Research Article
Fear-based Stress associated with Sleep Quality
1Columbus VA Ambulatory Care Center, 420 N. James Road, Columbus, OH, USA
2Suffolk University, 73 Tremont Street, Boston, MA, USA
*Corresponding author: Olivia H Tousignant, Clinical Psychology Doctoral Student, Suffolk University, 73 Tremont Street, Boston, MA, USA, E-mail: ohtousignant@gmail.com
Received: October 23, 2017 Accepted: November 02, 2017 Published: November 09, 2017
Citation: Sullivan EL, Tousignant OH, Fireman GD. Fear-based Stress associated with Sleep Quality. Madridge J Behav Soc Sci. 2017; 1(1): 11-18. doi: 10.18689/mjbss-1000103
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective: We examine within-group differences in cardiovascular reactivity and recovery patterns following fear- versus anger-inducing stress tasks as they relate to sleep experience.
Methods: Eighty-seven college student volunteers (64.4% female) were randomized to either a fear task or an anger task. Heart rate and blood pressure measurements were taken at baseline prior to task initiation, throughout the task, and during post-task recovery intervals. Additionally, prospective sleep data was collected daily for two weeks.
Results: For participants in the fear-inducing stress condition, significant elevation in systolic blood pressure persisted into the laboratory task recovery period and was negatively associated with sleep quality. This effect was not found for any other cardiovascular variables nor for participants in the anger-inducing stress condition.
Conclusions: Participant response to a stress task that evoked fear was related to sleep experience whereas anger response was not. Our results suggest that there is variability in the relationships between different negative emotional stressors and sleep experience, in particular when considering both reactivity to and recovery from the stressor. Interventions for sleep should target methods of recovery from stress.
Keywords: Sleep, Stress, Fear, Anger, Cardiovascular, Recovery.
Introduction
Many people believe that they will not sleep well if they have significant psychological stress in their lives [1]. The extant research literature generally supports this relation; however, some investigators have reported instances when stress has not been detrimental to sleep [2]. Research suggests that the relationship between stress and sleep is most detrimental when perceived stress and poor sleep compound each other in an unhealthy cycle undermining adaptive functioning [3]. The unhealthy cycle often involves an individualʼs cognitive interpretation of perceived triggers (i.e., stress) as threatening oneʼs homeostatic state, which elicits physiological arousal. Through this recursive cognitive-somatic cycle, increases in arousal may endure into the pre-sleep period (pre-sleep arousal, PSA) and delay sleep onset [4, 5]. This process can result in poor sleep experience that, in turn, triggers cognitive preoccupation with the effects of fatigue on subsequent waking functioning. However, it is unclear which stress-induced response patterns have the strongest associations with poor sleep experience. Exploring the strengths of associations between certain forms of stress and sleep experience may elucidate important targets of intervention.
Current theories of insomnia treat all stressors as having equivalent impacts on the body and on sleep. The current study explored whether different forms of stress have different effects on physiology and sleep processes, and it examined emotion-specific stress responses in relation to sleep. Previous stress-sleep research has investigated how poor sleep experience affects physiology. For example, Kato and colleagues [6] found that sleep disruption produced increases in resting blood pressure and no changes in heart rate. Franzen and colleagues [7] reported a main effect of sleep deprivation on elevated systolic blood pressure, specifically, during a ten-minute recovery period after stress induction. Recently, researchers who studied prospective measures of normal sleep patterns in a healthy sample did not find heart rate elevations or blood pressure reactivity following stress tasks, nor did they notice enduring effects of stress on systolic blood pressure following a five-minute stress recovery period [8]. However, Mezick and colleagues [8] did observe significant negative associations between sleep duration and both diastolic blood pressure and heart rate recovery from stress. Additional evidence suggests that people with poor sleep habits are susceptible to higher stress-related blood pressure elevations [9, 10]. These inconsistent physiological findings within stress-sleep studies suggest that an important variable may be missing from our current understanding.
Seemingly inconsistent physiological patterns found in the stress-sleep literature may be explained by emotion specificity, the concept that different stressors exert different influences on the body. Kreibigʼs review [11] of emotion specificity suggests that brain, behavior, emotion, and motivation determine the effect that each type of stressor has on the body. If different emotions have differing effects on the body, then it stands to reason that different emotional stressors may have differing effects on sleep. This study explored that possibility. Fear and anger were emotions of interest in the current study because of their integral role in the “fight-or-flight” response and because of evidence that they exert distinct effects on the body [12]. This study explored the role of emotion specificity in the relationship between stress and sleep.
