1Department of Translational Medicine, Lund University, Sweden
2Department of Microbiology and Immunology
3Chemical and Biological Engineering, University at Buffalo, USA,
4Abcombi Inc, USA
Streptococcus pneumoniae (the pneumococcus) is a human opportunistic pathogen of the respiratory tract that effectively colonizes the nasopharynx. When exposed to environmental stimuli, such as a virus infection or other inflammatory situations, colonizing bacteria can disperse and transition to infection of the sinuses, middle ears, lungs and blood. Current immunization strategies exploit the immunogenicity of the capsular polysaccharide conjugated to various toxoids. These vaccines have been very successful in protecting against invasive pneumococcal disease (IPD), but have the caveat that they show less pronounced overall protection against pneumonia, otitis media, and colonization and that they induce changes of the nasopharyngeal environment due to serotype replacement and increased colonization with other potential pathogens in the nasopharynx, such as Haemophilus influenzae and Staphylococcus aureus. To avoid potential deleterious effects on the nasopharyngeal environment we used transcriptional data of colonizing biofilm bacteria and bacteria dispersed from biofilms after exposure to virus infection or host factors associated with inflammation, cell damage, and virus infection, to identify antigens only expressed on disease-causing, biofilm-dispersed organisms. Using this approach, together with a novel liposome delivery system that does not require addition of adjuvant, we have identified several novel conserved antigens that when used for immunization showbroad protection against otitis media, pneumonia and IPD, without eliminating or affecting pneumococcal colonization. This disease-directed vaccine strategy offers a unique and dynamic vaccination option that eliminate the potential problems with serotype-replacement and is readily adaptable to other commensal pathogens in the future.
Biography:
Anders P. Hakansson trained and got his first faculty position and established his own lab at University of Alabama at Birmingham and Harvard Medical School, Buffalo, NY. He recruited back as full professor at Lund University in 2014 to present.
His lab is mainly focused on understanding host-pathogen interactions, with emphasis on respiratory pathogens, and to use this knowledge to develop novel preventive and therapeutic strategies to protect against bacterial infection. His work focuses on both the factors used by bacteria to establish colonization through biofilm formation and cause disease, and the host inflammatory response to colonization and infection. Better understanding of the mechanisms of transition to disease is used to develop novel vaccine strategies against pneumonia and otitis media; and to develop novel ways to provide novel therapeutic strategies against respiratory and other infections with antibiotic-resistant bacteria.
1Department of Nanoscience and Technology, Bharathiar University, Tamilnadu, India
2National Centre for Nanosciences & Nanotechnology, University of Mumbai, India
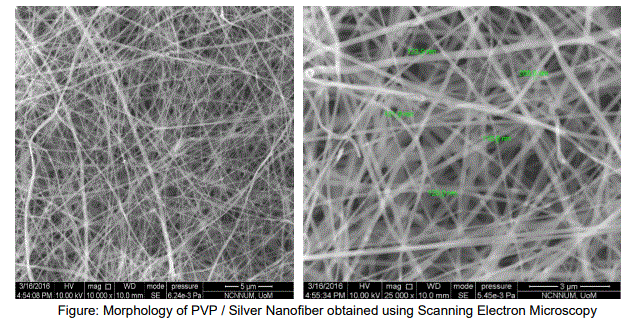
The electrospinning technique attracted much attention recently to the research due to the ability of converting materials to order of few nanometers with large surface areas, ease of functionalization for various purposes and superior mechanical properties. Also, the possibility of large scale productions combined with the simplicity of the process makes this technique very attractive for many different applications. Biomedical field is one of the important application areas among others utilizing the technique of electrospinning is used to produce fibers in the nanometer range by stretching a polymeric jet using electric field of high magnitude. Electrospinning leads to the formation of continuous fibers ranging from 100nm to 500nm. The ultra-fine fibers produced by electro spinning are expected to have two main properties, a very high surface to volume ratio, and a relatively defect free structure at the molecular level. Composites in the form of nanofibers were formed via electrospinning technique. Different ratios and different parameters pvp / silver composite nanofibers prepared in electrospinning. The prepared composite nanofibers were characterized using several techniques such as Scanning Electron Microscopy (SEM), X-ray Diffraction (XRD). It is anticipated that this PVP / silver nanofiber can be used in various applications such as clinical wound dressing, antimicrobial activity, bio adhesive, biofilm, and the coating of biomedical materials, etc. The morphology of PVP / silver nanofiber obtained using Scanning Electron Microscopy is show below.
