1International Centre for Genetic Engineering and Biotechnology (ICGEB), South Africa
2University of Cape Town, Institute of Infectious Diseases and Molecular Medicine (IDM), South Africa
Background: The ability of macrophages to modulate their phenotype in response to environmental signals from local tissues make them a crucial mediator of immune response, Mtb has several ways of subverting host immune responses; understanding the mechanisms and the genes involved is required for the development of novel strategies to combat the disease. Insulin-like growth factor 1(IGF-1) is one of the genes identified to be upregulated during alternative activation of macrophages in deepCAGE transcriptomics atlas on murine macrophages. IGF-1 in macrophages is downregulated upon infection with Mtb clinical and H37Rv strains. Lentivirus-mediated knockdown of IL-4 regulated IGF1 results in decrease in bacterial burden in bone marrow derived macrophages, and decrease pro-inflammatory cytokines. Chemical blocking of IGF-1 also led to decrease in bacteria growth, overall these results suggest that IGF-1 may play a role in the control of Mtb in vitro.
Methods: Primary macrophages, Bone marrow derived macrophages(BMDMs) were derived from 8-12week old BALB/C male mice, cultured for 10 days at 37°C in M-CSF supplemented medium for macrophage differentiation. BMDMs were harvested and plated overnight in the presence or absence of activators (100U/ml IL-4, IL-13 and IFNy) after 24hours of stimulation, the BMDMswere either left uninfected or infected with live logarithmic phase hyper virulent Mtb HN878 strain or H37Rv strain at a MOI 5:1 (bacilli: macrophage).Cells were transduced with shRNA containing lentivirus against IGF-1 gene for 10days, stimulated with Il-4 to induce expression of IGF-1, after 24hours cells were replenished with medium containing gentamicin.Following 4 hours, 3 days and 6 days infection, macrophages were lysed to determine bacterial growth, CFUs and cytokines were measuredusing ELISA. BMDMs IGF-1 receptor were chemically blocked with tyrophostin and CFUs evaluated.
Results: We validated the Deep CAGE by RTqPCR. We observed an increase expression of IGF-1 expression upon alternative activation with no change in the classicallyactivated BMDMs. Upon infection with both strains there was a down regulated expression of IFG-1. Knockdown of IFG-1 was confirmed with targeted shRNA containing lentivirus against IgF-1 with a corresponding reduction in CFUs at day 3 relative to the vector. IGF-1 chemical blocking resulted in reduction in CFU also. There was also a reduction in proinflammatory cytokines in the IGF-1 knockdown and blocked BMDMs.
Conclusion: We have shown that IGF-1 plays a role in the control of Mtb growth in primary macrophages. Further investigation on the mechanisms involved would suggest new strategies that can be used to design more effective drugs against Tuberculosis.
PnuVax, Canada and SaudiVax, Riyadh, KSA
Vaccine supply to the developing and lower income countries of the world continues to be inadequate and is limited by price, quantity and supply issues. Historically, vaccine supply in these areas was met with localized manufacturers, typically government sponsored ‘vaccine institutesʼ. However, the funding mechanisms for these local producers was inadequate to maintain increasing quality requirements, to meet growing population demands and to introduce newly developed vaccines. In the period between 1980 and now, vaccine manufacturing has been centralized in developed countries, with good effects on quality and new vaccine development, but by price, quantity and supply limitations leave many children with inadequate immunization, and new vaccines often take decades to diffuse worldwide. Today, with increased economic prosperity, improved education worldwide and the standardization of vaccine regulations and the expectations of National Control Authorities, it is now possible to once again localize vaccine manufacturing to overcome current limitations in the worldwide vaccine distribution system.
Biography:
Dr. Donald Gerson is President and CEO of PnuVax Inc, a developer and manufacturer of low-cost, high quality vaccines for the Developing World that has been supported by the Bill and Melinda Gates Foundation. Dr. Gersonʼs career has included: President and COO of Celltrion, a large-scale manufacturer of mammalian cell produced protein biopharmaceuticals in Songdo, Korea; Managing Director for Manufacturing at Wyeth-Lederle Vaccines in New York with responsibility for 50% if he US vaccine supply including DTP, OPV, Hib conjugate, PPV23, and process development and commercial production of the 1stPneumo-conjugate vaccine (Prevnar) and the first Meningococcal-C conjugate vaccine.
