Review Article
Experimental and Therapeutic Trials of Amygdalin
1Department of Medical Biochemistry & Molecular Biology, Faculty of Medicine, Assiut University, Egypt
2Students, Faculty of Medicine, Assiut University, Egypt
3Student, Faculty of Pharmacy, Assiut University, Egypt
*Corresponding author: Ragga H Salama, Professor, Head of Medical Biochemistry Department & Molecular Biology, Co-director of Medical Research Center, Faculty of Medicine, Assiut University, Egypt, Tel: +201063492008, E-mail: ragaa_2002@yahoo.com
Received: September 16, 2019 Accepted: October 18, 2019 Published: October 28, 2019
Citation: Salama RH, Ramadan AEG, Alsanory TA, Herdan MO, Fathallah OM, Alsanory AA. Experimental and Therapeutic Trials of Amygdalin. Int J Biochem Pharmacol. 2019; 1(1): 21-26. doi: 10.18689/ijbp-1000105
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Natural substances and alternative medications such as amygdalin gained huge popularity in treating various diseases due to wide availability and relatively low cost. Nevertheless, their use may cause serious side effects unless appropriate doses are considered. Therefore, this review illustrates the purported anticancer activity of amygdalin as well as its other effects on different body systems. For instance, the endocrine, urinary, genital and respiratory systems, considering its toxic side effects. Also, this review mentions a variety of clinical trials using amygdalin on both, humans and animals.
Keywords: Amygdalin; Vitamin B17; Cyanogenic Toxicity; Antitumor; Beta-glucuronidase; Rhodanese.
Introduction
Background
Although it is common to refer to amygdalin as laetrile, they are not the very same product from the biomedical point of view. Moreover, amygdalin is known under the misnomer vitamin B17, which is erroneous, as neither amygdalin nor laetrile is a vitamin [1]. Interestingly, the Egyptian papyri 5,000 years ago, did mention the beneficial use of derivatives of bitter almonds in treating skin tumors. Also, the Romans and Greeks connected some therapeutic properties to those derivatives [2]. Thereafter, those derivatives were known as amygdalin or vitamin B17, as bitter almonds are considered one of the richest sources of that [3,4]. Moreover, it was noticeable that some people and isolated tribes all over the world didnʼt have any cancer cases, such as the Abkhazians, the Hopi and Navajo Indians, the Hunzas, the Eskimos and the Karakorum. It turned out that they had in common a diet rich in amygdalin [2]. Consequently, many researchers and scientists all over the globe carried out various studies and clinical trials to prove its anticancer activity, and they found that amygdalin can specifically attack cancer cells without affecting other healthy cells [5], decrease the telomerase activity [6], and block the bladder cancer cell growth [7]. On the other hand, amygdalin showed serious side effects caused by cyanide compounds liberated after amygdalin degradation [8,9].
Sources of amygdalin
Amygdalin has a plant origin and particularly it is present in the kernels seeds of about 800 plants. Prunus seeds are one of the richest sources of amygdalin. Moreover, the seeds of bitter almond, apricots, cherries, sweet cherry, peaches, plums, nectarines and apples are also rich in amygdalin. Additionally, the seeds of olives, grapes and buckwheat contain amygdalin as well. Besides amygdalin, these seeds are rich in protein, polyunsaturated fats, and other nutrients and contain as much as 2 percent or more nitriloside [3,4,10].
