Research Article
Analytical methods Development and Validation of Crizotinib by RP-HPLC Technique
Department of Chemistry, Mother Teresa College of Pharmacy, N.F.C Nagar, Ghatkesar, Medchal, Telangana, India
*Corresponding author: Prapulla Putta, Associate Professor Department of Chemistry, Mother Teresa College of Pharmacy, N.F.C Nagar, Ghatkesar, Medchal, Telangana India, Tel: +91-92914-42653, E-mail: prapullapharmacy@gmail.com
Received: December 17, 2018 Accepted: January 18, 2019 Published: January 28, 2019
Citation: Putta P. Analytical methods Development and Validation of Crizotinib by RP-HPLC Technique. Int J Biochem Pharmacol. 2019; 1(1): 1-4. doi: 10.18689/ijbp-1000101
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
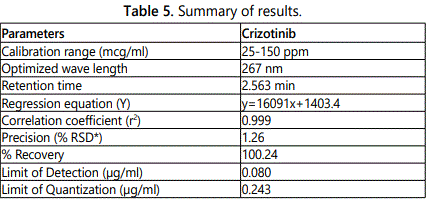
A simple, précised, accurate method was developed for the estimation of Crizotinib by RP-HPLC technique. Chromatographic conditions used are stationary phase BDS 250 × 4.6 mm, 5 µ. Mobile phase buffer: Acetonitrile in the ratio of 60:40 and flow rate was maintained at 1 ml/min, detection wave length was 267 nm, column temperature was set to 30°C and diluents was methanol: water. System suitability parameters were studied by injecting the standard five times and results were well (50:50), conditions were finalized as optimized method under acceptance criteria. Linearity study was carried out between 25% to 150% levels, r2 value was found to be as 0.999. Precision was found to be 1.26 for repeatability and 0.93 for intermediate precision. LOD and LOQ are 0.080 µg/ml. By using above method assay of marketed formulation was carried out 100.24% was present.
Keywords: Crizotinib; HPLC; ICH Guidelines; Method development.
Introduction
Chromatography is a method of separating a mixture of components into individual components through equilibrium distribution between two phases [1]. The technique of chromatography is based on the differences in the rate at which components of a mixture move through a porous medium (stationary phase) under the influence of some solvent or gas (mobile phase) [2]. Crizotinib: Crizotinib, is an anti-cancer drug acting as an ALK (anaplastic lymphoma kinase) and ROS1 (c-rosoncogene1) inhibitor [3]. The aim and objective of the work is to develop and validate a simple and economical RP-HPLC method as per ICH guidelines for the estimation of Crizotinib in drug and pharmaceutical dosage forms, and to study the linearity of the method (Figure 1).
Materials and Methods
Crizotinib was obtained as a gift sample from Wintac Ltd., (Bangalore). Acetonitrile served as solvent mixture was also obtained from CDH, New Delhi. All other chemicals/reagents were of analytical grade and were used without further purification.
Preparation of buffer
(0.1% OPA): 1 ml of Ortho phosphoric acid solution in a 1000 ml of volumetric flask is taken and add about 100 ml of milli-Q water and final volume make up to 1000 ml with milli-Q water [4].
Optimized chromatographic conditions
Column : BDS (250 × 4.6 µm)
Mobile phase : OPA buffer: Acetonitrile (60:40)
Flow rate : 1.0 ml/min
Detector : PDA 267 nm
Temperature : 30°C
Injection Volume : 10 µL
Method of validation
The proposed method was validated for various parameters such as linearity and range, accuracy, precision, robustness, ruggedness, sensitivity and specificity according to ICH Q2 (R1) guideline and USP guidelines [5].