1Department of Health Services Nakuru, Kenya
2Molo Sub County Hospital, Kenya
3Molo Sub County Health Office, Kenya
The World Health Organization estimates that 1.5 million deaths among children under 5 years were due to vaccine preventable diseases, representing 17 per cent of under-five child mortality worldwide. Until recently Kenya did not have a rubella immunization program or a surveillance system for congenital Rubella Syndrome. Rubella Cases are being detected using measles case based surveillance system. World Health Organization recommended that countries including Kenya should conduct a wide age range campaign with MR vaccine, 6 months prior to its absorption into routine. The Kenya Ministry of Health with World Health Organization and UNICEF conducted measles Rubella catch up immunization campaign in May 2016 targeting children 9 months to 14 years. Nakuru County vaccinated 102% while Molo sub county where the rubella outbreak occurred vaccinated 95%. Turi International School which has an eligible population of 1200 children 9 months to 14 years was totally missed out during the vaccination exercise due to vaccine hesitancy by the school management. Four months later rubella outbreak occurred affecting a total of 20 children aged between 2 years and 12 years. The Objective of this study is to demonstrate the best practices in rubella outbreak response and to document the need to prioritize efforts to address Challenges of vaccine refusal in future vaccination campaigns. The study was done in Molo Sub County in Nakuru County. Case definition was used to identify suspected cases. Data was collected in an excel line list and analyzed using SPSS version 20. Chi square was used to measure the association between the risk factor and the occurrence of rubella. Samples were analysed in KEMRI lab. Majority of the cases were from St Andrews school Turi, Children aged between 5 – 14 years were mostly affected. Those who did not receive the vaccine during the campaign were mostly affected. There is strong association between the occurrence of rubella (Chi 28.863, p 0.025) and non vaccination during the May 2016 MR Vaccination campaign. Following the outbreak, The county Rapid response team was convened, Surveillance intensified, health workers skills updated, Isolation ward was established, social mobilization and advocacy meetings were conducted. Finally, 5 days vaccination was conducted targeting children 9 months to 14 years. A total of 14,137 (116%) children out of the 12,130 were vaccinated and the outbreak was controlled within two weeks.
Biography:
Elizabeth Kiptoo is a holder of Master of Public health Epidemiology and disease control at the age of 43 years from Department of Health service, Nakuru County. She has worked in the field of public health for the last 22 years. She is a member of faculty associate of Mount Kenya University. A young researcher who has published two papers in reputed journals.
Mofid Children Hospital, Iran
Background and Aim: Tuberculosis(TB), caused by Mycobacterium tuberculosis is a global health problem. One-third of the worldʼs population is infected with Mycobacterium tuberculosis. In developing countries with high TB incidence, due to increased health expenses, the control of TB is sensitive issue. The BacilleCalmette-Guérin (BCG) vaccine; an attenuated live vaccine is only available effective vaccine for TB control. In some studies, Iran is using own locally BCG vaccine strain with unknown substrain. In present work we studied molecular characterizations of current BCG strain uses in Iran.
Materials and Methods: Sixty seven different vials ofBCG vaccine; 28 stocks and 39 non-stocks were selected. DNA was extracted by using modified CTAB method and PCR was done for detection of genes RD1, RD2, RD14 and DU1. A multiplex PCR was applied looking for four target regions including RD1, RD8, RD16 and SenX3–RegX3.The MIRU- VNTR typing was used to determine VNTR profile of BCG strains. The amplified RD16 region was sequenced for future confirmation.
Results: Our results showed that all studied batches were Mycobacterium bovis BCG and molecular analysis revealed Iranian vaccine strain possess RD1, RD8, RD16, SenX3–RegX3 and DU1 regions but not RD2 and RD14. The VNTR profile of BCG strains was 2-2-5-2-3-4-11. Sequencing of RD16 region showed that BCG vaccine strains are accordance with the BCG Pasteur 1173P2. Based on results, all of analyzed vaccine batches were compatible with BCG Pasteur 1173P2.
Conclusion: In conclusion based on our result, all of studied BCG strains; collected from different sources in Iran were recognized as the BCG Pasteur 1173P2 strain. No genetically diversity among stocks and non-stocks were detected. Key words: BCG vaccine, BacilleCalmette-Guérin، Gene polymorphism, Iran
Lovely Professional University, India
Eco-friendly synthesis of nanoparticles is the need of the society today. Present study has been undertaken to investigate the greener approach for the preparation of medicinally and chemically important nanoparticles. Waste biomaterial has been taken to synthesis nanoparticles. The synthesized nanoparticles are characterized by XRD, SEM, TEM studies. The particle size varied from 20-50 nm. These nanoparticles were studied for their catalytic properties and their antitubercular properties. Few of these are shown to possess potent antitubercular activity while others have catalytic property for dye degradation.