Fear and anger are central to the “flight-or-fight” stress response, respectively, and are generated by distinct aspects of the nervous and endocrine systems [13].Further, researchers have found evidence for cardiovascular response specificity for fear and anger [14, 15]. Contemporary constructionist models of emotion may help explain the response specificity finding. Constructionist models conceptualize emotions as existing along three dimensions: valence, arousal, and dominance [16, 17].Along these dimensions, fear is associated with negative valence, high arousal, and low dominance (i.e., submissiveness) and anger is associated with negative valence, high arousal, and high dominance [16, 18]. Differences in the dimension of dominance, in turn, may result in distinct behaviors, responses, and experiences [14, 15].
Anger and fear also are documented as common emotions experienced during sleep mentation, such as when having bad dreams and nightmares [19, 20]. Altogether, evidence suggests that waking experiences of anger and fear (i.e., experiencing a fight-or-flight response) may closely relate to sleep experience through persisting internal processes, such as sleep mentation. Although the exact role of cognition in the experience of emotion remains contested, it is generally accepted that cognitive appraisal operates in structuring emotional experience. Lazarus and Folkmanʼs Cognitive Appraisal Theory [21] denotes appraisal as a mediator of the stress reaction, central to the universal process by which people appraise the significance of a stressor to evaluate their personal well-being. Harveyʼs insomnia theory [4] implicates cognition as playing a central role in the initiation and perpetuation of a cycle of disturbed sleep. Therefore, when investigating relationships between fear, anger, and stress, it is fitting to implement tasks requiring deliberate cognitive engagement (e.g., memory and imagination) to induce fear and anger.
Within the procedures of recent studies on the relationship between stress and sleep, [7, 8] stress was induced using a two-part battery that included a feared speech task [22] and a word-interference cognitive Stroop task [23]. Despite the common comparison between fear and anger in stress literature, no study to date has compared the sleep-related effects of a fear-based stress induction task to an anger-based stress induction task. Our study adds to existing stress and sleep literature by exploring relationships between sleep and the variability of fear and anger stress reactions. Our procedure expands on the study design used by Mezick and colleagues [8] in which participants recorded seven days of sleep data at home and then presented to a laboratory for stress-induction and cardiovascular measurements. Further, we recruited a mixed-gender sample (rather than a primarily male sample [8]) and measured sleep experience prospectively for two weeks to better control for day-to-day variability in individual sleep patterns [24].
To examine commonly identified emotions related to the appraisal of stress we used cognitive tasks that induce fear and anger. First, we measured their effect on participantsʼ blood pressure and heart rate and then tested the relationship between those response patterns and subsequent sleep experiences. Using a healthy, young adult, mixed-gender sample, we compared relationships between sleep and fearversus anger-based cardiovascular reactivity and recovery. To our knowledge, the relationships between emotion-specific physiological recovery patterns and sleep experience have not yet been examined.
Based on previous research, we formulated three hypotheses. First, we expected to observe a negative association between prospective, self-reported stress and sleep variables such that when stress was high, sleep efficiency, duration, and quality would be poor. Second, we explored whether fear and anger would differentially affect heart rate and blood pressure reactivity and whether reactivity patterns would differentially relate to sleep experience. Finally, we predicted that delayed cardiovascular recovery from both fear and anger tasks—operationalized as a slow rate of return to baseline heart rate and blood pressure—would be significantly related to poor sleep.
Methods
The Institutional Review Board of the university where we conducted the study approved the investigation before implementation.
Participants
Eighty-seven college student volunteers between the ages of 18 and 25 years old (M = 19.32, SD = 1.68) who attended a mid-sized university in the northeastern United States provided data for the study. Thirty-one participants identified as male (35.6%) and 56 as female (64.4%). Sixty-two participants identified as White (71.3%), five as Black, five as Hispanic, five as Asian, five as “Mixed,” and five as “Other.” This gender and racial make-up is consistent with the universityʼs general population. At the time of sign up, experimenters requested that individuals who use medication(s) that depresses that central nervous system on a regular basis (e.g., benzodiazepines, alcohol, and/or recreational drugs) not participate in the study. Substances that influence the functioning of the central nervous system could have artificially influenced physiological measurements, obscuring the relationship between cardiovascular response, psychological stress, and sleep variables.
Procedure
For two weeks, participants completed daily stress and sleep measures upon waking. After completing these self-report measures via a secure, cloud-based web service, participants presented to a laboratory to engage in one of the two experimental stress tasks.
Prospective stress and sleep measures
The Daily Stress Inventory [25] (DSI) assessed daily psychological stress for two weeks. Participants indicated which items on a list of life events had occurred within a 24-hour period (the list contained 58 items with two “other” write-in options that accommodated additional items). For each event endorsed, participants included an impact rating ranging from 1 (occurred but was not stressful) to 7 (caused me to panic) on a Likert scale. Nineteen items on the measure were eliminated to decrease time burden for participants. Excluded items were expected to have low base rates in an undergraduate population. We calculated the frequency (FREQ) of stress events, the sum (SUM) of event impact ratings, and the average impact rating (AIR) of stress events. AIR was calculated via dividing SUM by FREQ.