Method of linearity and range
The linearity of an analytical procedure is its ability (within a given range) to obtain test result which are directly proportional to the concentration of an analyte in the sample. The range of an analytical procedure is the interval between the upper and lower concentration of an analyte in the sample for which it has been demonstrated that the analytical procedure has a suitable level of precision, accuracy and linearity. The linearity of the analytical method was demonstrated over the concentration range investigated by triplicate analysis (n=3) at a concentration range of 2-20 µg/ml. The absorbance obtained at respective concentration was recorded, and the graph is plotted as concentration (µg/ml) versus absorbance. The linear regression equation and the coefficient correlation were obtained from the UV probe software [6].
Method of accuracy
The accuracy of an analytical procedure expresses the closeness of agreement between the value which is accepted either as a conventional true value or an accepted reference value and the value found. This is sometimes termed trueness. The accuracy of proposed method was determined on the basis of recovery study. Recovery study was carried out by spiking standard working solution to sample solution (formulation) at three different levels 80%, 100% and 120%. The final concentration of Crizotinib was determined at each levels of the amount; three determinations were performed. The percentage recovery was calculated as mean ± standard deviation [7].
Method of precision
The precision of an analytical procedure expresses the closeness of agreement (degree of scatter) between a series of measurements obtained from multiple sampling of the homogeneous sample under the prescribed conditions. The precision of the method was demonstrated by intra-day and inter-day variation studies. In the intra-day precision study, three different solutions of same concentration were prepared and analysed in the same day (morning, noon and evening), whereas in the inter-day precision study, the solutions of same concentration were prepared and analysed, for three consecutive days, and the absorbance were recorded. All study was performed in triplicates. The result was indicated by calculating percentage RSD [8].
Method of robustness
The robustness of an analytical procedure is a measure of its capacity remains unaffected by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage [9].
Method of ruggedness
The ruggedness is a degree of reproducibility of test result under verification of condition like a different analyst, different instruments and different days [10].
Assay procedure
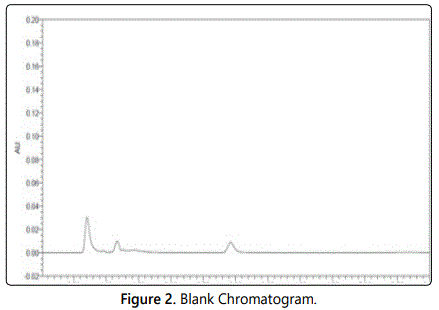
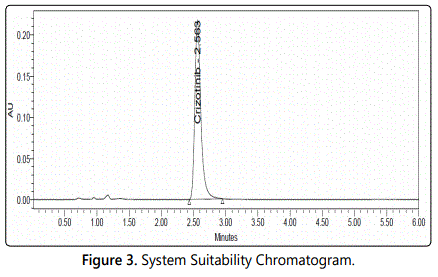
Column is equilibrated for 30 min with mobile phase. 20 µl of diluent as blank was injected into the system and recorded the chromatogram for a run time of 30 min (Figure 2). 20 µl of standard preparation-1 was injected into the system and recorded the chromatogram for a run time of 30 min. 20 µl of standard preparation-2 was injected into the system and recorded the chromatogram for a run time of 30 min. Test is valid only when the match factor is in between 0.98 to 1.02. 20 µl of standard preparation-2 into the system was separately injected for four times and recorded each chromatogram for a run time of 30 min. Test is valid only when the five standard preparation-2 injections pass the system suitability [11] (Figure 3).
Results and Discussion
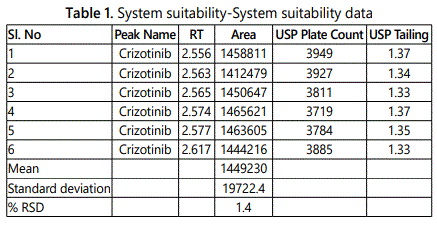
Results were shown in the below given tables and figures. Table 1 shows system suitability data.
Method of precision
Table 2 shows the repeatability data.

