Research Article
Socio-Demographic and Maternal Risk Factors of Malaria among Pregnant Women attending AnteNatal Care in Zamfara State, Nigeria
1
All Babies Are Equal, Non-Governmental Organization New Incentives, Zamfara State, Nigeria
2
Department of Community Health, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor
Darul Ehsan, Malaysia
3
Department of Planning, Research and Statistics, Ministry of Animal Health and Livestock Development, Gusau, Zamfara State, Nigeria
*Corresponding author: Hejar Abdul Rahman, Department of Community Health, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 Serdang, Selangor, Darul Ehsan, Malaysia, E-mail: hejar@upm.edu.my
Received: September 12, 2017 Accepted: December 27, 2017 Published: January 2, 2018
Citation: Hadiza K, Rahman HA, Hayati KS, Ismaila UG. Socio-Demographic and Maternal Risk Factors of Malaria among Pregnant Women attending Ante-Natal Care in Zamfara State, Nigeria. Madridge J Womens Health Emancipation. 2018; 2(1): 41-45. doi: 10.18689/mjwh-1000109
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Malaria is a major global health concern. It is one of the worldʼs most prevalent serious infectious diseases, with approximately 250 million cases and one million deaths per year. The aim of this study is to determine the socio-demographic and maternal risk factors associated with malaria among pregnant women attending ante-natal care in Zamfara State, Nigeria.
An unmatched case control study was conducted among pregnant women attending ante-natal care in Zamfara State, Nigeria. A total of 581 pregnant women both cases and controls were obtained using multistage random sampling. Cases and controls were defined as pregnant women attending ante-natal care from the selected general hospitals in Zamfara State, confirmed with and without malaria respectively, using Giemsa staining method based on their medical records. Face to face interview and self-administered pretested questionnaire in English and Hausa languages were used to obtain information on socio-demographic characteristics and maternal history of the respondents from May to August 2014. Data was analysed using SPSS version 21, using simple descriptive and multivariate logistic regression were employed to determine the predictors of malaria.
The overall response rate was 89.8%. Pregnant women ≤ 25 years of age (AOR=1.695, 95% CI=1.031, 2.789, p=0.038), informal education (AOR=9.390, 95% CI=5.516, 15.985, p<0.001), unemployed (AOR=25.948, 95% CI=14.831, 45.398, p<0.001) and first trimester (AOR=1.856, 95% CI=1.126, 3.060, p=0.015) were the risk factors.
This study has identified the risk factors of malaria in Zamfara State, Nigeria. The findings in this study can be used by policy makers in planning how to tackle the factors associated with malaria among pregnant women in Zamfara State.
Keywords: Malaria; Pregnancy; Socio-demography; Maternal history; Nigeria.
Introduction
Malaria still remains a key public health issue globally. In 2013, about 3.3 billion people were at risk of malaria infection. The World Malaria Report indicated that Africa bears the heaviest burden and the highest risk of malaria infection. Hence, about 82% of the reported malaria cases and about 90% of the reported deaths occur in Africa, majority were children below five years and pregnant women living in malaria-endemic regions [1]. In 2013, there were approximately 198 million cases and 584,000 deaths [1], it was estimated that one death occurs each 30 seconds with 90% of malaria deaths occurring in Sub-Saharan Africa, and 90% of malaria deaths are pregnant women and children [2]. Nigeria and the Democratic Republic of the Congo together accounted for 39% of the worldwide total of predictable malaria deaths and 34% of cases in 2013 (WHO 2014). Malaria control still remains a challenge in Africa, as evidenced by the 163 million estimated cases and 528,000 deaths in 2013 [1].
Nigeria is included among the 45 countries that are endemic for malaria, and about 97% of the population were at risk especially children and pregnant women [3-5]. The geographical location of Nigeria makes the environment suitable for malaria transmission throughout the country, only 3% of the population living in mountains southern Jos, Plateau State, at an altitude ranging from 1,200 to 1,400 metres, are at moderately low risk of malaria. Due to the large population, about 140 million people lives in areas of high malarial transmission [6].