The Sleep/Dream Checklist [26] (SDC) measured sleep duration, sleep efficiency, and sleep quality. Sleep duration represented the average number of minutes slept across the two-week period, which was calculated by dividing the total minutes of sleep reported by the number of days on which the participant completed a survey. Sleep efficiency was calculated as a percentage via dividing sleep duration by total time spent in bed, and multiplying that value by 100. Sleep quality was measured on an nine-point Likert scale with ratings from poor to excellent. We calculated an average sleep quality score across the two-week period.
Experimental tasks
Participants were asked to refrain from caffeine use, tobacco use, and strenuous exercise the night before and the day of their experimental task participation. Before beginning a stress task procedure, experimenters attached electrocardiogram (EKG) leads and a blood pressure cuff to participants. Then, for fifteen minutes, participants sat comfortably in an upright position and listened to soothing music. Experimenters advised participants to remain still during measurement. An automated television display signaled the beginning and end of the fifteen-minute period. Experimenters excluded the first five minutes of measurement from study analyses to account for acclimation. The subsequent ten minutes provided baseline cardiovascular data.
Participants then completed one of two experimental tasks. The independent variable, emotion specificity, was manipulated through use of either a fear or anger stress-induction task. Block randomization determined participant assignment to condition. For the fear task, experimenters instructed participants to prepare a speech and then allowed three minutes to deliver the speech to a video camera while the experimenter rated participantsʼ speaking performance. Experimenters instructed participants to imagine a scenario in which a fictitious security guard had accused them of shoplifting. Participants constructed a speech to defend their innocence. Before the speech preparation period, a video recording cued participants to begin preparing the speech. A timer on a television counted down from two to zero minutes to mark the preparation period, and then from three to zero minutes to mark the speech period. During both periods, the timer font changed from white to red when thirty seconds remained. At the end of the preparation period, a video recording instructed participants to begin the speech. At the end of the speech period, a video instructed participants to stop speaking. In view of participants, experimenters evaluated speeches by silently marking a rating form, and they did not provide performance feedback to participants. A similar task has evoked fear in prior studies [7].
For the experienced anger task, experimenters allotted two minutes to think about a recent experience of feeling angry. As in the fear task, a visual two-minute timer appeared on a television screen to mark the time available for recall. After two minutes, a video recording cued participants to describe the experience on video in the presence of an experimenter for three minutes. A timer on the television screen marked the time available for the discussion. At the end of the three-minute period, a video recording informed participants that the discussion period ended. Experimenters did not evaluate participantsʼ performance during the anger task. A similar task has evoked anger in prior studies [27, 28].
Following both tasks, participants quietly listened to soothing music for ten minutes while seated comfortably in an upright position. This period represented recovery time, which provided variables for recovery analyses and ensured that participants would leave the laboratory feeling relatively calm. After recovery, experimenters removed the EKG leads and blood pressure cuff, then debriefed participants.
Apparatus
A Lablinc V (Vertical) modular physiological measurement device (Coulbourn Instruments) provided heart rate, systolic blood pressure, and diastolic blood pressure measurements for all participants. To measure these cardiovascular variables, experimenters used the most commonly applied equipment settings. For HR, “gain x1000” was set to zero (sb 0/30), the high cutoff for the filter was set to forty hertz (Hz), and the low cutoff of the filter was set to eight Hz. The coupling value was set to one Hz, Gain x10, and percent gain to 100. An automatically inflating cuff was used to obtain arterial BP. WinDaq Waveform Browser playback and analysis software provided live EKG feeds and recorded heart rate, systolic blood pressure, and diastolic blood pressure values (DATAQ Instruments, Akron, Ohio 44333 http://www.dataq.com/products/software/playback.htm).
Experimenters used a standard Lead (EKG) placement for three electrodes to measure heart rate reactivity and recovery [29]. Experimenters placed two electrodes above a bone on the surface of the forearm near the radial artery of each distal radius (i.e., the underside of each wrist). Next, they placed the reference lead near the right ankle above the nearest bone that is superior to the lateral malleolus. They taped all leads to participantsʼ arms and legs to minimize electrode movement, and they asked each participant to minimize body movement throughout physiological measurement. Heart rate measurements were sampled continually every one-tenth of a second from the beginning of the acclimation period (i.e., fifteen minutes prior to starting the task) through the end of the recovery period (i.e., ten minutes after finishing the task). Systolic blood pressure and diastolic blood pressure were measured at five-minute intervals. Blood pressure readings occurred at minute zero of acclimation, and at minutes five, ten, and fifteen for the baseline period. Task period measurement occurred after task completion. Blood pressure measurement occurred at the beginning of the post-task recovery period as well as at minutes five and ten (the last minute) of the recovery period.