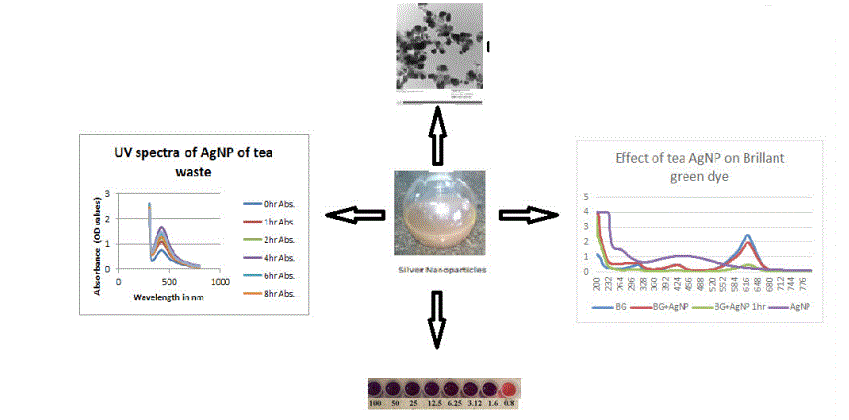
1CSIR-Institute of Microbial Technology, Chandigarh, India
2Department of Pulmonary Medicine, Government Medical College Hospital, Chandigarh, India
3Department of Microbiology and Immunology, Peter Doherty Institute for Infection and Immunity, The University of Melbourne, Australia
The clinical trials suggest that BCG fails to protect against tuberculosis (TB) in TB-endemic population. Recent studies advocate that non-tuberculous mycobacteria (NTM) and latent Mycobacterium tuberculosis (Mtb) infection interferes in the antigen processing and presentation of BCG to induce protective immunity against Mtb. Thereby, indicating that any vaccine that require exhaustive antigen processing may not be efficacious in TB-endemic zones. Recently, we have demonstrated that single epitope based vaccine (L91), conferred better protection than BCG. In this study, we constructed a multi-stage based multi-epitope vaccine, comprising of promiscuous MHC-I and MHC-II binding peptides of active (TB10.4, Ag85B) and latent (Acr1) stages of Mtb antigens respectively, conjugated to TLR-2 agonist Pam2Cys (L4.8). L4.8 significantly elicited both CD8 and CD4 T cell immunity as evidenced by increase in enduring polyfunctional CD8 and CD4 T cells. L4.8 efficiently declined Mtb-burden and protected animals better than BCG and L91, even at late stage of Mtb infection. The animal data related to T cells, were replicated using PBMCs of BCG-vaccinated healthy subjects. This study emphatically denotes that L4.8 can be a promising future vaccine candidate for controlling active and latent TB.
Biography:
Javed N. Agrewala, born in Agra in the Indian state of Uttar Pradesh, graduated in science from Dr. B. R. Ambedkar University in 1980 and earned a masterʼs degree from the same institution in 1982 after which he did his doctoral studies at Sarojini Naidu Medical College to secure a PhD in 1986. In 1989, he joined Institute of Microbial Technology, Chandigarh as a faculty member and scientist where he has been working since then and serves as the chief scientist and professor. In between, he had two sabbaticals, initially at Royal Postgraduate Medical School of Hammersmith Hospital (1994–1996) and later at Trudeau Institute (2001–2002). In 2014, he was short-listed among the three possible candidates to become the vice chancellor of the University of Kashmir but the position eventually went to Khurshid Iqbal Andrabi. At IMT, he heads a laboratory, The Agrewala Lab, where he hosts a number of researchers and students engaged in the studies on self-adjuvanting peptide vaccines and immunomodulation therapy and serves as a biosafety officer.
Tamil Nadu Veterinary and Animal Sciences University (TANUVAS), INDIA
Tamil Nadu is gifted with 11.23 mill. heads of cattle, 2.03 mill. buffaloes, 8.09 mill. sheep, 9.41 mill. goat and over 74 mill. hybrid chicken and about 33 mill. indigenous chicken. For livestock and poultry several vaccines and different adjuvants were developed and in use. For cattle,Inactivated Johneʼs Disease (JD) vaccine using intermediate strain; heat killed Myco.avium subsp. paratuberculosis vaccine with Chitosan adjuvant for cattle, sheep and goat; inactivated Leptospirosis vaccine are in use. For sheep, Multivalent Blue Tongue (BT) vaccine with Montanide adjuvant ; biofilm vaccine for sheep pasteurellosis with Montanide adjuvant; homologus vaccine for Peste des petits (PPR) ruminants. For Swine,BEI inactivated Classical Swine Fever (CSF) vaccine and PK-15 cell adapted live CSF vaccine with Montanide adjuvant. For poultry Inactivated Fowl Cholera vaccine with Aluminium hydroxide gel adjuvant; vero cell adapted heat killed Newcastle Disease (NCD) vaccine with aluminium hydroxide gel adjuvant; nano vaccine for NCD with calcium phosphate adjuvant; D52 oral pellet NCD vaccine, inactivated Infectious Bursal Disease (IBD) vaccine and inactivated Hydro pericardium syndrome (leechi disease) vaccine are used. For Japanese quails Manhemia haemolytica vaccine with Aluminium hydroxide gel adjuvant of TANUVAS. NCD is endemic in india. In EU between1986 to1992, NCD outbreak was <10.From 1993,it was >375.Scientists advised to avoid LaSota vaccine, since the ICPI value was 0.4,hence prohibited. Seed material used for NCDV vaccine should have <0.4 ICPI value. Australia was free of NCD from 1968 to 1997 for 30 years. But, during 1998-2000 period witnessed the incidence of NCD. Due to mutation of a virulent NCD virus circulating in Australia. Safe vaccines for NCD as per Australian plan was seed material isolated from fowl with ICPI value 0. To ensure purity,safety, potency of vaccines, ICPI value of seed material should be 0 or near 0. The origin of virus should be from poultry. NCD virus adapted by men mutates every 5 years. In one instance in 1993, isolates of NCD virus LaSota/RDVF/RDVK were lentogenic by monoclonal antibody typing, but biologically all were velogenic. one such vaccine strain was developed by TANUVAS isolated from fowl with ICPI value 0. As per international norms if any isolate of NCD virus with ICPI value of 0.7 and above the farm should be closed. In 1993, during IBD outbreak, virus grown in chicks and bursal tissue homogenate was used as IBD killed vaccine for control. No prophylactic vaccination against AI.It is suggested to focus on autogenous vaccine to prevent/control AI.SAN eggs may be an alternative to SPF eggs. A few more points on NCD viz. maternal antibody of commercial chicks is 8 to 9[log 2],on 5th day of age, NCD live vaccine is given. This will be neutralised by maternal antibody. Correct mode of vaccination is spray vaccination since it will work even in presence of maternal anti body. Alternatively, mix the NCD live and killed vaccine and use or on the same day give NCD live vaccine i/o and killed vaccine s/c routes. Central and state level reference lab. for validation of vaccines safety/purity/potency. Periodical update of Pharmacopia. Correct mode of maintenance of passage level of seed material and antigen content of live and killed vaccine.