Malaria in pregnancy is a serious health problem, both for the pregnant women and her foetus with 11% of maternal deaths occurring annually in Nigeria [7, 8]. Malaria in pregnancy has been identified in many studies to worsen certain pregnancy outcomes, leading to an increase in morbidity and mortality. Pregnant women are susceptible to malaria as pregnancy decreases a womanʼs immunity to malaria, making them more vulnerable to anemia, placental parasitic infestation and increasing the risk of illness leading to death. For the unborn babies, maternal malaria increases the risk of spontaneous abortion, stillbirth, premature delivery and low birth weight. Hence, is a leading cause of child and maternal mortality [9].
The increased risk of malarial infection among pregnant women could be related to illiteracy, low educational status, unemployment, low income and gravidity of the pregnant women [10-12].Young maternal age has been identified as an independent risk factor for malarial infection[13,14]. Malaria also exerts a huge social and economic burden on families, communities, and the country at large, causing an annual loss of about 132 billion naira in payments for treatment and prevention as well as hours not worked [15].
Recently, the worldʼs first malaria vaccine has been approved and given a positive opinion by the Europeʼs drug regulator, European Medicines Agency (EMA), setting the stage for a final assessment by the World Health Organization (WHO). If the vaccine gains approval from the WHO, then GlaxoSmithKline will market the drug to individual African countries. The EMA declares the vaccine, Mosquirix, is safe for use in children aged 6 weeks to 17 months [16, 17].
Materials and Methods
Study design
An unmatched case control study was conducted among pregnant women attending ante-natal care in six general hospitals Zamfara State, north-western Nigeria.
Data collection
There are three senatorial zones and 14 local governments in Zamfara State. Two local governments were selected from each of the three senatorial zones using simple random sampling and each of the local governments had one general hospital. An estimated sample size of 243 was obtained and 10% attrition ratio (24) was added for non-response [18, 19]. Using 1:1 ratio, 267 cases and 267 controls were obtained, given a total sample size of 534.
A total of 581 eligible respondents were selected using multistage random sampling. Based on the medical records of the pregnant women attending ante-natal care in each of the selected hospital, the respondents were sub-grouped into cases and controls based on their malarial status using Giemsa staining method. Those who were positive were classified as cases while those who were negative were the control group. Using SPSS, simple random sampling was used to select the study subjects within each group based on their record.
Face to face interview and self-administered pretested questionnaire in English and Hausa languages was used to obtain information on socio-demographic characteristics such as age, ethnicity, religion, marital status, educational status, occupation and monthly income. Maternal history of the respondents which includes gravidity and trimester of the respondents was also obtained.
Ethical Clearance
Consent was obtained from relevant institutions. The participants were provided with consent form and fact sheet containing information about the study, signed consent was obtained from the participants before the questionnaire administration and confidentiality of the information obtained from the pregnant women was assured.
Data Analysis
The data was analyzed using statistical package for social sciences (SPSS) version 21, a simple frequency analysis was used to describe the socio-demographic characteristics of malaria for the selected case group (n=261) and control group (n=261). Chi-square test was used to investigate the relationship between the two groups; simple logistic regression was used to determine the risk factors and multivariate analysis using forward stepwise (likelihood ratio) was used to determine the predictors of malaria; p < 0.05 was taken as significant level.
Table 1 below shows the association between socio demographic characteristics of the respondents and malaria. There were statistical significance between lower age ≤ 25 years old and malaria, (χ2 = 17.835, df = 3, p < 0.001). Pregnant women who attended religious schools only were found to be significantly associated with malaria (??2= 166.619, df = 4, p <0.001). Unemployment and having a family income of < 5000 naira were also significantly associated with malaria respectively (χ2 = 220.519, df = 2, p <0.001), (2 = 353.841, df = 2, p <0.001).