Chemistry
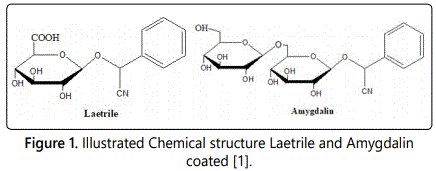
Structure, formula and physical properties: Amygdalin (C20H27NO11) belongs to the group of aromatic cyanogenic glycoside. The chemical name of amygdalin is (R)-α-[(6-O-β-D-glucopyranosyl-b-Dglucopyranosyl)-oxy]-(phenyl) acetonitrile, also named d-(-)-mandelonitrile-β-D-gentiobioside. Meanwhile, the active form of amygdalin is R-amygdalin right-handed structure, which is the natural form (Figure 1). Amygdalin is colorless and has a molecular mass of 457.4 gmol-1, melting point of 213°C and its Chemical Abstracts Service (CAS) number is 29883-15-6. Although amygdalin is insoluble in non-polar solvents like chloroform, it is highly soluble in ethanol and moderately soluble in water. Amygdalin also named laetrile or vitamin B-17. But the names laetrile, vitamin B-17, and amygdalin are not the same product. As amygdalin is a cyanogenic glucoside, and its purified form is called laetrile which refers to the terms levorotatory and mandelonitrile. Whereas, laetrile is a semi synthetic cyanogenic glucuronide, therefore it is structurally different from amygdalin. Laetrile in USA and Mexico are different in the process of synthesis. As in USA, Laetrile is a partially synthetic (man-made) form of amygdalin, while in Mexico it comes from crushed apricot pits. E.T. Krebs Jr gave the term vitamin B-17 to laetrile, indicated laetrile as a vitamin or nutritional supplement rather than being a drug. But in fact, it is mistakenly referred to as vitamin B17, since the compound is not a vitamin [5,8,11].
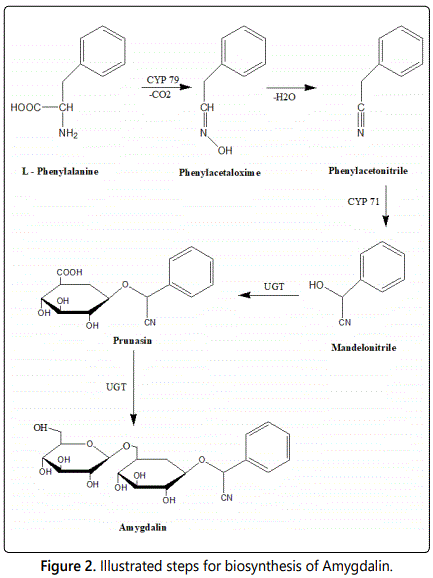
Biosynthesis of amygdalin: In amygdalin, the amino acid phenylalanine undergoes hydroxylation by a CYP79 enzyme into phenyl acetaldoxime, which is further hydroxylated by a CYP71 enzyme to mandelonitrile. Subsequent attachment of one glucose molecule to the α-hydroxyl group of mandelonitrile, catalyzed by a uridine diphosphate glucose-glucosyl transferase (UGT) leads to prunasin (d-(-)-mandelonitrile-β-d-glucoside, CAS number 99-18-3, 295.3 g mol-1). On addition of another glucose molecule to the 6ʼ-hydroxyl group, it is finally converted to amygdalin forming the di-glucoside gentiobiose (Figure 2). The biosynthesis of amygdalin in plants follows the general scheme for CNGs i.e. consecutive hydroxylation of an amino acid by cytochrome P450 (CYP) enzymes to an oxime and subsequently α-hydroxy nitrile, followed by glycosylation of the latter [1].
Extraction
Amygdalin was obtained from seeds and protein isolates. The process of extraction of amygdalin was prepared as follows: the first step is to mix 550 mg of seeds with 15 mL of MeOH or 40 mg of protein isolates with 5 mL of MeOH to prepare a solution from which amygdalin was extracted . The second step is to cover the solution and keep it for 24 hours in a water bath with agitation at 60 ± 2°C. Then, the solution was centrifuged for 10 min at 4000 g. After that, the supernatants were injected into a 1100 series High Performance Liquid Chromatography (HPLC). Chromatographic conditions were as follows: isocratic elution with water:MeOH 65:35; flow-rate, 1 mL min-1; temperature, 25°C; injected volume, 10 μL; UV detection, 218 nm [10].