Biography:
Mannu Babu born in 1956 earned his Ph.D. in 1993 from TANUVAS. Studied PG Diploma in Personnel Management and Labour Law. Retired from service, currently he is serving as consultant and faculty. He has filed 3 patent on rural women friendly technologies. Co-authored 2 books. Published more than 60 research papers. Visited Uganda, Malawi, Kenya, Austria, Argentina and Bangladesh for professional works
Northern University, Pakistan
The infections caused after 24 hours while staying inside the hospital are called hospital acquired infections (HAIs) or nosocomial infections (NIs). HAIs are maximum in developing countries which lack facilities and methods to reduce NIs. About 70% NIs are seen fewer in developed countries. It has been identified that 5-10% of NIs were from developed countries like North America and Europe counties, whereas in Asia, Africa and Latin America 40% of patients in hospital have hospital acquired infections. In present research nosocomial pathogens in intensive care units (ICUs) of the tertiary health care hospital from Abbottabad city. The study is divided into two portions. i.e. 1st samples were collected from patientʼs body aspirates in ICU and 2nd environmental samples include surfaces and air sampling from ICU. From body aspirates (urine, sputum, and blood and pus samples) like, Escherichia coli, Pseudomonas aurogenosa, Staphylococcus epidermitis, and Staphylococcus aurous, and Klebsella spp were identified. Most of the nosocomial species were from pus in female samples 76.2% and 74.44% from male pus. Second largest amount were from sputum samples 44.6% in male, 40.2% in female samples. From urine and 16.42%, 30% and 15% were found from male urine, female sputum, and female blood respectively. Environmental samples include Escherichia coli, Staphylococcus aurous and Apergillusspecie. These microbes were studied before and after cleaning practices of the ICUs. All of these micro-organisms were identified from floor, air and different instruments present inside the ICU of the hospital. These microbes were cultured on Nutrient agar (NA), mannitol salt agar (MSA), eosin methylene blue agar (EMB) and sabouraud dextrose agar (SDA). Large numbers of Staph aureus, E. coli and Apergillus specie were identified from floor, air and different instruments. On NA, EMB agar and SDA the reduction rate of bacteria are shown between Air contamination, lab coat and patient bed 18.51 ×105 < NC, 5.57 ×105 < 18.72×105, 5.87×105 < 16.86×105 and 3.71 ×105 < 9.14×105, 1st instrument, floor and 3rd instrument like, 2.75 × 104 < 8.76 × 104, 4.50 × 104 < 9.6× 104 and 3.10 × 104 < 5.3× 104 and air, floor samples and 3rd instrument NC=NC, 45.76 × 104 < 52.6× 104 and 9.90 × 104 <13.25× 104 respectively, before and after cleaning practices of ICUs. These microbes were not fully cleared after cleaning because of the use of only water instead of any disinfectant usage.
College of Veterinary Medicine, Haramaya University, East Africa
Epizootic lymphangitis is an economically important disease in some areas of the world, particularly where large numbers of horses, donkeys, or mules are present. Epizootic lymphangitis results from infection by a dimorphic fungus, Histoplasma capsulatum var. farciminosum.
Although the disease is prevalent in the tropics, so far there is no vaccine for this disease, no effective treatments except with sodium iodide for 1-3 months. Mortality is 100% only prevalence of the disease is known very little in all studies.
A crude formalin killed vaccine was tried. Apparently there seems to occur protection. No disease was observed after challenge with unskilled pathogen.
Thus we strongly call for partners to work together to produce the vaccine.