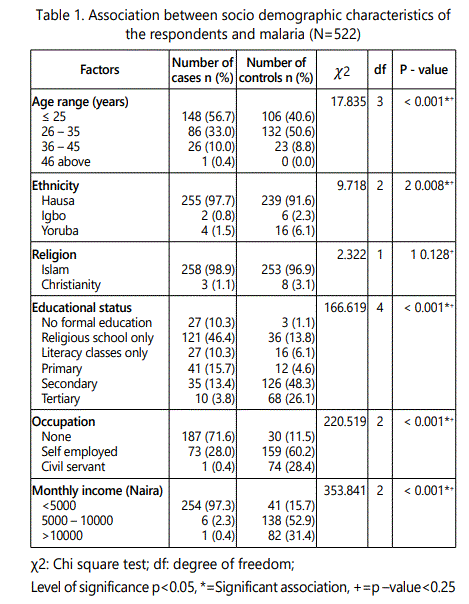
Table 2 below shows that majority of the respondents were in their first trimester 120 (46.0%) cases and 63 (24.1%) controls ( = 27.754, df = 2, p < 0.001).
Simple logistic regression analysis
Table 3 shows the risk factors for malarial infection among pregnant women using logistic regression analysis namely respondents below the age of 26 years, (COR = 1.829, 95% CI = 1.292 - 2.588, p < 0.001), respondents with informal education (COR = 6.810, 95% CI = 4.602 - 10.078, p < 0.001) and those in their first trimester compared to second and third trimesters (COR = 2.027, 95% CI = 1.427 - 2.87, p < 0.001).
Multivariable analysis
Table 3 also shows the predictors for malaria; variables that had p-value < 0.25 from simple logistic regression analysis were used in multiple logistic regressions, to determine the adjusted odds ratio [20]. The final model was constructed and the results were obtained using forward stepwise (likelihood ratio). After the adjustment for the potential confounders, variables with a significant association in the analysis p < 0.05 were retained.
As observed in this study, young maternal age ≤ 26 years were more likely to get malaria than those with <26 years (AOR = 1.695, 95% CI = 1.031, 2.789, p = 0.038). The risk of having malaria was 9 times higher among those with informal education than those with formal education (AOR = 9.390, 95% CI = 5.516, 15.985, p < 0.001), unemployed (AOR = 25.948, 95% CI = 14.831, 45.398, p < 0.001) and first trimester (AOR = 1.856, 95% CI = 1.126, 3.060, p = 0.015).
Discussion
In the present study, pregnant women < 26 years of age were found to be at risk of malaria infection. This is in conformity with the findings of a previous study conducted in Kano and Oyo State, Nigeria, which reported higher malarial infection among younger pregnant women as compared to older pregnant women [21, 22]. It has also been reported in Kaduna and Lagos State, Nigeria that respondents with 40 years and above were among the low risk group as compared to younger age. 13, 14. Adults living in malaria endemic regions have a protective immunity against malarial infection. This shows that risk of malaria decreases with increase in age. Clinically, the presentation of malaria infection varies by age and the status of the pregnancy with older age providing some protection against malaria [23, 24]. The immunity accumulates with repeated pregnancies, so long as there is exposure to malaria infection. It has also been described as advanced depressed immunity due to delayed antibody expression or lack of awareness on the necessary preventive measures in pregnancy, which could be responsible for the increased malaria parasitemia in the primigravidae [25].
Primigravidae have also been found to be at greatest risk of malaria in pregnancy due to the absence of specific immunity to placental malaria, which is acquired from exposure to malaria infection during pregnancy. The findings in this study is similar to that of Amuta, in Makurdi, Benue State, Nigeria, who reported pregnant women in primigravida and gravida 2 had the highest risk of malarial infection followed the multigravidas [10]. In line with this study, similar reports shows primigravidae had greater risk of having malaria than the multigravida [14, 26]. This correlate with a study in Katsina State, who reported higher risk of malaria infection among primigravidae than multigravida, this may be due to the fact that pregnant women at primigravidae are mostly stressed up as a results of the physiological changes that may occur during their first pregnancy which lowers their immunity [11].