Administration
There are two ways of amygdalin administration; it can be given orally as a pill, or by injection (intravenous or intramuscular). However, the commonest way of administration is initially by intravenous injection for a period of time followed by oral maintenance therapy. Giving amygdalin orally is thought to cause much higher levels of cyanide poisoning while amygdalin injection produces little breakdown to yield the hydrogen cyanide. This is because that laetrile is hydrolyzed in the intestine by enzymes (beta-glucosidases) that activates the release of cyanide from laetrile and converts laetrile into toxic substance (hydrogen cyanide). This enzyme presents in the intestinal bacteria and some commonly eaten plant [5].
Pharmacokinetics
After the ingestion of either laetrile or amygdalin, they both get metabolized by hydrolysis via the enzymes of the duodenal and intestinal alkaline juice, producing D-glucuronic acid and L-mandelonitrile, the latter is further hydrolyzed to benzaldehyde and hydrogen cyanide (Hydrocyanic acid or HCN) which if taken in an abundant amount, will result in the cyanogenic toxicity [11]. Additionally, the catalytic degradation of amygdalin by Aspergillus nigerʼs extracellular enzymes resulted in four products that were identified as mandelonitrile, prunasin, benzaldehyde and phenyl-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-acetonitrile (PTMT) [12].
In an experiment done by Li et al. [13], after dosing rats orally with 20 mg/kg of amygdalin, the pharmacokinetic results exposed a short Tmax (less than 2 hours) which indicated that amygdalin may be absorbed rapidly after oral administration with an immediate plasma detection of amygdalin within 5 min. Meanwhile, rats had a long t1/2 value of amygdalin, approximately 8.45 hours, which indicated that elimination of amygdalin may be slow.
In another study on healthy Chinese human subjects prepared by Li et al. [14], the researchers used Huoxue-Tongluo lyophilized powder for injection (HTLPI) in which amygdalin is a bioactive component, since HTLPI is a mixture of Paeoniae Radix Rubra and Persicae semen. The study showed that sex did not affect the pharmacokinetic properties of amygdalin significantly. Meanwhile, the single-dose phase of the study exposed that the mean maximum plasma concentration as well as the mean area under the plasma concentration-time curve of amygdalin had a proportional escalation with each dose increase, whereas, after multiple-dose administration of 6 g HTLPI, the steady state concentration was obtained by day 4. Also, no significant systemic accumulation was noticed after repeated dosing with 6 g HTLPI, as the mean pharmacokinetic parameters achieved on day 1 were similar to those on day 7. Moreover, the study showed that approximately 79.6% of the administered amygdalin was excreted unchanged in urine within 24 hours. Eventually, the researcher didnʼt observe any serious adverse events during the whole study.
Also, Shalayel [11] concluded that the amygdalin content of bitter apricot seeds can affect the bodyʼs control of various minerals, nitrogen, and possibly the acid-base balance as well. Although a 42-day administration of apricot seeds at a dose of 60 mg/kg of body weight decreased the urinary calcium excretion significantly, this decline moved the raised mean value in control collection to the usual physiological range, additionally; urinary changes were detected regarding urea and phosphorus levels.
Theories that explain its Purported Anticancer Activity
Laetrile proponents have suggested different theories explaining its alleged anticancer activity.
• One of them is “the trophoblastic theory of cancer”. This theory states that all cancers arise from primordial germ cells (cells that would normally give rise to eggs or sperms), some of which become spread throughout the body during embryogenesis and, accordingly, they are not restricted to the testicles or ovaries. Laetrile use is explained by the suggestion that malignant cells are special in having levels higher than normal of an enzyme called beta-glucuronidase and that they are defective in another enzyme called rhodanese (thiosulfate sulfurtransferase). Another opinion states that laetrile is modified in the liver, and then beta-glucuronidase enzyme breaks down the modified compound, producing cyanide. Thereafter, rhodanese converts cyanide into the relatively harmless compound thiocyanate. Thus, the imbalance in these two enzymes shows that cancer cells are more affected by the toxic effects of laetrile than normal cells.