York University, Canada
Visceral leishmaniasis (VL or kalaazar) is the most serious form of human leishmaniasis, responsible for over 20,000 deaths annually, and post kalaazar dermal leishmaniasis (PKDL) is a stigmatizing skin condition that often occurs in patients after successful treatment for VL. Lack of effective or appropriately targeted cell mediated immunity, including CD8+ T cell responses, underlies the progression of VL and progression to PKDL, and can limit the therapeutic efficacy of anti-leishmanial drugs. Hence, in addition to the need for prophylactic vaccines against leishmaniasis, the development of therapeutic vaccines for use alone or in combined immuno-chemotherapy has been identified as an unmet clinical need. Here, we report the first clinical trial of a thirdgeneration leishmaniasis vaccine, developed intentionally to induce Leishmania-specific CD8+ T cells.
Biography:
Mohamed Osman is a senior vaccinologist in the Centre for Immunology and Infection (CII). Mohamed obtained his MSc from London School of Hygiene and Medicine, and his PhD from Queenʼs University of Belfast. Mohamed was a senior research fellow at the University College London, overseeing the immunology on Phase IIb CMV vaccine clinical trial in organ transplant patients. Mohamed has over 10 years experience in human vaccine testing, development and research. He has been at the York University since 2011
Cellular and Molecular Biology Research Center, Iran
Background: Hepatitis C virus is a blood borne disease estimated to infect more than 350 million people globally and is a leading cause of liver cirrhosis, transplantation and hepatocellular carcinoma. Current gold-standard therapy often fails, has significant side effects in many cases and is expensive. The fact that a significant proportion of infected people spontaneously control HCV infection in the setting of an appropriate immune response suggests that a vaccine for HCV is a realistic goal but no vaccine is currently available. The present study was designed to investigate the possibility of a new vaccine against the hepatitis C virus based on mRNA encoding of membrane antigens (Core &E2) of hepatitis C virus.
Material & Methods: Nucleotide sequence of mRNA encoding core and E2 antigen of HCV virus by bioinformatics program was design and in pGE plasmid vector was prepared .Then in vitro transcription reactions are used to synthesize mRNA from this recombinant DNA template. Nanoparticles encapsulated this mRNA synthesized delivered to Monocytes isolated from human buffy coatand the activation and differentiation of monocyte to dendritic cells was examined.
Results: The results showed that the combination of this antigens that were expressed in dendritic cells by synthetic mRNA were able to activation and differentiation of dendritic cells. With respect to the unstable structure of mRNA, the use of PLGA nanoparticles as delivery system to dendritic cells is an efficient method. It is recommended to assess the appropriate response of the immune system, study be performed In vivo and Ex vivo. Maybe if properly stimulate the immune system,this combination to be introduced as a good candidate RNA vaccine against hepatitis C.
Keywords: RNA vaccine, Hepatitis C virus, Dendritic cell
Catholic University of Salvador-Bahia, Brazil
Emergent, re-emergent and neglected diseases: a literature review. Emergent, re-emergent and neglected diseases include a group of infectious diseases which correspond to those that affect, respectively, the populations in three distinct ways: by never been described in literature, which means that are no preparation by specific groups of health attention in the world; by have been already affected a great number of populations around the world and, after a hard work of health organizations in the world, had its casesʼ number diminished or eradicated, but, after a few years, presenting lots of cases; and by not having combat and preventive therapy, mainly because it affects poor countries and people, this way being less lucrative to the pharmaceutics industries. In Brazil, we find all three classifications of these diseases in whole extension, but with specific points depending on the countryʼs region. Brazil is among one of the Latin American countries most affected by infectious diseases and therefore more affected by emerging, reemerging and, mainly, neglected diseases.
Descriptors: Infectious Disease; Emergent; Re-emergent; Neglected; Public Health
Biography:
Postdoctoral Researcher University Barcelona: Research Group: Intercultural Spaces, Languages, Cheers and Identities: Department Humanities-Barcelona. Doctorate in Education and Society University of Barcelona . Graduated in Nursing from the Catholic University of Salvador, Specialization in Public Health.Pedagogue. Degree in Social and Cultural Anthropology University of Barcelona. Coordinator and Professor of the Nursing Faculty of the Catholic University of Salvador, Collaborating Professor of the University of Barcelona - Governmental Institute for Social Policies. She has experience in Collective Health, Education, Social Intervention and Citizenship, Oppressed Theater, Research Methodology, working mainly in the following subjects: Education, Health Intervention and Citizenship.
Laboratoire pathogénèse et vaccination lentivirales: PAVAL lab, Université grenoble alpes, France
The pandemic of human immunodeficiency virus type 1 has caused infection in near 80 million individuals worldwide and the death of near half of these infected people while the other half is living with HIV-1 infection thanks to innovative antiviral therapies. Although these therapies were highly effective at controlling active virus replication, they failed to eradicate the reservoirs of latent virus that rebounds upon interruption of therapy. A large variety of classical and innovative strategies of vaccine development has been used and failed to generate a safe and efficacious vaccine that could efficiently control HIV in infected people or protect risk populations from HIV acquisition.