Manipulation check
To verify that the anger and fear tasks significantly elevated heart rate, systolic blood pressure, and diastolic blood pressure relative to baseline, a series of paired samples t-tests was conducted. Experimenters also conducted paired samples t-tests to compare baseline heart rate, systolic blood pressure, and diastolic blood pressure to carryover heart rate, systolic blood pressure, and diastolic blood pressure (Table 1). Comparisons were analyzed across and within tasks. Overall, heart rate, systolic blood pressure, and diastolic blood pressure were significantly higher during the stress tasks than during the baseline period.
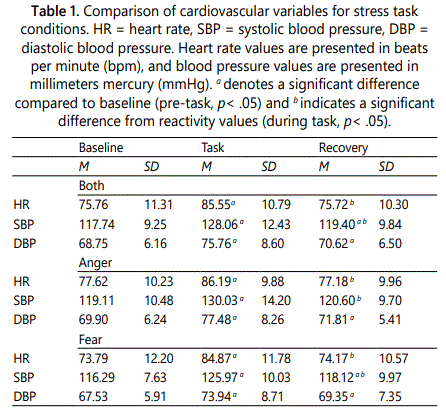
Results
Alpha criterion for significance was set to 0.05 for all analyses.
Descriptive sleep analyses
Participantsʼ average daily sleep duration ranged from 273.93 minutes (4.57 hours) to 616.27 minutes (10.27 hours), M = 455.36 minutes (7.59 hours) and SD = 65.37 minutes (1.09 hours). Daily sleep efficiency ranged from 68% to 98% (M = 89%, SD = 6%). Daily reported sleep quality ranged from 3 “fair sleep” to 9 “excellent sleep,” M = 5.09, SD = 1.08.
The process of random assignment to stress task condition was effective. Independent samples t-tests revealed that for both the anger and fear task conditions, participants exhibited equivalent baseline heart rate, t (68) = 1.43, p = .16, systolic blood pressure, t (68) = 1.28, p = .20, and diastolic blood pressure, t (68) = 1.62, p = .11. There were no statistically significant differences in age between task conditions, t(68) = 1.03, p = .31. A chi-square analysis revealed no statistically significant differences in gender between task groups, χ2 = 2.03, p = .15.
We conducted three standard multiple regression analyses to evaluate associations between prospective stress ratings and sleep experience. We entered FREQ and AIR (from the DSI) as predictor variables and sleep duration, sleep efficiency, and sleep quality as outcome variables in each equation. We excluded the DSI SUM score from these analyses because it has been found to correlate highly with AIR [25] as a measure of perceived stress impact.
High prospective daily stress related to poor sleep quality (R = .43, p = .001) and poor sleep efficiency (R = .32, p = .03). The coefficients for both FREQ and AIR significantly related to sleep quality (p = .04 and p = .003, respectively). The partial correlation coefficient for the relationship between FREQ and sleep quality was r = -.25 and between AIR [25] and sleep quality was r = -.35. Only the coefficient for FREQ significantly correlated with sleep efficiency (p = .02). The partial correlation coefficient for FREQ and sleep efficiency was r = -.29. An omnibus regression equation using SD as an outcome variable did not reach significance. Consistent with our first hypothesis, both a high frequency of stressful events and high daily stress impact ratings related to poor sleep quality and efficiency.
To evaluate the hypothesis that cardiovascular reactivity would predict sleep experience, we subtracted withinparticipant heart rate and blood pressure values during tasks from baseline values to create a simple delta (change) variable. We computed six standard multiple regression equations (three for the fear task and three for the anger task) by entering delta heart rate, delta systolic blood pressure, and delta diastolic blood pressure as predictor variables with the three sleep variables as outcomes. Cardiovascular reactivity did not correlate with any of the study sleep variables (anger task, R = .13, p = .91; fear task, R = .22, p = .67), which did not support our hypotheses that cardiovascular reactivity would relate to poor sleep.
Finally, we assessed the hypothesis that poor recovery (i.e., carryover) from cardiovascular stress (i.e., slow return of heart rate and blood pressure variables to baseline values) would relate to poor subjective sleep experience. Carryover values were calculated by subtracting participantsʼ average heart rate and blood pressure values obtained during the recovery period from those obtained during task periods. These differences reflect cardiovascular arousal from the stress tasks that persisted into the recovery period. We entered carryover heart rate, carryover systolic blood pressure, and carryover diastolic blood pressure as predictor variables into multiple regression equations with the three sleep variables as outcomes. Within the anger task, carryover arousal was not associated with sleep experience. Within the fear task, the omnibus regression equation demonstrated a statistically significant relationship between carryover arousal and sleep quality, with carryover systolic blood pressure emerging as a unique predictor of sleep quality (β = -0.462, p = .01).