Danylo Halytsky Lviv National Medical University, Ukraine
Possibility of intensification of antiviral therapy of chronic hepatitis B (HBV)with intradermal immunization with inactivated autoleukocytes has been investigated. It was also taken into consideration that autoleukocytes may be used as an individual virus-containing material (“personified vaccine”).
Patients with HBV were immunized thrice with the interval 30-40 days in long-lasting (over 2 years) antiviral therapy with tenofovir, however, DNA HBV replication continued (further reduction of viral load ceased). In a group of 8 patients, DNA HBV of whom constituted ≥3х104 IU/ml, it became less 1000 IU/ml in 5 (62.5%) patients, and in 3 (37.5%) it was detected with only supersensitive PCR method (sensitivity 5 IU/ml). In 16 patients of the second group, DNA HBV amount of whom was ≤ 2 х 103 IU/ml, in 11 (68.75%) the virus was detected only with supersensitive PCR method, in 5 patient (31.25%) – it was not revealed anymore. In 6 patients of the third group, in whom HBV DNA was detected only with supersensitive PCR method, in 2 – the result became negative (33.33%). Stability of the effect persisted for a year (period of monitoring). Thus, autoleukocyte immunization significantly intensifies efficacy of antiviral HBV therapy.
Biography:
HerasunOlexandrBorysovych was born in 1977; in 2000 graduated from Lviv medical university, in 2000-2001 – intern, 2001-2003 – clinical residency at the department of infectious diseases; worked as a doctor of Lviv regional center of HIV/AIDS; since January 2004 – an assistant at the department of infectious diseases; in 2007 defended PhD research paper on the theme “Clinical-epidemiological and immunopathogenic peculiarities of HBe-negative HBV-DNA-positive chronic hepatitis B”; participated in international programs of investigation of chronic hepatitis and HIV-infections; since 2015 – an associate professor; conducts classes and delivers lectures to students and doctors of the faculty of postgraduate education; has 81 publications.
DanyloHalytskyLviv national medical university, Ukraine
Efficacy of treatment of autoimmune processes by the method of intradermal immunization with inactivated autoleukocytes has been investigated. Amount of cryoglobulins decreased by ≥ 40% in 73 patients (out of 84; 86.90%) with cryoglobulinemia syndrome after single immunization; normalization was observed in 90% of patients after the second or the third immunization (interval 3-4 weeks). At the same time, clinical signs of cryoglobulinemia syndrome significantly decreased. A number of spermatozoa increased to 20 mln/ml and higher, their motility and percentage of normal forms improved in 29 men (out of 38; 76.32%) with idiopathic oligoteratozoospermia due to inhibition of cryoglobulin synthesis.
Influence on autoimmune processes is also confirmed by reduction of antinuclear antibodies (ANA) and antibodies against thyroglobulin and thyroid peroxidase in the blood of 75-80% of patients after single immunization, complete normalization of indices was observed in 25-30% of patients. The method was also tested for treatment of autoimmune hepatitis. After autoleukocyte immunization, condition in 10 out of 12 patients (83.33) significantly improved, stable remission was observed in 4 (33.33%) patients (term of monitoring – a year).
Vaccination also causes inhibition of excessive synthesis of TNF-alpha. Thus, after single immunization of patients with psoriasis (35), decrease in the level of pro-inflammatory cytokine occurred in all patients; the index reached the norm in 23 (65.71%) patients. At the same time, signs of psoriatic arthritis decreased and long-lasting remission occurred. Presence of viruses and their antigens in leukocytes enables to use such vaccination as a treatment vaccine in patients with frequently recurrent types 1 and 2 herpes. Immunization was tested on patients, who did not respond properly to antiviral therapy. Thus, in patients with labial herpes (n=21), stable remission occurred in 17 patients (80.95%) after treatment, and in patients with genital herpes – in 8 out of 11 (72.72%).