The main reasons of these failures are could be explained by the biologic properties of the virus and the complex host/virus interactions that the virus establishes during infection. Indeed, following the initial picture of viremia early post infection, the virus causes slow low chronic infection associated with a variety of strategies that help the virus to escape the host defences and to progressively eradicate or dysfunction the key cells of the hostʼs immune system. Existence of a swarm of genetically and antigenically distinct variant in contaminated fluids during virus transmission adds further complications. Innovative strategies and vaccine prototypes are strongly needed to face these constraints and control this type of pathogens. We used the innovative DNA vaccine strategy to engineer lentivector-based DNA vaccine prototypes against HIV-1. We successfully tested these prototypes for their immunogenicity and protection against pathogenic viruses in animal models. Our latest prototype provided the demonstration in our recent experiment that a single DNA immunization was sufficient enough to induce long lasting immunity in all immunized macaques. Vaccine induced T cell immunity contained a variety of CD4+ and CD8+ T cells with both memory and effector phenotypes of cells including cells with high capacity of differentiation/proliferation and self-renewal. Humoral responses contained binding antibodies both to Gag and Env but were not associated with any neutralizing activity. As expected all 6 control and 6 vaccine macaques were all infected with the heterologous SIVmac251 challenge virus, since the design of this T-cell based vaccine was not for eliciting neutralizing antibodies capable of preventing acquisition of infection. Importantly however, the viremia was significantly lower in vaccinated group of macaques than that in controls after virus acquisition and then all 6 vaccinated animals progressively cleared their virus infection to barely detectable levels. Virus control was fully independent from neutralizing antibodies since all sera during 80 weeks before challenge and those collected up to 48 weeks post-challenge lacked any neutralizing activity against the challenge or the homologous virus to the vaccine. However, this low initial viremia in vaccines correlated with augmented IgA/IgG ratios in the rectal mucosa of all vaccinated animals compared with those of the controls. The clearance of viremia was found to be associated with increased central memory CD4+ and CD8+ T cells endowed with high capacity of proliferation upon homeostatic and antigenic stimulations.
Taken altogether, the data of this pilot study clearly demonstrate that a single immunization with our lentivector DNA vaccine has initiated long lasting immune responses that lack neutralizing antibodies but still significantly reduced the initial viremia by controlling virus acquisition and progressively cleared the challenge virus infection to barely detectable viremia.
Biography:
Yahia chebloune has completed his Ph.D at the age of 30 years from claude bernard lyon-1 university and postdoctoral studies from university of kansas school of medicine. He is research director at the CNRS and joseph fourier university of grenoble, leading the group of pathogenesis and lentivirus vaccination. He has published more than 80 papers in reputed journals and serving as an editorial board member of repute. His recent work is focusing on development of HIV-1 vaccine using the naturally attenuated animal lentivirus, caev as model and to study the mechanisms involved in latency/persistence of HIV/SIV in relation with pathogenesis
1Pasteur Institute of Iran, Iran
2Microbiology research center (MRC) Pasteur Institute of Iran
3Mazandaran University of Medical Sciences, Iran
4Pasteur Institute of Iran, Iran
5University of Medical Science, Iran
6Department of Medical Diagnostic Sciences, Belgium
Staphylococcus epidermidis as an opportunistic pathogen and the leading cause of morbidity and premature mortality in patients with medical .Developing a strategy to raise opsonic antibodies against polysaccharide intracellular adhesion (PIA) could be promising for elimination of colonizing and biofilm forming S.epidermidis. Following the purification of truncated resist protein and PIA, for the first time, PIA were conjugated to recess as a safe carrier to increase the immunogenicity of PIA and we evaluated it efficacy in mice. Construction of the conjugate was analyzed by using Fourier Transform Infrared spectroscopy (FTIR) and Proton Nuclear Magnetic Resonance spectroscopy (H1- NMR) methods. Afterwards, the immune response was evaluated by measuring total IgG, IgG2a, and IgG2b titers. Immunization of mice with the PIA-rSesC conjugate raised the levels of opsonic antibodies, and the vaccinated mice were protected.when challenged intravenously by wild type S. epidermidis strain 1457. Further studies indicated that the conjugated vaccine could eliminate S. epidermidis biofilm formation in in vitro and in vivo assays. This survey confirms the proposal that immunization of mice with PIA-rises conjugate vaccine could be secure and protected against Staphylococcus epidermidis infection.
Biography:
Dr. Mohammad Ahanjan, born in 1963, Ph.D of Microbiology, associate professor and scientific member of Microbiology and Virology department of medicine faculty of Mazandaran university of medical sciences and member of Infectious Diseases Research Center with focus on nosocomial infection.Manager of Education development office (EDO) of medicine faculty and referee of some scientific journals.Submitted some antibacterial resistant new Genes in NCBI (AHJ.MAZUMS, TOILEE.IR).