• Another theory states that laetrile, or amygdalin/vitamin B-17, is the missing vitamin needed by the body to restore health. Accordingly, this theory assumes that cancer is a metabolic disorder caused by the vitamin deficiency. Despite the experimental evidence which indicates that the level of intake of individual vitamins can influence the development of cancer, there is no evidence that laetrile is essential for normal metabolism.
• Also, there is a theory which suggests that cyanide released from laetrile increases the acidic content of tumor cells, leading to the disruption of lysosomes releasing their contents. Thereby, ceasing the tumor growth and destructing cancer cells. According to this theory, another consequence of the lysosomal destruction is stimulating the immune system [5].
• Moreover, amygdalin could greatly reduce the action of telomerase enzyme which presents in higher levels in cancer cells (∼8–13 fold). Therefore, it reduces the expression of telomerase reverse transcriptase and telomerase RNA component. As a result, it caused suppression of cancer cell growth. Therefore, Moon et al. [6] regarded amygdalin as a powerful treatment for cancer.
Toxicity of amygdalin
Degrading 1 g of amygdalin liberates 59 mg hydrogen cyanide (HCN) which is present in its dissociated form as cyanide [1,15]. Despite the fact that cyanide has a beneficial effects in fighting cancer, it is regarded as a great risk due to its toxic side effects, principally, when amygdalin is ingested orally, since the cyanide released after oral ingestion is way larger than that liberated through the intravenous route, not only due to the rapid action of gut microflora bacteria in releasing cyanide [9,16] but also as a result of chewing or grinding [1].
The highest dose of amygdalin that did not cause any unacceptable side effects in mice, rabbits and dogs was 3 g/kg of intravenous and intramuscular injection, and was 0.075 g/kg when given orally; also, the maximum tolerance dose of human intravenous injection of amygdalin was around 0.07 g/kg. Additionally, after treating mice by inhibiting the intestinal bacteria, the oral administration of 300 mg/kg did not lead to death, on the other hand, the mortality increased by 60% using the same dose in untreated mice. Moreover, systemic toxicity in humans occurred following the oral administration of amygdalin at a dose of 4 g per day through a period of half a month, or a month of intravenous injection. Nevertheless, after the stoppage of amygdalin intake or when the daily oral dose is reduced to 0.6~1 g, the toxicity disappeared. Furthermore, the response of the digestive system toxicity is more frequent and accompanied by changes of a trial premature beats [16].
In a case report by Sauer et al. [8] on a 4-year old child receiving complementary and alternative medicine treatment for a malignant brain disease, the childʼs parents gave him amygdalin intravenously and orally in the form of apricot kernel, which resulted in acute cyanide poisoning that finally led to a severe encephalopathy. Thereafter, the childʼs condition improved rapidly after sodium thiosulfate administration. Moreover, according to Sauer et al. [8], the cyanide toxicity leads to the dysfunction of the oxidative phosphorylation process within the cells, consequently, the clinical symptoms of mild cyanide poisoning are nausea, drowsiness, headache, metallic taste, dizziness, hyperpnea, anxiety, and mucous membrane irritation. Therefore, Milazzo and Horneber [9] regarded that the risk–benefit balance of using amygdalin for treating cancer was accordingly negative.
Additionally, it is important to consider the ingestion of apricots among the differential diagnoses in patients having severe lactic acidosis with oxygen saturation being normal. As in a case report by Dalk et al. [17], the patientʼs parents reported that the patient had extracted and eaten the cores of 3 apricot kernels before showing the severe acidosis. Moreover, the patientʼs blood pH and the cardiovascular functions reviled significant improvement following the initiation of hemodialysis. Therefore, the case report concluded that in severe cases of cyanide poisoning when there is no access to hydroxocobalamin with no response to supportive therapy, hemodialysis could be considered as an approach to get rid of the toxic metabolic byproducts and the free blood cyanide.