Biography:
G(H)erasunBorys Abramovich (born in 1938, Kremenchug, Poltava region), graduated from Lviv medical institute in 1963; PhD (1971), associate professor (1984), MD (1985), professor (1987), honorary professor of DanyloHalytsky Lviv national medical university (2007); epidemiologist in Lviv region (1963-68); postgraduate student (1968-71); assistant (1971-83), professor (since 1986) at the department of infectious diseases, DanyloHalytsky Lviv national medical university (since 1986), chief editor of the journal “Gepatologia” (since 2008).Main directions of scientific investigations: problems of hepatology and autoimmune processes, caused by infectious diseases; total number of publications – 300. More information: Whoʼs Who in the World 2015, 32nd Edition.
Food and Drug Administration, USA
Development of vaccines against emerging and re-emerging human pathogenic virusesis a public health imperative. As most licensed vaccines are effective through eliciting neutralizing antibodies, assays that can quantify neutralizing antibodies will likely be crucialin demonstratingvaccineeffectiveness. Neutralization assays traditionallyinvolve infectious virus, and the assay most commonly used is the plaque-reduction neutralization test. For viruses that can be propagated under standard laboratory conditions, such as Biological Safety Level 2 (BSL-2) conditions, the native virus can be used. This is the case for flaviviruses, such as Zika virus (ZIKV). However, for select agents such as Ebola virus (EBOV), this must be doneunder BSL-4 conditions. Because BSL-4 laboratories are uncommon, we have taken an alternative approach. Our approach uses a replication-competent hybrid virus whose genome carries the envelope gene from the pathogenic virus on the genetic backbone of a non-pathogenic virus. We use vesicular stomatitis virus (VSV), since VSV has the advantage that it can, once its own envelope gene is deleted, accommodate envelope genes from various virusesandincorporate this envelope into the viral particle. The resulting chimeric virus uses the heterologous envelope for entry and is neutralized by antibodies that neutralize the native virus. We have constructed hybrid VSVs carrying the envelope genes for several filoviruses. The readout for infectivity is by reverse transcriptase quantitative PCR (RT-qPCR), an approach we established for other viruses. The primers are directed against VSV genome rather than against the specific envelope gene, and thus the assay can be readily adapted to any virus whose envelope can be incorporated into the VSV particle. There are several advantages of our method. First, using PCR as readout allows the assay results to be obtained within 24 hours and frequently within 8 hours. Second, the sample for the assayis a crude lysate from the infected cells, which obviates the need to purify the nucleic acids and thus the assay is amenable to automation and high throughput. Third, the assay as done is in 96-well format, but could be adapted to a 384-well format for the evaluation of multiple sera. To demonstrate the utility of the approach, we have assessed neutralizing antibodies against several filoviruses and have shown that the antibody titersobtained using our assay are in agreement with those obtained using other assays. Thus, we conclude that our approach could be an alternative to other neutralization assays.
Biography:
He obtained his PhD from the MRC Mammalian Genome Unit, University of Edinburgh, UK. Dr Peden carried out post-doctoral work in the Department of Molecular Biology, University of Edinburgh, UK, and then at the Department of Molecular Biology and Genetics with Dan Nathans at the Johns Hopkins University School of Medicine, Baltimore. He spent a year at the Pasteur Institute (1988/89) with Luc Montagnier working on HIV, after which he spent 5 years at the NIH continuing work on HIV-1 and HIV-2 before moving the CBER/FDA in 1994. Currently, he is Chief of the Laboratory of DNA Viruses in the Division of Viral Products, Office of Vaccines Research and Review, CBER, FDA, where his major research focus is on safety issues associated with cell substrates for vaccine production and developing assays to evaluate vaccine efficacy.