Sharling and colleagues also reported highest risk of malaria during pregnancy among primigravidae which might be due to lack of specific immunity to placental malaria which is normally acquired during pregnancy when exposed to malaria parasite. The immunity gathers with consecutive pregnancies, as long as there is exposure to exposure to malarial infection [27, 28]. This could be due to the fact that the multigravidas are likely to be much more familiar with preventive and control measures against malaria due to information they acquired during the previous ANC visits as compared to primigravidae who were attending for the first time. Similarly, multigravidaʼs developed more immunity to malaria during pregnancy than primigravida and gravida 2 due to their fast exposure [29].
The low rate of malarial infection among the multigravidas might be as a result of the inhibition of the factor responsible for the susceptibility of primigravida to malaria infection which is known as type 1 cytokine response (interferon, interleukin 2 and 12).
Our finding revealed that pregnant women with low educational background were important risk factor for malaria. It was observed that pregnant women who attended religious schools only had the highest risk of acquiring malaria compared to those with secondary or tertiary school certificate. The high infection rate among pregnant women with no formal education in this study was similar to that of Gajia, who reported 54.7% of the pregnant women attended Quranic schools only [21]. Another study reported majority of the pregnant women attending antenatal care had only primary school certificate [12].
Similarly, a study in Benin shows significant association between pregnant women with tertiary level of education than those with lower level of education (p = 0.002) [30]. This might be due to the fact that pregnant women with tertiary school certificate are more likely to reside in areas that do not support mosquito breeding thatʼs areas with good sanitary hygiene better sanitary conditions [31]. However, this contradicts the findings of Iriemenan in Lagos, Nigeria, who found no significant association between the educational status of the pregnant women and malaria. A study conducted in Kano State, Nigeria, also reported no significant difference between the occurrence of malaria infection among the educated and non-educated pregnant women, those who had tertiary, secondary or primary school certificates were not health educated [11]. This might also be possibly as a result of being more exposed to malaria parasite due to high population density, high rain fall, poor environmental conditions and life style in the area.
The high infection rate among unemployed pregnant women in this current study was similar to that Oyefabi, who reported 15% of the respondents were physical working class, 17% craft women, 77% full house wives, 32% traders, health workers 0.2%, 0.3% teachers and students 0.3% [12]. The findings in this study is similar to that reported in Lagos which showed statistically significant association between the unemployed pregnant women attending ante natal care and malaria infection (χ2 = 13.797, df = 6, p = 0.032).31 This study reveals that unemployment was an important risk factor for malaria in pregnancy, which corroborates the findings of Oyefabi and colleagues who reported a statistically significant association between the unemployed compared to employed pregnant women (AOR = 8.437, 95% CI:1695 - 42.007, p = 0.009) [12]. The employment status of an individual is one of the contributing factors of the personʼs financial background. A pregnant woman with well- established economic status is likely to have better quality of life such as proper health care services, ability to buy insecticide spray, mosquito coil and insecticide treated bed nets (ITNs). Malaria causes financial strain yearly on the Nigerian economy with an estimate of US $835 million spent for the prevention, treatment, control and loss of income due failure to work as a result of illness [32].
In the current study, it was observed that pregnant women in their first trimester had the highest risk of malarial infection. This is in line with the study of Amuta and colleagues who reported higher risk of malarial infection among pregnant women in their first trimester as compared to those in their second and third trimester. This might be due to the fact that the pregnant women started taking intermittent preventive treatment (IPT) during their second and third trimester which helps to reduce the risk of malarial infection [11]. On the contrary, a study conducted in Lagos, Nigeria, reported higher risk of malaria during third trimester of pregnancy [33]. It has been reported that pregnant women in their second trimester were found to be the highest risk group of infection followed by third trimester while those in first trimester were the lowest risk group [14]. Previous study reported malarial infections vary at different trimesters of pregnancy, which might be as a result of some factors such as exposure to mosquito bite which might be due to the environment, use of preventive measures and immunogenetic constitution of the pregnant women [10].