Observation
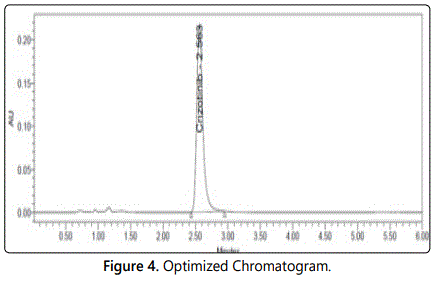
Crizotinib eluted with good peak shape and retention time and tailing was passed (Figure 4).
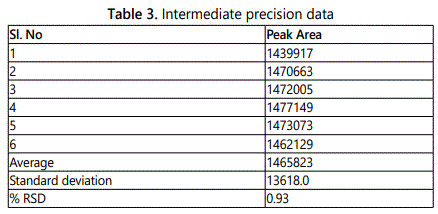
Intermediate precision (Table 3)
Six working sample solutions of 100 ppm are injected on the next day of the preparation of samples and the % amount found was calculated and %RSD was found to be 0.93 and chromatogram was shown in figure 3.
Method of linearity
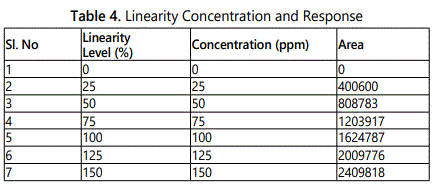
To demonstrate the linearity of assay method, inject 5 standard solutions with concentrations of about 37.5 ppm to 225 ppm of Crizotinib. Plot a graph to concentration versus peak area. Slope obtained was 21035 Y-Intercept was 892.5 and Correlation Co-efficient was found to be 0.999 and Linearity plot (Tables 4 and 5).
Conclusion
Chromatographic conditions used are stationary phase BDS 250 × 4.6 mm, 5 µ. Mobile phase buffer: Acetonitrile in the ratio of 60:40 and flow rate was maintained at 1 ml/min, detection wave length was 267 nm, column temperature was set to 30°C and diluents was methanol: Water (50:50), Conditions were finalized as optimized method. System suitability parameters were studied by injecting the standard five times and results were well under the acceptance criteria. Linearity study was carried out between 25% to 150% levels, r2 value was found to be as 0.999. Precision was found to be 1.26 for repeatability and 0.93 for intermediate precision. LOD and LOQ are 0.080 µg/ml and 0.243 µg/ml respectively. By using above method assay of marketed formulation was carried out 100.24% was present.
References
- Sethi PD. Quantitative analysis of Drugs & Pharmaceuticals. 3rd edition, CBS publishers and distributors, New Delhi. 2001: 1-120.
- PubChem. Compound Summary for CID 11626560: Crizotinib. Open Chemistry Database.
- Ramachandra B, Naidu NVS. Validation of RP -HPLC Method for Estimation of Dasatinib In Bulk and Its Pharmaceutical Dosage Forms. Int J Pharm Bio Sci. 2014; 4(1): 61-68.
- Konam K, Jadhav D. Development and validation of a RP- HPLC method for determination of dronedarone in bulk and pharmaceutical formulation Int J Pharm Bio Sci. 2014; 4(1): 179-185.
- Skoog DA, Holler FJ, Nieman TA. Principles of Instrumental Analysis. 7th edition, Cengage Learning. 2017: 973-995.
- Selvakumar S, Ravichandran S, Matsyagiri L. Development and Validation of analytical method for Simultaneous estimation of Ornidazole and Cefixime trihydrate tablet dosage forms by UV spectroscopy. Asian J Pharm Ana. 2016; 6(4): 246-252.
- Matsyagiri L, Jagadeesh P, Mounika B, et al. Effect of solvents on Spectrophotometric Estimation of Tinidazole in bulk and dosage forms. World Journal of Pharmaceutical Research. 2018; 7(9): 1742-1754.
- Jadhav PB, Shejwal V. Development and Validation of an RP-HPLC Method for Crizotinib. IJPPR Human. 2017; 9(2): 100-106.