Discussion
Consistent with prior research, a strong association between stress and poor sleep experience emerged. Interestingly, for both the anger and fear stress induction tasks cardiovascular reactivity was not associated with poor sleep experience. There was also no significant association between sleep experience and cardiovascular recovery from the anger task. In contrast, there was a significant association between cardiovascular recovery from the fear stress task and sleep experience. Participants who demonstrated slower recovery from the fear task reported poorer prospective sleep quality. This finding suggests that individual differences in cardiovascular recovery patterns following fear-related stress experiences may have a relationship with sleep disruption, whereas anger-related stress may not. This distinction between cardiovascular reactivity and recovery has received limited attention in the literature and highlights the value of techniques that focus on stress recovery and sleep health.
The cognitive model of insomnia [4] best contextualizes the results of the current study. In this model, perceived stressors are precipitating factors of sleep disruption when they lead to repetitive negative thought (i.e., cognitive arousal), such as rumination and worry. These cognitive processes trigger somatic arousal, which may be interpreted as an indicator of threat that initiates more rumination and worry and contributes to a maladaptive cycle that disrupts sleep processes. Although this study does not investigate rumination and worry, it assesses the perceived frequency of stressors and their cumulative stress impact. Both measures were negatively associated with sleep experience. This finding is consistent with those of previous studies [30, 31] and suggests that individual differences in reaction to stress may moderate the influence of stress on sleep. Specifically, whereas stress appears to be a detriment to sleep for most people, individuals with prolonged reactions to stress may experience the worst sleep problems. The results of this study suggest that one avenue through which perceived stress influences sleep is through delayed cardiovascular recovery following fear. Furthermore, the results of this study suggest that each emotion may elicit differing effects upon the body and sleep. There is historical precedent for this supposition.
In the early twentieth century, researchers began investigating the nuanced cardiovascular responses of emotions. Marston [32] found that both anger and fear are accompanied by a rise in systolic blood pressure such that anger is associated with a rise of shorter duration while fear is associated with a rise of longer duration. Later, anger and fear were found to be associated with increased epinephrine and norepinephrine with inconsistent response patterns and intensities [33, 34]. Expanding on evidence of qualitative differences in physiological responses following anger and fear states, Ax [35] proposed that investigating psychophysiological patterns of specific emotions would be a worthwhile endeavor for the field. For some time, standard stimulus induction paradigms were used to study emotional experiences, but they did not capture individual differences inherent in emotional experiences [36].
Recognizing this lack, Roberts and Weerts [37] introduced individually tailored imaginal fear and anger emotioninduction tasks to assess cardiovascular responding. In their study, Roberts and Weerts [37] found no significant differences in heart rate changes following fear and anger induction yet noted significantly greater increases in diastolic blood pressure following anger induction compared to fear induction. They also found an equivalent increase in systolic blood pressure across fear and anger inductions. Although these findings differed from the current studyʼs results, they support a hypothesis that different emotions may provoke differing physiological responses. Unfortunately, the researchersʼ limited sample size (n = 16) restricted the power of the results. Using a larger sample size, Stemmler [12] found increases in heart rate, systolic blood pressure, and diastolic blood pressure following fear and anger induction. More recently, Lupis, Lerman, and Wolf [38] found a significant positive association between anger and heart rate for men but not women, and no significant association between fear and heart rate or blood pressure within the sample. Overall, additional research on cardiovascular response specificity following fear and anger stress-inductions may elucidate important mechanisms that govern the emotional branches of the fight-or-flight stress response and sleep.
Considering that the relationship between stress and sleep has potential to promote optimal functioning [3], it is worthwhile to continue investigating how anger and fear translate into sleep states (e.g., through nightmares) to shape intervention priorities. Rather than stress in general being associated with poor sleep, it may be the case that carryover responses induced by certain forms of emotional stress have the strongest associations with poor sleep. Indeed, the current study results suggest that it may not be the individualʼs initial reaction to fear that is most related to sleep experience, but rather, the process of calming down (i.e., recovering to baseline). This distinction indicates that sleep interventions may be most beneficial when they help a client return to baseline cardiovascular functioning following a stress experience rather than minimizing a clientʼs reactivity to stimuli. Our findings support the trend of teaching relaxation techniques as adaptive coping skills. It may also be beneficial to study the effects of rumination and worry on the relationship between stress and sleep since these two processes may interfere with relaxation.