Conclusion and Recommendation
This study has revealed the most important sociodemographic and maternal risk factors associated with malaria among pregnant women in Zamfara State, Nigeria. These are pregnant women <26 years of age, informal education, unemployment and first trimester of pregnancy. Public health physicians need to implement sustainable health education campaigns and health promotion activities. Health promotion activities targeting high risk groups like pregnant women should be given especially during antenatal visits, apart from ensuring effective case management. Meanwhile, efforts should be made by towards the vector control by maintaining the sanity of the environment, promotion of literacy by starting from early age in school and also providing employment opportunities in order to reduce the level poverty in the state. Finally, much attention needs to be paid to remote areas in order to achieve the global targets.
Acknowledgements
The authors would like to thank the Hospital Service Management Board (HSMB), Zamfara State, Nigeria, the hospital managements as well as the laboratory technicians of the hospitals involved for their kind assistance during the period of the research. Also, this study would not have been possible without the co-operation of the pregnant women.
Conflict of Interest
The authors confirm that there is no conflict of interest regarding this manuscript.
References
- WHO. World Malaria Report. 2013.
- Tillotson GS. Foreword: 10-year Special Edition. J Expert review of antiinfective therapy. 2012; 10(11): 1219-1220.
- Agomo CO, Oyibo WA, Anorlu RI, Agomo PU. Prevalence of malaria in pregnant women in Lagos, South-West Nigeria. Korean J Parasitol. 2009; 47(2): 179-183.
- Aregawi M, Cibulskis RE, Otten M, Williams R. World Malaria Report 2009. World Health Organization; 2009.
- Duffy PE, Fried M. Malaria in the pregnant woman. Malaria Drugs Disease and Post- Genomic Biology. 2005; 169-200.
- Polsa P, Spens K, Soneye A, Antai I. Comparing the perceived quality of private and public health services in Nigeria. Journal of Management Policy and Practice. 2011; 12(7): 18-26.
- Nzeako SO, Nduka FO, Origie OA. Prevalence of Malaria in Pregnant Women Attending Ante Natal Care at University of Port Harcourt Primary Health Care Centre Aluu, Port Harcourt, Rivers State, Nigeria. International Journal of Scientific Research in Environmental Sciences 2013; 1(10): 268- 372. doi: 10.12983/ijsres-2013-p268-272
- World Malaria Report 2010. World Health Organization Geneva.2012.
- World Health Organization. Malaria fact sheet no. 94. WHO, Geneva 2010.
- Amuta E, Houmsou R, Wama E, Ameh M. Malarial Infection among Antenatal and Maternity Clinics Attendees at the Federal Medical Centre, Makurdi, Benue State, Nigeria. Infect Dis Rep. 2014; 6(1): 5050. doi: 10.4081/idr.2014.5050
- Bawa J, Auta T, Liadi S. Prevalence of malaria: Knowledge, attitude and cultural practices of pregnant women in Katsina metropolis, Nigeria. European Scientific Journal. 2014; 10(21).
- Oyefabi A, Sambo M, Sabitu K. Effect of primary health care workers training on the knowledge and utilization of intermittent preventive therapy for malaria in pregnancy in Zaria, Nigeria. Journal of Medicine in the Tropics. 2015; 17(1): 4-11.
- Umaru ML, Uyaiabasi GN. Prevalence of Malaria in Patients Attending the General Hospital Makarfi, Makarfi Kaduna–State, North-Western Nigeria. American Journal of Infectious Diseases and Microbiology. 2015; 3(1):1-5.