Founder & CEO Cleanrooms Containments, India
The presentation shall give an Over view of Bio-Containments and its necessity, how to select suitable containment, how to stop outbreaks by establishing effective, energy efficient, safe and certified research and manufacturing facilities while handling Human/Veterinary pathogens.
Presentation shall cover methods and technologies implemented globally for Bio-containments, Research facilities. Constructional features of BSL-2, BSL-3, BSL-3Ag, BSL-4 facilities, some typical layouts, material of construction of these facilities, HVAC requirements, and various primary, secondary and tertiary containment methods shall be discussed.Bio-containments are for Bio-safety, they are different from Clean rooms. Misperception while establishing Bio-containment leads to failure of bio safety.
Biography:
Mr. Ravi Kumar Tummalachalra is a Graduate Mechanical Engineer, expert in Design& construction of Bio-Containments (BSL-3 Ag, BSL-3, BSL-2 facilities), Clean rooms, Vivariums (Animal facilities), HVAC systems and energy efficient systems design. In the past worked for many years with various vaccine manufacturing companies in India, having 16 yrs of experience in Design and Construction of Clean rooms and Bio containments and Animal Research Facilities. Currently Founder and CEO of Clean rooms Containments since 2011 He is the Member of ABSA (American Biological Safety Association), EBSA (European Biological Safety Association), IVBWG (International Bio-safety Working Group), Society for Bio safety India, ASHRAE (American Society for heating, Refrigerating& Air-conditioning Engineers
1Laboratory of Parasitic Chemotherapy and Entomology, Department of Pathobiology, Faculty of Veterinary Sciences, BahauddinZakariya University, Pakistan
2Department Clinical Sciences, Faculty of Veterinary Sciences, BahauddinZakariya University, Pakistan
Introduction: Warble fly disease or Grub disease is equally affecting the cattle, buffalo and goats at Pakistan especially in Southern Punjab. The area mostly under attack is near rivers belt of River Sind, River Chennab area and Suleyman mountainous area. The disease has economic effect on the growing economy of the livestock based country/ region. The disease is endemic for centuries in the subcontinent and area reported.
Materials and Methods: For the affective control of P. silenusbraurthe somatic vaccine was developed first time in Pakistan. The somatic antigen of the 2nd stage larva was purified by ultracentrifugation at the speed of 3000 rpm for 10 minutes. The supernatant was obtained and desiccated. The 1 μgm of the dried somatic material was mixed with saponins of Sapindustrifoliatus (A new candidate for adjuvant) and injected subcutaneously at 0.5 ml to the suspected Capra hirsaharboring migratory larva of P.silenusbraur.
Result: The injected animals showed specific immunity along with increase in Neutrophils. The animals under experiment could not show the nodules or eruptions of L3 in skin of the animals. The study proved that somatic vaccine against P.silenusbraur showed 85 to 95 controls in grub eruption that could be a good candidate for the effective control of P.silenusbraurL3 along with Sapindustrifoliatusa new candidate in adjuvants family.
Discussion: Warble fly inflicts economic losses in livestock and tannery industry. It is imperative to control the problem and to address the issues timely. The fly is endemic in area and has been reported from adjacent countries including Iran but little data from Afghanistan about the disease. The ivermectin remained a drug of choice for decades to control the disease but emergence of resistance is a constraint. To address the dipteran disease which completes its partial life cycle inside the host can be justified by the vaccine. This is first time that warble fly in long hairy goats of the area has been treated effectively by the vaccine.
PSMMC, Riyadh, Saudi Arabia
Background: Seasonal influenza outbreak in healthcare facilities disturbs work fluency and jeopardizes patientsʼ safety (1). Thus, influenza vaccination of healthcare workers (HCWs) is recommended by the World Health Organization (WHO), United States (US) Center for Disease Control and Prevention (CDC) and the immunization guidelines set by many countries including Saudi Arabia (2). Despite this strong recommendations influenza vaccination rate among HCWs is still suboptimal all over the globe (3).
Objectives: To estimate the rate of influenza vaccination uptake by HCWs in WHC. The study is also intended to determine the role of sociodemographic factors, work related factors and health status of HCWs on acceptance of influenza vaccine. Furthermore, it also aim at studying the effect of the HCWsʼ beliefs about influenza vaccine and influenza disease on acceptance of influenza vaccine by HCWs VII
Method: A Cross-sectional study was carried out between 27 May and 15 June 2017. A random job-stratified sample of 240 HCWs was selected from HCWs at Al-Wazarat Health Care Center-PSMMC. 25 items self-administered questionnaire was used to collect the data. SPSS use to data analysis.
Results: Out of the 240 randomly selected HCWs, 83% have completed and returned the questionnaires. A statistically significant association existed between most of the socio-demographic factors and vaccination acceptance. However, Age, marital status and income were not significantly association with the vaccination status. A statistically significant association existed between almost all work and heath related factors and acceptance of the flu vaccine. Unexpectedly the only statistically insignificant association was found between chronic diseases and flu vaccination uptake. There is a positive association existed between believe in vaccine preventability, effectiveness and safety while negative with reduced susceptibility to flu disease. Our results showed that, VIII Predictors of vaccination are non-Saudi nationality, Increasing vaccination belief and clinical.