Individual differences in coping resources may help explain why stress affects the sleep of some people but not others. For example, disrupted sleep may reflect the increased perception of stress [39] and people with more negative affect may experience a stronger relationship between stress and sleep [40]. The primary and unique finding from the current study – that a significant negative association with cardiovascular recovery and sleep quality emerged for participants in the fear stress condition – suggests that different forms of emotion-related stress may elicit distinct physiological, psychological, and/or behavioral responses [12] with varied influences on sleep. It may be the case that different emotions require different types of coping strategies to facilitate healthy stress processing and sleep. The current findings suggest that people with stress-related sleep difficulties may benefit from interventions targeting waking fear responses. Considering that poor sleep may impair the ability to regulate negative emotions [41], it may be effective to utilize distress tolerance and emotion-focused coping strategies aimed at reducing the duration and intensity of fear-based physiological arousal.
A second interest of this investigation was whether traitlike cardiovascular reactivity to stress predicted poor sleep experience. Within this sample, cardiovascular reactivity to stress did not predict sleep experience, which is inconsistent with previous research. Differences in study design may explain the contrast. For example, experiments that have measured stress reactivity immediately before bedtime in a laboratory setting have found an association between physiological arousal and disrupted sleep [42]. The lack of association between cardiovascular reactivity and sleep variables in the current study may be explained by the singular measurement of reactivity, compared to the measurement of recovery, which was taken at multiple time points. A more reliable exploration of the relationship between stress reactivity and sleep may require multiple measurements of heart rate and blood pressure immediately following stress induction [28]. Although we did not find an association between cardiovascular reactivity and sleep experience, the findings on recovery suggest that stress is highly relevant, and that fear-inducing stress has potential to prolong the elevation of systolic blood pressure and disrupt sleep. The current study adds to the literature by demonstrating that fear-inducing stressors have longer lasting cardiovascular impacts than anger-inducing stressors. Future research may benefit from using experimental designs to clarify the causal mechanisms involved in relationships between perceived stress, sleep, and cardiovascular variables.
In recent studies, sleep deprived participants exhibited heightened cardiovascular reactivity [7] and prolonged blood pressure recovery following stressful experiences [8]. These findings indicate that the strength of the relationship between cardiovascular arousal and sleep quality increases as people are deprived of sleep. One speculation on the causal nature of this relationship may be that lack of sleep elicits hypervigilance and hypothalamic-pituitary-adrenal (HPA) axis activation [43], such that any perceived stressor signifies the presence of threat and, thus, more readily maintains arousal. The need for hypervigilance may be conveyed through cellular inflammatory signaling that has been found to contribute to cardiovascular disorders in people who are sleep deprived [44].
Furthermore, people who have not been sleep deprived may process certain forms of stress more easily. Our findings suggest that fear-induced stress may signify a need for hypervigilance in healthy sleepers and initiate high blood pressure for a relatively longer period compared to anger-induced stress. Suggesting a self-reinforcing cyclical mechanism, prior research has shown that people with high blood pressure may have a hyperreactive cardiovascular system and experience heightened HPA axis responses to stress [45]. Considering that fear and anger are generally considered equivalent on dimensions of arousal (high) and valence (negative), it may be that the low degree of perceived control associated with fear (relative to the high perceived control associated with anger) is the component of the emotional experience that relates most closely to sleep and blood pressure elevation. Our study suggests that intervention efforts aimed at increasing clientsʼ self-efficacy could improve sleep experiences of people who take longer to recover to baseline blood pressure following stress experiences.
Future Directions and Limitations
The primary use of undergraduate students limits the external validity of the results since sleep habits of college students may not represent an older adult population. Participants were generally healthy sleepers without known sleep disorders, which prevents the application of our findings to individuals with sleep problems. It may be beneficial to investigate processes involved in the relationship between stress and sleep that affect both good and poor sleepers. Examples of these processes may include rumination, worry, and/or general coping style [4, 46, 47].
The one-time induction of fear and anger limits the degree to which we can extract information about directionality within the stress-sleep relationship. Future studies could also assess personality characteristics and other individual difference variables as moderators of the relationship between the fightor-flight response and sleep experience. For example, hostility may be associated with cardiovascular stress recovery [48], suggesting that this emotion-related interpersonal style could be relevant to the stress-sleep literature. In a hypothetical study designed to measure differences in trait hostility, participants could be separated into high and low hostility groups. Each group would undergo fear or anger induction before bedtime, group membersʼ sleep would be measured via actigraphy during the night, and self-report sleep variables would be assessed in the morning. Inclusion of a control condition (i.e., no emotion induction) may reveal the degree to which stressful threat-based emotions, together, affect functioning.
The use of self-report sleep measures can be considered both a strength and limitation of our study. Sleep measures were taken over the course of fourteen days, which enhanced reliability. However, participantsʼ answers may have been influenced by error variance typical of self-report measures, such as social desirability and demand characteristics [49]. It may be beneficial to use multimethod sleep assessments, including subjective and objective measures [50]. Supplementing self-reports with objective sleep measures (e.g., actigraphy and/or polysomnography) and more ecologically valid, objective cardiovascular measures (e.g., ambulatory monitoring) may help elucidate nuanced patterns within relationships between stress, cardiovascular reactivity and recovery, and sleep.