Experimental Trials of Amygdalin
Generally, Amygdalin was shown to have multiple beneficial effects on many systems and diseases as in: the digestive system, where it shows soothing and defensive effect; the urinary system as it promotes apoptosis of human renal fibroblast and improves kidney function; the respiratory system, has been used for treatment of asthma, bronchitis, emphysema and leprosy in addition to an antitussive effect [18]. Also, in diseases such as cancer, controversial reports show that it might reduce pleural effusion in lung cancer patients. In inflammation, amygdalin suppresses both its response in human epidermal keratinocytes and the expression of TNF-α and IL-1β in LPS-treated RAW 264.7 cells. Additionally, amygdalin was reported to prevent alloxaninduced diabetes [19].
The endocrine system
Various doses of Amygdalin caused ovarian granulose cells to release estradiol-17β but not progesterone depending on the dose. It also regulated steroid production in porcine ovaries. Moreover, amygdalin enters in pharmacological components of the crude ingredients of Keishi-bukuryo-gan, a Japanese herbal medicine used for induction of ovulation in women with infertility. The rough constituent of Keishi-bukuryo-gan affected steroidogenesis in pre-ovulatory follicles and corpus luteum in the ovaries of rats both in vivo and in vitro. Therefore, the natural substance in bitter almond seeds may be involved in mechanisms of folliculogenesis in rabbitʼs ovary through repression of FSH [11,20]. However, intramuscular and oral application of amygdalin did not significantly affect the plasma levels of some endocrine regulators (progesterone, 17β-estradiol, testosterone), thyroid (triiodothyronine, thyroxin, thyroid-stimulating hormone), anterior pituitary hormones (prolactin, luteinizing hormone), or the average body weight of rabbits used in the experiment [15].
The urogenital system
Blocking fibrosis in chronic kidney disease: It was proven that Amygdalin has a strong antifibrotic activity and can be used for treatment of patients with kidney fibrosis. Since cultured interstitial fibroblast cells, when treated with amygdalin, showed diminished proliferation and transforming growth factor production (TGF)-β1. Additionally, in obstructive neuropathy trials on rats, following ureteral obstruction, application of amygdalin immediately removed the extracellular matrix accumulation. Also, on the 21st day, amygdalin reduced the renal injury in general. Consequently, amygdalin could attenuate kidney fibroblast activation and renal interstitial fibrosis in rats [21].
Reduction of bladder cancer growth: It was shown that Amygdalin can stop cancer progression by down modifying cdk2 and cyclin A. As amygdalin, dose dependently, inhibited growth and proliferation of the bladder cancer cell lines and largely delayed cell cycle advance with a G0/G1 arrest. Additionally, molecular estimation implied diminished phosphorAkt, phosphoRictor and loss of Cdk and cyclin components [22].
Induction of apoptosis in human cervical cancer cells in vivo: Application of amygdalin stopped the growth of HeLa cell xenografts through apoptosis, as it greatly inhibited the viability of HeLa cell line in cervical cancer. Using immunohistochemistry, the increased activity of caspase-3 confirmed the development of apoptosis in those cells. Further studies showed reduction of antiapoptotic protein Bcl-2 and increase in proapoptotic Bax protein in the amygdalin-healed HeLa cells implying process of apoptosis [23].
Effect on prostate cancerʼs cell cycle in vitro: With optimum results at 10 mg/ml, amygdalin reduced tumor cell growth and apoptosis of PC3 and LNCaP but not of DU-145 cells (which are the classical cell lines of prostate cancer), stopping colony formation in cell lines altogether. It also demonstrated high antitumor activity in both castration-sensitive and castration-resistant PCa cell lines [24].
Also, amygdalin significantly modulated the chemotaxis and adhesion of androgen resistant prostate cancer cell lines, DU-145 and PC3. Nonetheless, the amygdalin exposure resulted in quite different responses in the two cell lines. On one hand, in the DU-145 cell line, amygdalin reduced tumor-endothelial cell interaction, adhesion to collagen, chemotaxis, and migration. On the other hand, adhesion of PC3 increased following 2-week long-term exposure to amygdalin [25].