1Instituto Finlay de Vacunas, Biocubafarma, Cuba
2Centro Nacional deInvestigaciones Científica, Biocubafarma, Cuba
3Instituto de Medicina Tropical Pedro Kouri, Ministerio de Salud, Cuba
A clinical trial was conducted to assess the safety, reactogenicity and immunogenicity of the live attenuated vaccine candidate Vibrio cholerae 638 (CV638), in healthy children and adolescents from both sexes, ranging 5 to 17 years old from the Cienfuegos Province, Cuba. An oral single dose of CV638 was administered to 40 out of 54 subjects of the study group, and the 14 subject of the control group, received the placebo. No serious adverse events nor clinical laboratory results out of range with clinical significance were reported. The percentage of volunteers with any adverse event did not reach significant differences among groups, being mild in most of the subjects. The most frequent adverse events in the group that ingested CV638 were meteorism (25.64%) and vomit (17.95%), while in the group that ingested placebo were fever (21.46%) and abdominal pain (4.29%). The one hundred percent of 10 to 17 years old adolescents, the other ones of 9 to 11 years old and the ones 5 to 8 years old children of the study group, seroconverted at 14 days after to be ingested the CV638. It is concluded that CV638 is safe, little reactogenic, and immunogenic in healthy children and adolescents of both sexes.
Biography:
Hilda García has completed his Masterʼs Degree in Bacteriological and Micological Sciences and Doctorʼs Degree in Health Science Pedro Kourí Institute (IPK), Cuba. 1996-1997& 2000-2004
Degree in Biochemistry University of Havana, Cuba. 1979-1984.
His Research is based on Genetic manipulation of Vibrio cholerae for vaccine development. Construction of Live attenuated El Tor Candidate Vaccine Strains. Selection of attenuated Vibrio cholerae strains to obtain oral attenuated candidate vaccines against cholera. Process development for cuban cholera vaccine based on the attenuated strain Vibrio cholerae 638. Clinical evaluation of a vaccine candidate based on the attenuated strain 638 Vibrio cholerae O1 El Tor Ogawa. Total publications 40
1Department of Virology, Faculty of Veterinary Medicine, Cairo University, Egypt
2Department of Immunology, Animal Health Research Institute, Egypt
3Reference Laboratory for Veterinary Quality Control on Poultry Production (RLQP), Animal Health Research Institute, Egypt
In this study, a recombinant DNA plasmid was constructed, encoding for HA1 of a selected Egyptian H5N1 virus (isolated during the 2012 outbreaks). In the immunization and challenge experiments, SPF chickens received 1 or 2 doses of H5-DNA plasmid prime, and boosted with the inactivated H5N2 vaccine. Haemagglutination inhibition (HI) titers, protection levels, and the magnitude of virus shedding were compared to that of the chickens that received either DNA plasmid or inactivated H5N2 vaccine alone. H5N1 virus A/chicken/Egypt/128s/2012 (H5N1) HPAI clade 2.2.1/C was used for the challenge. Chickens immunized with 1 or 2 doses of H5-DNA vaccine failed to overcome the challenge with 0% and 10% protection, respectively. Quantitative real-time reverse transcription-PCR revealed virus shedding of 2.2 x 104 PCR copies/ml 3 days post challenge (dpc) in the only surviving bird from the group that received 2 doses of plasmid. However, chickens immunized with 1 or 2 doses of H5-DNA plasmid as prime and inactivated H5N2 vaccine as booster, showed 80% protection after challenge, with a viral shedding of 1.2 x 104 PCR copies/ml (1 dose) and 1.6 x104 PCR copies/ml (2 doses) 3 dpc. The surviving birds in both groups did not shed the virus at 5 and 7 dpc. In H5N2-vaccinated chickens, protection levels were 70% with relatively high virus shedding (1.8x 104 PCR copies/ml) 3 dpc. HI titers were protective to the surviving chickens. This study reports the efficacy of H5-DNA plasmid to augment reduction in viral shedding and to provide better protection when applied in a prime-boost program with the inactivated AI vaccine.
Biography:
Basem Ahmed is the assistant lecturer of Virology from Faculty of Veterinary medicine in Cairo university. His main Research interest is in Molecular Virology, Vaccinology, Immunology.