Conclusion
Although there is strong and consistent evidence across studies for the relationship between stress and sleep, the current study highlights that this association is complex. Importantly, we found that fear and anger producing stress did not have equivalent effects on sleep. Rather, it appears that fear-related stress is more problematic for sleep than is angerrelated stress. Future research is needed to confirm this finding and further clarify mechanisms that create a differential impact on sleep. The current findings highlight the value for clinicians in practice to mitigate the effect of fear-related stress on sleep, possibly in a manner that enhances clientsʼ sense of control and acceptance in response to fear-inducing stressors.
Formatting of Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Kim EJ, Dimsdale JE. The effect of psychosocial stress on sleep: A review of polysomnographic evidence. Behavioral Sleep Medicine. 2007; 5: 256-278. doi: 10.1080/15402000701557383
- Garde AH, Albertsen KA, Persson R, Hansen AM, Rugulies R. Bi-directional associations between psychological arousal, cortisol, and sleep. Behavioral Sleep Medicine. 2012; 10: 28 -40. doi: 10.1080/15402002.2012.636272
- Harvey AG. A cognitive model of insomnia. Behavior Research and Therapy. 2002; 40: 869-893. doi: 10.1016/S0005-7967(01)00061-4
- Nicassio P, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: The development of the pre-sleep arousal scale. Behaviour Research and Therapy. 1985; 23(3): 263-271. doi: 10.1016/0005-7967(85)90004-X
- Kato M, Phillips BG, Sigurdsson G, et al. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000; 35: 1173-1175. doi: 10.1161/01.HYP.35.5.1173
- Franzen PL, Gianaros PJ, Marsland AL, et al. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosomatic Medicine. 2011; 73(8): 679-682. doi: 10.1097/PSY.0b013e31822ff440
- Mezick EJ, Matthews KA, Hall MH, Jennings R, Kamarck TW. Sleep duration and cardiovascular responses to stress in undergraduate men. Psychophysiology. 2014; 51(1): 88-96. doi: 10.1111/psyp.12144
- Massar SA, Liu JC, Mohammad NB, Chee MW. Poor habitual sleep efficiency is associated with increased cardiovascular and cortisol stress reactivity in men. Psychoneuroendocrinology. 2017; 81: 151-156. doi: 10.1016/j.psyneuen.2017.04.013
- Ulmer CS, Bosworth HB, Germain A, et al. Associations between sleep difficulties and risk factors for cardiovascular disease in veterans and active duty military personnel of the Iraq and Afghanistan conflicts. Journal of Behavioral Medicine. 2015; 38(3): 544-555. doi: 10.1007/s10865-015-9627-4
- Kreibig SD. Autonomic nervous system activity in emotion: A review. Biological Psychology. 2010; 84: 394-421. doi: 10.1016/j.biopsycho.2010.03.010
- Stemmler G, Heldmann M, Pauls CA, Scherer T. Constraints for emotional specificity in fear and anger: The context counts. Psychophysiology. 2001; 38(2): 275-291. doi: 10.1111/1469-8986.3820275
- Lӧvheim H. A new three-dimensional model for emotions and monoamine neurotransmitters. Medical Hypotheses. 2012; 78(2): 341-348. doi: 10.1016/j.mehy.2011.11.016
- Chatelain M, Silvestrini N, Gendolla GH. Task difficulty moderates implicit fear and anger effects on effort-related cardiac response. Biological Psychology. 2016; 115: 94-100. doi: 10.1016/j.biopsycho.2016.01.014
- Montoya P, Campos JJ, Schandry R. See red? Turn pale? Unveiling emotions through cardiovascular and hemodynamic changes. Spanish Journal of Psychology. 2005; 8(1): 79-85. doi: 10.1017/S1138741600004984
- Jerram M, Lee A, Negreira A, Gansler D. The neural correlates of the dominance dimension of emotion. Psychiatry Research: Neuroimaging. 2014; 221: 135-141. doi: 10.1016/j.pscychresns.2013.11.007
- Russell JA. A circumplex model of affect. Journal of Personality & Social Psychology. 1980; 39: 1161-1178. doi: 10.1037/h0077714
- Russell JA, Mehrabian A. Distinguishing anger and anxiety in terms of emotional response factors. Journal of Consulting and Clinical Psychology. 1974; 42(1): 79-83. doi: 10.1037/h0035915
- Merritt JM, Stickgold R, Pace-Schott E, Williams J, Hobson JA. Emotion profiles in the dreams of men and women. Consciousness and Cognition. 1994; 3: 46-60. doi: 10.1006/ccog.1994.1004