Effect on breast cancer in vitro
Amygdalin showed antitumor activity against breast cancer cells through their sensitization to oxidative stress in vitro. Also, amygdalin caused differential inhibition in the proliferation of the breast cancer cell lines MCF-7 and T47D. This differential inhibition may be attributed to a difference in its sensitization capability to oxidative stress. Consequently, the mechanism of amygdalin against breast cancer cells is mainly through the induction of oxidative stress [26].
The respiratory system
Amygdalin has antitussive and anti-asthmatic effects provided that it is given orally, because Hydrocyanic acid result from hydrolysis of amygdalin could suppress the respiratory cent. Therefore, diminish the respiratory movement. Additionally, an experiment on animals with respiratory distress syndrome found that amygdalin could enhance the formation of pulmonary surfactant and relieve the disease [16]. Additionally, amygdalin has a repressive action on lipo polysaccharide-produced acute lung injury by inhibiting NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and NLRP3 gene signaling pathways [19].
Moreover, amygdalin might be used as a drug for the treatment of COPD. As amygdalin, to a certain extent, could inhibit the epithelial–mesenchymal transition process induced by cigarette smoking both in vivo and in vitro. This effect may be attributed to amygdalinʼs ability to inhibit both the expression of TGF‐β1 and the phosphorylation of smad2/3, which is associated with inhibition of the TGF‐β/smad pathway [27].
The effects on the digestive system
When rats were treated orally with 500 mg/kg of pepsin hydrolysate of almond water-solution on carbon tetrachloride, the action of pepsin was inhibited by the benzaldehyde resulted from disintegration of amygdalin. Additionally, the level of AST and ALT decreased, hydroxyproline content raised and the extension of euglobulinlysis time was lowered. Also, it could diminish the proliferation of connective tissue of rat liver. Moreover, amygdalin was found to be an effective medication for chronic gastritis and atrophic gastritis in rats [16].
Also, amygdalin possessed a significant protective effect for chronic liver injury in rats. Furthermore, amygdalin consumption reversed most of the pathological changes of the LPS-induced liver injury of rats. Additionally, the beneficial effects of amygdalin may be attributed to its strong anti-inflammatory action and improvement of hepatic dysfunction through the inhibition of PI3K/AKT, JAK2/STAT3 and NF-κB signaling pathways [28].
Moreover, amygdalin reduced the production of inflammatory factors of pancreatic fibrosis and improved the microcirculatory disturbance with attenuating pancreatic stellate cells activation. The probable mechanism is by regulating the expression of ET-1 and CGRP in vivo [29].
Improving the immune function of organism
Amygdalin could meaningfully increase polyhydroxyalkanoates which stimulate peripheral blood T-lymphocytes to secrete IL-2 and IFN-γ, and then reduce the secretion of TGF-β1. Moreover, it enhances the expression of regulatory T cells in treatment of atherosclerosis. Consequently, amygdalin has a valuable role in ameliorating the immune function [16].
Effect on neurodegenerative diseases
According to Cheng et al. [30], amygdalin plays a positive role in treating neurodegenerative disorders, such as Parkinson disease, because it enhances NGF-induced neurite outgrowth in addition to protecting the cells from 6-hydroxydopamine produced neurotoxicity via inducing calreticulin expression.
Conclusion
Amygdalin gained wide popularity owing to its purported anti-cancer activity and its natural presence in seeds of many fruits. Therefore, theories tried to explain this activity by the presence of a certain enzyme in cancer cells and its lack in other normal cells. This enzyme degrades amygdalin into the active anti-cancer compound cyanide. Despite the beneficial effect of cyanide against cancer, it can cause many harmful side effects and lead to toxicity, especially if taken orally. Nevertheless, amygdalin showed other beneficial effects on different body systems besides its anti-cancer activity, as inhibiting renal fibrosis, antiasthmatic action, improving the immune function and antiparkinsonian effect. Consequently, amygdalin reveals promising outcomes as anti-cancer treatment but with its side effects in mind, appropriate doses and good management of its harmful consequences needed. Moreover, further research is required to find a means by which we can overcome these side effects and assure its safety as a dependable anti-cancer treatment.
