Bone. 2018; 114: 1-13. doi: 10.1016/j.bone.2018.05.025
- Henriksen K, Karsdal MA, Martin TJ. Osteoclast-derived coupling factors in bone remodeling. Calcif Tissue Int. 2014; 94(1): 88-97. doi: 10.1007/s00223-013-9741-7
- Xie H, Cui Z, Wang L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014; 20(11): 1270-1278. doi: 10.1038/nm.3668
- Vi L, Baht GS, Whetstone H, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res. 2015; 30(6): 1090-1102. doi: 10.1002/jbmr.2422