Kenya Agricultural and Livestock Research Organization Perkerra Research Center, Kenya
A post vaccination assessment of contagious caprine pleuro-pneumonia serum antibody persistence following vaccination withCaprivax® F38 Biotype vaccine was conducted at the Kenya Agricultural and Livestock Research Organization - Perkerra Research Center between the months of April, 2014 and March, 2015 to determine the effectiveness of the vaccination program in place. A total of 42 goats of different ages were recruited and put in a longitudinal study with two groups; the vaccine group and non-vaccine group considered as negative control. A baseline assessment of antibody titre was done prior to administration of treatments. Post vaccination serum sampling was conducted monthly and antibody titres assessed using monoclonal antibody based competitive Enzyme-linked immuno-sorbent assay. A significantly higher mean percentage inhibition titre of 71.8% was recorded in goats in the vaccine group compared to 58.6% in goats in the non-vaccine group (p < 0.0001). The magnitude of the difference in the mean percentage inhibition titre levels was large (eta squared = 0.847) indicating a large effect of vaccination in antibody titre levels and hence the effectiveness of using the Caprivax® vaccine. Mortalities were recorded in goats in young age category that received the vaccine for the first time in life, suggesting the need for a repeat vaccination to improve protection using this vaccine.
Department of Food Sciences, Cornell University, USA
For over two decades now, plants have been explored for their potential to act as production platforms for biopharmaceuticals, such as vaccines and monoclonal antibodies. Without a doubt, the development of plant viruses as expression vectors for pharmaceutical production have played an integral role in the emergence of plants as inexpensive and facile systems for the generation of therapeutic proteins. More recently, plant viruses have been designed as non-toxic nanoparticles which can target a variety of cancers and thus empower the immune system to slow or even reverse tumor progression. The following presentation describes the employment of plant virus expression vectors for the treatment of some of the most challenging diseases known today. The presentation concludes with a projection of the multiple avenues by which virus nanoparticles could impact developing countries.
Biography:
Kathleen Hefferon received her PhD from the Department of Medical Biophysics, University of Toronto. She worked as a postdoc, then received a faculty position at the Departments of Nutritional Sciences and Food Sciences at Cornell University. Kathleen is also a visiting faculty member at the University of Toronto, where she teaches virology. Kathleen has four patents and has written and edited six books. Kathleen is currently the co-editor of the Encyclopedia of Food Security and Sustainability. Her research interests include viruses, vaccines, infectious disease, cancer, global public health and food/energy security.
Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences, China
Riemerella anatipestifer infection causes high mortality for ducks which results in major economic losses in the duck industry. In this study, we identified a mutant strain RAM1 by Tn4351 transposon mutagenesis, in which the M949_1603 gene encoding glycosyl transferase was inactivated. PCR analysis revealed that M949_1603 gene is specifically existed in R. anatipestifer serotype 1 strains. RAM1 presented no reactivity to the anti-lipopolysaccharide (LPS) MAb in an indirect ELISA. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting demonstrated that RA-M1 LPS had a deficiencyin ladder-like binding pattern to rabbit antiserum against R. anatipestifer serotype 1 strain CH3, indicating that the O antigen structure of RA-M1 was changed. RA-M1 showed significant attenuated virulence in ducks and higher sensitivity to normal duck serum, compared with its parent strain CH3. Furthermore, cross-protection of RA-M1 for R. anatipestifer serotypes 1, 2, and 10 strains was evaluated. Ducks that received two immunizations with inactivated RA-M1 vaccine were 100 % protected from challenge with R. anatipestifer serotype 1 strain WJ4, serotype 2 strain Yb2, and serotype 10 strain HXb2. No changes were observed in the liver, heart, or spleen samples from the protected ducks during autopsy and histological examination. Furthermore, vaccination generated high antibody titers of 1:12,800 against serotypes 1, 2, and 10 strains and enhanced production of interleukin 2 (IL-2) and IL-4 in ducks. These results suggested that M949_1603 gene is associated with serotype 1 O-antigen biosynthesis, and mutant RA-M1 could be used as a novel cross-protection vaccine candidate to protect ducks against R. anatipestifer infection.
Biography:
Shengqing Yu is the Professor, Head of Department of Veterinary Public Health, Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences. She has done her Ph.D From Totori university Japan with Virus pathogenesis and Immunology as major research interest. She worked as a post doctoral fellow in NIDCD/NIH, USA and Childrenʼs hospital Los angeles, USA. She also worked as Research associate and Research assistant from Jiangsu Institute of Poultry science china.