References
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Acute health risks related to the presence of cyanogenic glycosides in raw apricot kernels and products derived from raw apricot kernels. EFSA Journal. 2016; 14: 1-17. doi: 10.2903/j.efsa.2016.4424
- Enculescu M. Vitamin B17/Laetrile/Amygdalin (a Review). Bulletin UASVM Animal Science and Biotechnologies. 2009; 66: 1-2.
- Yamshanov VA, Kovanʼko EG, Pustovalov YI. Effects of Amygdalin from Apricot Kernel on Transplanted Tumors in Mice. Bull Exp Biol Med. 2016; 160(5): 712-714. doi: 10.1007/s10517-016-3257-x
- Del Cueto J, Ionescu IA, Pičmanová M, et al. Cyanogenic Glucosides and Derivatives in Almond and Sweet Cherry Flower Buds from Dormancy to Flowering. Front Plant Sci. 2017; 8: 800. doi: 10.3389/fpls.2017.00800
- PDQ Integrative, Alternative and Complementary Therapies Editorial Board. Laetrile/Amygdalin (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US); 2002. ID: NBK65988.
- Moon JY, Kim SW, Yun GM, et al. Inhibition of cell growth and downregulation of telomerase activity by amygdalin in human cancer cell lines. Anim Cells Syst (Seoul). 2015; 19: 295-304. doi: 10.1080/19768354.2015.1060261
- Makarević J, Rutz J, Juengel E, et al. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS One. 2014; 9(8): e105590. doi: 10.1371/journal.pone.0105590
- Sauer H, Wollny C, Oster I, et al. Severe cyanide poisoning from an alternative medicine treatment with amygdalin and apricot kernels in a 4-year-old child. Wien Med Wochenschr. 2015; 165(9-10): 185-188. doi: 10.1007/s10354-014-0340-7
- Milazzo S, Horneber M. Laetrile treatment for cancer. Cochrane Database Syst Rev. 2015; (4):CD005476. doi: 10.1002/14651858.CD005476.pub4
- García MC, González-García E, Vásquez-Villanueva R, Marina ML. Apricot and other seed stones: amygdalin content and the potential to obtain antioxidant, angiotensin I converting enzyme inhibitor and hypocholesterolemic peptides. Food Funct. 2016; 7(11): 4693-4701. doi: 10.1039/c6fo01132b
- Shalayel MHF. Beyond Laetrile (Vitamin B-17) Controversy-Antitumor Illusion or Revolution. British Medical Bulletin. 2017; 5(1): 3.
- Chang J, Zhang Y. Catalytic degradation of amygdalin by extracellular enzymes from Aspergillus niger. Process Biochem. 2012; 47(2): 195-200. doi: 10.1016/j.procbio.2011.10.030
- Li X, Shi F, Gu P, Liu L, He H, Ding L. A sensitive LC-MS/MS method for simultaneous determination of amygdalin and paeoniflorin in human plasma and its application. J Pharm Biomed Anal. 2014; 92: 160-164. doi: 10.1016/j.jpba.2014.01.020
- Li X, Shi F, Zhang R, et al. Pharmacokinetics, Safety, and Tolerability of Amygdalin and Paeoniflorin After Single and Multiple Intravenous Infusions of Huoxue-Tongluo Lyophilized Powder for Injection in Healthy Chinese Volunteers. Clin Ther. 2016; 38(2): 327-337. doi: 10.1016/j.clinthera.2015.12.005
- Halenar M, Chrastinová L, Ondruška L, et al. The evaluation of endocrine regulators after intramuscular and oral application of cyanogenic glycoside amygdalin in rabbits. Biologia. 2017; 72(4): 468-474. doi: 10.1515/biolog-2017-0044