- Fireman GD, Levin R, Pope AW. Narrative qualities of bad dreams and nightmares. Dreaming. 2014; 24(2): 112-124. doi: 10.1037/a0035791
- Marsland AL, Manuck SB, Fazzari TV, Stewart CJ, Rabin BS. Stability of individual differences in cellular immune responses to acute psychological stress. Psychosomatic Medicine. 1995; 57: 295-298. doi: 10.1097/00006842-199505000-00012
- Bush G, Shin LM, Holmes J, Rosen, BR, Vogt BA. The multi-source interference task: Validation study with fMRI in individual subjects. Molecular Psychiatry. 2003; 8: 60-70. doi: 10.1038/sj.mp.4001217
- Brantley PJ, Waggoner CD, Jones GN, Rappaport NB. A daily stress inventory: Development, reliability, and validity. Journal of Behavioral Medicine. 1987; 10(1): 61-74. doi: 10.1007/BF00845128
- Levin R, Fireman G. Nightmare prevalence, nightmare distress, and selfreported psychological disturbance. SLEEP. 2002; 25(2): 205-212. doi: 10.1093/sleep/25.2.205
- Lobbestael J, Arntz A, Wiers RW. How to push someoneʼs buttons: A comparison of four anger-induction methods. Cognition and Emotion. 2008; 22(2): 353-374. doi: 10.1080/02699930701438285
- Rutledge T, Linden W, Paul D. The stability of cardiovascular reactivity: Effects of task type and family history over a three-year interval. International Journal of Behavioral Medicine. 2001; 8: 293-303. doi: 10.1207/s15327558ijbm0804_4
- Marston WM. Sex characteristics of systolic blood-pressure. Journal of Experimental Psychology. 1923; 6: 387-419. doi: 10.1037/h0070058
- Ax AF. The physiological differentiation between fear and anger in humans. Psychosomatic Medicine. 1953; 15: 433-442. doi: 10.1097/00006842-195309000-00007
- Ax AF. Goals and methods of psychophysiology. Psychophysiology. 1964; 1: 8-25. doi: 10.1111/j.1469-8986.1964.tb02616.x
- Lang PJ. A bio-informational theory of emotional imagery. Psychophysiology. 1979; 16: 495-512. doi: 10.1111/j.1469-8986.1979.tb01511.x
- Roberts RJ, Weerts TC. Cardiovascular responding during anger and fear imagery. Psychological Reports. 1982; 50: 219-230. doi: 10.2466/pr0.1982.50.1.219
- Lupis SB, Lerman M, Wolf JM. Anger responses to psychosocial stress predict heart rate and cortisol stress responses in men but not women. Psychoneuroendocrinology. 2014; 49: 84-95. doi: 10.1016/j.psyneuen.2014.07.004
- Friedman L, Brooks JO III, Bliwise DL, Yesavage JA, Wicks DS. Perceptions of life stress and chronic insomnia in older adults. Psychology and Aging. 1995; 10(3): 352-357. doi: 10.1037/0882-7974.10.3.352
- Fortunato VJ, Harsh JR. Stress and sleep quality: The moderating role of negative affectivity. Personality and Individual Differences. 2006; 41: 825-836. doi: 10.1016/j.paid.2006.03.024
- Mauss IB, Troy AS, LeBourgeois MK. Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cognition and Emotion. 2013; 27(3): 567-576. doi: 10.1080/02699931.2012.727783
- Minkel J, Moreta M, Muto J, et al. Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychology. 2014; 33(11): 1430-1434. doi: 10.1037/a0034219
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R et al. Sleep loss activates cellular inflammatory signaling. Biological Psychiatry. 2008; 64(6): 538-540. doi: 10.1016/j.biopsych.2008.05.004
- Nyklíček I, Bosch JA, Amerongen AV. A generalized physiological hyperactivity to acute stressors in hypertensives. Biological Psychology. 2005; 70: 44-51. doi: 10.1016/j.biopsycho.2004.11.013
- Sadeh A, Keinan G, Daon K. Effects of stress on sleep: The moderating role of coping style. Health Psychology. 2004; 23(5): 542-545. doi: 10.1037/0278-6133.23.5.542
- Yeh Z, Wung S, Lin C. Pre-sleep arousal as a mediator of relationships among worry, rumination, and sleep quality. International Journal of Cognitive Therapy. 2015; 8(1): 21-34. doi: 10.1521/ijct.2015.8.1.21
- Suarez EC, Williams RB. Situational determinants of cardiovascular and emotional reactivity in high and low hostile men. Psychosomatic Medicine. 1989; 51: 404-418. doi: 10.1097/00006842-198907000-00004
- Jackowska M, Ronaldson A, Brown J, Steptoe A. Biological and psychological correlates of self-reported and objective sleep measures. Journal of Psychosomatic Research. 2016; 84: 52-55. doi: 10.1016/j.jpsychores.2016.03.017