- Agomo CO, Oyibo WA. Factors associated with risk of malaria infection among pregnant women in Lagos, Nigeria. Infect Dis Poverty. 2013; 2:19: 1-8. doi: 10.1186/2049-9957-2-19
- Jimoh A, Sofola O, Petu A, Okorosobo T. Quantifying the economic burden of malaria in Nigeria using the willingness to pay approach. Cost Eff Resour Alloc. 2007; 5(6): 1-8. doi:10.1186/1478-7547-5-6
- Dockrill P. Worldʼs first malaria vaccine approved by European regulators. 2015.
- Lorenzetti L. Worldʼs first malaria vaccine approved by European Regulators. 2015.
- Suresh KP, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012; 5(1): 7-13. doi: 10.4103/0974- 1208.97779
- Schlesselman J. Case-control studies design conduct analysis. In: Paul DS, editor. Monographs in Epidemiology and Biostatistics Oxford University, New York; 1982.144 -150.
- Hosmer DW, Lemeshow S editors. Applied Logistic Regression. Second ed. San Francisco: John Wiley & Sons; 2000.
- Gajida AU, Iliyasu Z, Zoakah A. Malaria among antenatal clients attending primary health care facilities in Kano state, Nigeria. Ann Afr Med. 2010; 9(3): 188-93. doi: 10.4103/1596-3519.68352.
- Akinleye SO, Falade CO, Ajayi IO. Knowledge and utilization of intermittent preventive treatment for malaria among pregnant women attending antenatal clinics in primary health care centers in rural southwest, Nigeria: a cross-sectional study. BMC Pregnancy Childbirth. 2009; 9: 28. doi: 10.1186/1471-2393-9-28
- Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. The Lancet infectious diseases. 2007; 7(2): 93-104. doi: 10.1016/S1473-3099(07)70021-X
- Kovacs SD, Rijken MJ, Stergachis A. Treating severe malaria in pregnancy: a review of the evidence. Drug Saf. 2015: 38(2):165-81. doi: 10.1007/ s40264-014-0261-9.
- Okpere EE, Enabudoso EJ, Osemwenkha AP. Malaria in pregnancy. Nigerian Medical Journal. 2010; 51(3):109-113.
- Alaku IA, Abdullahi AG, Kana HA. Epidemiology of Malaria Parasites Infection among Pregnant Women in Some Part of Nasarawa State, Nigeria. Developing Country Studies. 2015; 5(2): 30-33.
- Beeson J, Duffy P. The immunology and pathogenesis of malaria during pregnancy. Immunology and Immunopathogenesis of Malaria. 2005; 187-227.
- Sharling L, Enevold A, Sowa KMP, Staalsoe T, Arnot DE. Antibodies from malaria-exposed pregnant women recognize trypsin resistant epitopes on the surface of Plasmodium falciparum -infected erythrocytes selected for adhesion to chondroitin sulphate A. Malaria Journal. 2004: 3:31. doi: 10.1186/1475-2875-3-31
- Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub- Saharan Africa. Clin Microbiol Rev. 2004; 17(4): 760-769. doi: 10.1128/CMR.17.4.760-769.2004
- Oladeinde BH, Omoregie R, Odia I, Oladeinde OB. Prevalence of Malaria and Anemia among Pregnant Women Attending a Traditional Birth Home in Benin City, Nigeria. Oman Med J. 2012; 27(3): 232-236. doi: 10.5001/omj.2012.52
- Iriemenam NC, Dosunmu AO, Oyibo WA, Fagbenro-Beyioku AF. Knowledge, attitude, perception of malaria and evaluation of malaria parasitaemia among pregnant women attending antenatal care clinic in metropolitan Lagos, Nigeria. J Vector Borne Dis. 2011; 48(1):12-7.
- Babalola M. An Examination of the Association between Malaria Knowledge and Bed Net Use of Pregnant Women Receiving Antenatal Care at Federal Medical Centre, Abeokuta, Nigeria. 2013.
- Falade CO, Tongo OO, Ogunkunle OO, Orimadegun AE. Effects of malaria in pregnancy on newborn anthropometry. J Inf Dev Ctries. 2010; 4(7): 448-453.