Conclusion: The vaccination rate was higher among, males, Muslims, married, graduated and clinical worker participants. The factors affecting awareness about influenza vaccine was gender, nationality, religion, job category and education level.
Keywords: Seasonal Influenza, Vaccination, Healthcare Workers
Family and Community Medicine Dept; PSMMC, Riyadh, Saudi Arabia
Background: Since its discovery in 2012, MERS presents a challenge for HCWs and for epidemiologists. Its spread in Arabian Peninsula, North Africa, East Asia and Europe represents a burden both Health care economic and human resources Therefore, good knowledge, positive attitude, and healthful practice of healthcare workers (HCWs) regarding MERS-CoV are a cornerstone in prevention of virus spread and disease outbreak.
Objectives: The objective of this study is to determine the knowledge and attitude of MERS- COV among healthcare worker in primary care center of PSMMC, Riyadh, Saudi Arabia.
Methods: A cross sectional study was performed in primary care center of PSMMC, Riyadh, Saudi Arabia. A total of 233 healthcare workers were selected to participate in this study. Knowledge and attitude were assessed by using self-administered and pretested questionnaire. Descriptive statistics were carried out to express participantsʼ demographic information, mean knowledge score and mean attitude score of HCWs.
Result: Participants demonstrated good knowledge and positive attitude towards MERS. Majority of the respondents had gained knowledge about MERS from internet as shown by this study. The most number of correct responses were gathered from the question about the symptoms of MERS followed by the question on developing severe acute respiratory illness. The lack of respondentsʼ knowledge was shown in the question asking about the incubation time of MERS-CoV, about 82.4% of the HCWs answered it incorrectly.
The most positive attitude of HCWs was regarding the use of protective equipment when dealing with MERS patient (4.64±0.64). Overall, gender and experience were the two demographic variables significantly associated with the mean knowledge and attitude scores.
Conclusions: The findings of this study showed that healthcare workers in Wazarat primary care center of PSMMC, Riyadh, Saudi Arabia, have good knowledge and positive attitude towards MERS. Yet there are areas where low knowledge and negative attitude of HCWs was observed. However, studies are required to assess the knowledge and attitude of HCWs at national level so that effective interventions could be designed as surveillance and infection control measures are critical to global public health.
Keywords: Knowledge, attitude, MERS-CoV, healthcare workers
1Secretaria de Salud de Bogotá-Colombia, South América
2Patholab-LTDA. Clínica de la Mujer, Bogotá-Colombia, South América
3Ginesalud IPS. Bogotá-Colombia, South América
4Instituto de Genética Veterinaria Fernando Dulup. Universidad Nacional de la Plata (Argentina), South América
Summary:
Introduction World wide cervical Cancer is the third type of cancer affecting women. Several studies showed that in cervical cancer hpv prevalence iscloseto 90%, demonstrating that hpv infection is necessary but insufficientfor cervical cancer development. So, the development of molecular approaches to detect viral dna from oncogenic hpv types showed the benefit of diagnosingthem in high grade lesions (cin-2 and cin-3), considered cervical cancer precursors. Since these molecular techniques are sensitive, reliable and consistent, they were evaluated as tools in secondary prevention strategies and also as approaches to studing the prevalence and incidence of the viral genotypes present in sexually active women.
Objective To establish the prevalence of HPV infection in paraffin embedded samples and cervical smears obtained from women with normal cervical mucosa, high and low grade cervical lesions and cervical cancer.
Methods All women belong to the public net for cervical cancer promotion and prevention program, bogotá and collected between 2015 and 2016.paraffin embedded samples were processed according totomassino et al. and also using the QIAamp DNA FFPE Tissue Kit. conventional PCR was used to amplify a región of the l1 gene from HPV using the my09/my011 primers. HPV positive samples were then genotyped using the linear array kit from roche.
Results A total of 3528 samples, includingbiopsies and cervical smearswereanalyzed. HPV prevalence was 34.3% (1211/3528). HPV 16 was the most prevalent genotype found in the analyzed population, reaching 42.4% of the samples, followed by HPV 58 (10.6%), HPV 52 (8.9%) and HPV 31 (6.8%). Single infections were detected in 54.8%, (663/1211). Thehighest HPV prevalence was observed in those women among 14 and 25 years old (22.5%; n=272). The highest HPV positivity was observed in those samples classified as CIN II and CIN III (58.9%; n=274). CIn II/CIN III samples also showed the highest proportion of oncogenic HPV types.
Conclusions This is the first retrospective analysis of the HPV genotypes in the sexually active women population from Bogotá, Colombia. This information could contribute to the implementation of integrated actions in order to strengthen the Cervical Cancer Detection and Control Program from the Capital City.
Keywords: Cervical Cancer, Cervical Intra epitelial lesions, HPV, Molecular detection