1David H. Koch Institute for Integrative Cancer Research USA
2,3Beth Israel Deaconess Medical Center, Boston, MA, USA
3CHU de Liege -University of Liege, AIDS Laboratory Reference, Liege, Belgium
Infection of HIV causes decline of CD4 T-cells number and impaired immune function that leads to onset of AIDS. Apart from HIV-1-mediated killing a more comprehensive explanation has appeared that includes chronic immune activation and T cell exhaustion as a central feature in HIV-1 pathogenesis. While highly active antiretroviral therapy (HAART) markedly reduces viral load, T cell activation levels and soluble markers of inflammation remain abnormally high. Markers of chronic activation, such as CD38, PD-1 or HLA-DR on T cells, appear to be better predictors for clinical progression during HIV infection than HIV RNA levels and CD4 T cell counts alone. Therefore, a better understanding of HIV-induced immune activation and the design of new immunomodulatory approaches in combination with HAART are needed.
We have generated an efficient model of human stem cells (HSCs) engraftment in NOD/LtsZ-scidIL-2Rnull (NSG) mice that supports chronic HIV infection with high plasma viral loads. HIV-1 infection in these humanized mice is characterized by widespread immune activation with increased expression of PD-1, HLA-DR, CD38, CD69, CD25 and other immune activation markers. These humanized mice provide an effective in vivo system for the assessing novel approaches for their potential in suppressing chronic immune activation during HIV-1 infection, in absence of interference of antiretroviral therapy. In this study, we evaluated in vivo the benefits of two novel approaches aimed at reducing HIV-induced immune activation.
Minocycline is an antibiotic of the tetracycline family with anti-inflammatory and immunomodulatory properties affecting CD4 T cells activation by a mechanism involving the inhibition of the NF-AT1 transcription factor activity. We hypothesized that this antibiotic could suppress the HIV-1-induced chronic immune activation and thus, limit the HIV pathogenesis when combined to HAART. Therefore, we treated HIV-1 (JRCSF) infected-humanized NSG mice with minocycline (100mg/kg/day) for 60 days. We next evaluated the expression, by flow cytometry, of several T cells activation markers together with CD4+ T cells counts. Our data suggest that minocycline is effective in suppressing HIV-1 induced immune activation in peripheral blood and lymphoid organs (spleen, lymph nodes and bone marrow). Levels of cellular immune activation markers such as PD-1, HLA-DR, CD38, CD69, CD25, CD28 and CTLA-4 were significantly lower in minocycline treated group. These immunological benefits of minocycline were correlated with higher CD4+ T cell counts in the treated group.
The immune activation which is associated with retroviral infection is also associated with increased levels of intracytoplasmic cyclic AMP which could act as a positive feedback loop in the infection since several reports have suggested that cAMP and downstream signaling pathways play an important role in the permissivity of susceptible cells to HIV infection and replication. We have used a peptide which prevents the binding of the catalytic subunit of PKA type I to its anchoring protein and therefore blocks most effects of cyclic AMP within lymphocytes and monocytes (RIAD peptide). Mice were treated with 3.5 mg/kg of RIAD peptide weekly. Treatment of humanized mice with RIAD peptide limited viral replication after high dose of HIV intraperitoneal challenge and reduced the intracytoplasmic levels of cyclic AMP.
Further experiments are needed to better appreciate the therapeutic potential of these novel therapies in the suppression of HIV-induced chronic immune activation.
Biography:
Maneesh singh, Postdoctoral Associate, at David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Boston where his work focused on developing humanized mouse model of osseous metastasis cancer as a novel preclinical platform to test synergistic chemoimmunotherapeutic regimens. He has received Mastersʼs degree in Botany from Purvanchal University in India. In 2004, he joined Center for Cellular and Molecular Biology, Hyderabad in India were he worked for molecular diagnosis of Japanese encephalitis and Chikungunya virus during 2005 and 2006 outbreaks in India. He received his Ph.D. in are immunology and virology in 2013 from University of Liege, Belgium. His research focuses on the development of humanized mice model for HIV/Cancer research.