- Song Z, Xu X. Advanced research on anti-tumor effects of amygdalin. J Cancer Res Ther. 2014; 1: S3-7S. doi: 10.4103/0973-1482.139743
- Dalkiran T, Kandur Y, Ozaslan M, Acipayam C, Olgar S. Role of Hemodialysis in the Management of Cyanide Intoxication From Apricot Kernels in a 3-YearOld Child. Pediatr Emerg Care. 2018. doi: 10.1097/PEC.0000000000001644
- Liu C, Li X, Yang H, Mao X, Wang J, Gao W. Effect of Natural betaGlucosidase Inhibitors in Reducing Toxicity of Amygdalin in Persicae Semen. Phytother Res. 2017; 31(5): 771–777. doi: 10.1002/ptr.5798
- Zhang A, Pan W, Lv J, Wu H. Protective Effect of Amygdalin on LPSInduced Acute Lung Injury by Inhibiting NF-κB and NLRP3 Signaling Pathways. Inflammation. 2017; 40(3): 745-751. doi: 10.1007/s10753-017-0518-4
- Halenár M, Medveďová M, Maruniaková N, Kolesárová A. Ovarian hormone production affected by amygdalin addition in vitro. Journal of Microbiology, Biotechnology and Food Sciences. 2015; 4(2): S19-S22. doi: 10.15414/jmbfs.2015.4.special2.19-22
- Guo J, Wu W, Sheng M, Yang S, Tan J. Amygdalin inhibits renal fibrosis in chronic kidney disease. Mol Med Rep. 2013; 7: 1453-1457. doi: 10.3892/mmr.2013.1391
- Makarević J, Rutz J, Juengel E, et al. Amygdalin influences bladder cancer cell adhesion and invasion in vitro. PLoS One. 2014. 9(10): e110244. doi: 10.1371/journal.pone.0110244
- Chen Y, Ma J, Wang F, et al. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol Immunotoxicol. 2013; 35(1): 43-51. doi: 10.3109/08923973.2012.738688
- Makarević J, Tsaur I, Juengel E, et al. Amygdalin delays cell cycle progression and blocks growth of prostate cancer cells in vitro. Life Sci. 2016; 147: 137-142. doi: 10.1016/j.lfs.2016.01.039
- Mani J, Neuschäfer J, Resch C, et al. Amygdalin Modulates Prostate Cancer Cell Adhesion and Migration In Vitro. Nutr Cancer. 2019; 12: 1-10. doi: 10.1080/01635581.2019.1637442
- Abboud MM, Al Awaida W, Alkhateeb HH, Abu-ayyad AN. Antitumor Action of Amygdalin on Human Breast Cancer Cells by Selective Sensitization to Oxidative Stress. Nutr Cancer. 2018; 71(3): 1-8. doi: 10.1080/01635581.2018.1508731
- Wang Z, Fang K, Wang G, et al. Protective effect of amygdalin on epithelial–mesenchymal transformation in experimental chronic obstructive pulmonary disease mice. Phytother Res. 2019; 33(3): 808-817. doi: 10.1002/ptr.6274
- Yang Y, Zhao J, Song X, et al. Amygdalin reduces lipopolysaccharideinduced chronic liver injury in rats by down-regulating PI3K/AKT, JAK2/STAT3 and NF-κB signalling pathways. Artif Cells Nanomed Biotechnol. 2019; 47(1): 2688-2697. doi: 10.1080/21691401.2019.1634084
- Zhang X, Hu J, Zhuo Y, et al. Amygdalin improves microcirculatory disturbance and attenuates pancreatic fibrosis by regulating the expression of endothelin-1 and calcitonin gene-related peptide in rats. J Chin Med Assoc. 2018; 81(5): 437-443. doi: 10.1016/j.jcma.2017.09.005
- Cheng Y, Yang C, Zhao J, Tse HF, Rong J. Proteomic identification of calcium-binding chaperone calreticulin as a potential mediator for the neuroprotective and neuritogenic activities of fruit-derived glycoside amygdalin. J Nutr Biochem. 2015; 26(2): 146-154. doi: 10.1016/j.jnutbio.2014.09.012