University of Tennessee Medical Center, USA
The issues for investigators and for sites are somewhat unique for vaccine studies. The large number of subjects enrolled in a very short time, the number of minor AEs, the large total number and different types of specimens to process and the need for 1-5 year subject retention. Many issues arise around specific populations especially the elderly. Speed and competitive enrollment verses a controlled calculated pace is a discussion mostly unique to vaccines. Other issues while not unique to vaccine studies, such as data entry, supplies, I.P., and randomization plans are amplified due to the large number of subjects these studies enroll. This session will discuss these differences and offer protocol considerations to assist with protocol development and execution.
Biography:
William B. Smith, MD, FACC, is currently a Professor of Medicine at the University of Tennessee Medical Center in Knoxville, Tennessee. Dr. Smith is board certified in Nephrology, Internal Medicine, Cardiology and Critical Care Medicine.
Dr. Smith is the President and Founder of New Orleans Center for Clinical Research and Volunteer Research Group located within the University of Tennessee Medical Center. Dr. Smith has been involved as a Principal Investigator in over 1800 clinical research studies including cardiac disease, renal/hepatic disease, healthy volunteers, diabetes, womenʼs health, HSDD, obesity, Parkinsons, Alzheimers, Multiple Sclerosis, smoking cessationandnumerous variations of PK trials.Dr. Smith has extensive experience conducting complex trials, including, Phase 1/special population trials, First-in-Human, POC, SAD-MAD and with vaccines, including Smallpox, H5N1, H1N1, Seasonal flu, Dengue Fever, TDAP, Bubonic Plague, Ebola, and others.
1CSIR-Institute of Microbial Technology, India
2Government Medical College and Hospital, India
3Department of Microbiology and Immunology, the University of Melbourne, Australia
Background: Vaccines have been successful in worldwide eradication of dreaded diseases like smallpox, diphtheria, tetanus, yellow fever, whooping cough, polio, and measles. Unfortunately, such triumph has not been achieved in controlling tuberculosis (TB) globally. Bacillus Calmette Guérin (BCG) is the only available vaccine against TB. Paradoxically, BCG has deciphered successful results in the Western population but has failed in TB-endemic areas. Hence, it is quite crucial to understand the immunity responsible for controlling Mycobacterium tuberculosis infection and factors responsible for the failure of BCG in TB-endemic countries. Consequently, introducing radical changes in the vaccines that would impart protection in the populations where BCG has failed. One of the main reasons considered for BCG failure in TB-endemic areas is impediment by environmental mycobacteria in its processing by antigen presenting cells and generation of memory T-cell response.
Methods: The peripheral blood mononuclear cells of sputum positive pulmonary TB patients and their house-hold contacts were separated by ficoll-hypaque gradient method. The cells were cultured with L91 and proliferation was monitored by CFSE-dye dilution assay and phenotypic markers by flowcytometry using fluorochrome tagged appropriate antibodies and their isotype-matched controls.
Results: Keeping in view the shortcomings of BCG, we developed a unique lipopeptide (L91) by linking the promiscuous peptide (sequence 91-110) of 16 kDa antigen of Mycobacterium tuberculosis to Toll-Like Receptor-2 agonist Pam2Cys. L91 does not require extensive antigen processing and targets and activate dendritic cells. This is evidenced by the fact that L91 significantly improved the activation and proliferation of polyfunctional Th1 and Th17 cells of the TB patients and their house-hold contacts. Furthermore, L91 surmounts the barrier of major histocompatibility complex polymorphism. Importantly, this peptide has self-adjuvanting property and induces enduring memory T cell response, which is significantly better than BCG.
Conclusion: L91 can be a potent future vaccine candidate against tuberculosis in TB-endemic and non-endemic zones.
Biography:
Javed N Agrewala did his BSc, MSc and PhD from Agra University, Agra. In 1989, he joined as a Scientist at the CSIR-Institute of Microbial Technology, Chandigarh. He was a Visiting Scientist at the Royal Postgraduate Medical School, Hammersmith Hospital, London, UK [1994-1996] and Trudeau Institute, Saranac Lake, USA [2001-2002]. Currently, he is working as a Chief Scientist at the CSIR-Institute of Microbial Technology, Chandigarh. He received the Shanti Swaroop Bhatnagar award, National Bioscience award for career development and new idea research talent award. He received a fellowship from Medical Research Council (MRC), UK