Research Article
Seroprevalence of Hydatidosis in Camels of Assuit Province, Egypt
1Department of Medical Parasitology Faculty of Medicine Assiut University, Egypt
2First researcher, Animal Health Research Institute, Assiut Laboratory, Egypt
3Department of Medical Parasitology Faculty of Medicine South Valley University, Egypt
*Corresponding author: Ahmed Kamal Dyab Haemaei, Professor and Head, Department of Medical Parasitology, Faculty of Medicine (71526), Assiut University, Egypt, Tel: 00201018614645, Fax: 0020882350177, E-mail: ahmedsaf2001@yahoo.com; ahmed2015@aun.edu.eg
Received: March 16, 2017 Accepted: May 5, 2017 Published: May 12, 2017
Citation: Dyab AK, Mohamed GM, Abdella OH. Seroprevalence of Hydatidosis in Camels of Assuit Province, Egypt. Madridge J Vaccines. 2017; 1(1): 5-8. doi: 10.18689/mjv-1000102
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The present investigation was conducted (during PM examination of the slaughtered carcasses) to assess the prevalence of hydatidosis in camels sacrificed in Assuit Governorate Egypt and to evaluate the sensitivity and specificity of Indirect Enzyme-linked immunosorbent assay (ELISA) in identifying camels infected with hydatid cysts before slaughtering using antigens were precipitated from HCF. Hydatid cyst count and characterization were conducted based on routine meat inspection. Slaughterhouse samples of 200 camels were collected through weekly visits. Hydatid cysts in livers, lungs and kidneys were detected and counted, also the fertility rate of the cysts was examined. Out of these, 12 (6%) were found to harbour hydatid cyst, in livers 9(75%), lungs 2(17%) and kidney 1(8%).On the other side, fertile cysts 5(41.7%) were found more frequently in livers 4(33.3%) than in lungs 1(8.3%), while sterile cysts7(58.3%) found in livers, lungs and kidneys 5(41.7%), 1(8.1) and 1(8.3%) respectively. In addition to PM examination, Enzymelinked immunosorbent assay test (ELISA) was developed to the same camels for serological detection of hydatid cyst infection but in alive state. 16(8%) of the 200 camels were found harbouring hydatid cysts were serologically positive when screened for hydatidosis by ELISA test. Four animals (2%) out of the 188 non-infected camels gave serologically positive result. It is suggested that the ELISA as a serological assay, is a valuable method with high diagnostic efficiency for serodiagnosis of hydatid disease. The public health importance of hydatidosis as well as some recommended measures for controlling of the disease were discussed.
Keywords: Diagnosis; Hydatid cyst; ELISA P.M; Camels.
Introduction
The main source of animal protein is livestock and their products. Parasitism is one of the main constraints limiting livestock production. Mortality of animals from parasitic diseases may not be alarming at time but their direct effects in terms of reduced milk, meat, wool, hide production, infertility and loss of stamina of working animals [1].
Cystic Echinococcosis (CE) is a chronic zoonotic parasitic helminthic disease due to infection with the larval stage (hydatid) of the dog tapeworm Echinococcus granulosus. The parasite has a global distribution but is particularly prevalent in rural areas where it is transmitted in a cycle between the dog, the definitive host and man, sheep, camel, and other ruminants act as intermediate hosts causing major economic and health problems [2]. The life cycle of Echinococcus granulosus involves domestic and wild carnivores as definitive hosts, which are infected by the ingestion of the hydatid cyst, which may be present in the tissues of infected animals with viable protoscoleces producing adult stage in the intestine. Dogs are the main source of infection, although in some areas jackals, hyenas, foxes, and wolves could also play a role as definitive hosts. A wide range of domestic, wild mammals and humans act as intermediate hosts for this parasite where the larval stages (hydatid cyst) develop after ingesting the eggs [3]. Accidental rupture of hydatid cyst during trauma can provoke severe anaphylactic reactions in human [4]. Cystic echinococcosis accounts for more than 95 % of the estimated 2–3 million human global cases affected by Echinococcus parasites [5]. Hydatid cysts can be found in many tissues, most often in the liver, lung, mediastinum, peritoneum and nearly every site of the body [6]. In animals, hydatid cyst usually remain as asymptomatic disease producing no clinical symptoms and its course is slow. In domastic animals diagnosis is almost made during postmortem, small unilocular cysts are usually not diagnosed in young animals until middle life or later [7]. Animals infected with this cysts often suffer from reductions in live weight gain, in milk yielding, in the fertility rates, in the value of wool or other products [8]. On the other hand the main clinical symptoms in humans include liver dysfunction, lung problems, ascites, abdominal pain, hepatomegaly, splenomegaly, central nervous system disorders [7]. The larva of Echinococcus granulosus, may grow for 5 to 20 years without being detected. Surgical excision of the cyst is the only effective treatment, but in many cases the disease recurs because the contents of th5e cyst may be escape during the operation [8]. Identification of sensitive and specific methods for immunodiagnosis of hydatid cysts is affected by the degree of sensitivity and specificity of the used antigens. These methods are able to exclude false negative or false positive reactions caused by infection with other cestoda or even other helminthes [9]. Immunodiagnostic techniques such as Enzyme-linked immunosorbent assay (ELISA) is used for the diagnosis of hydatidosis in human and animals. This serological test provides extremely useful diagnostic method for the disease [10]. The current study aimed to assess the usefulness of ELISA test for serodiagnosis of Cystic echinococcosis in camel infected with hydatid cysts before slaughtering and to assess the prevalence of hydatidosis in camels sacrificed in Assuit governorate. Hydatid cyst count and characterization were conducted based on routine meat inspection.
Materials and Methods
The camels used in this study came from different location of Assuit Governorate Egypt, to the abattoirs (Assuit and Bany-ady abattoirs). After slaughtering, the animals were examined for the presence of hydatid cyst in the livers, lungs and other organs. Infected animals were recorded and the infected organs were collected. Any cyst found was collected in normal saline. The surface of a randomly selected cyst of each infected organ was sterilized by alcoholic-iodine solution to reduce intra-cystic pressure, and then the cyst was penetrated by a needle and cut given with scalpel and blade, then the content (fluid and germinal layers) was transferred into sterile container and examined microscopically for the presence of protoscolices. The viability of protoscolices was determined by using eosin exclusion 10% solution. This test for cell death, viable protoscolices do not take eosin stain [11].
Serological survey
Indirect enzyme-linked immunosorbent assay (ELISA) was developed for serological detection of hydatid cyst infection in alive camels. (Employed to determine the prevalence of specific antibodies against hydatidosis in sera collected from alive camels). In indircet ELISA kits the wells were coated by hydatid cyst fluid (HCF) antigens were precipitated from HCF [12]. The antigen was prepared to used local antigen which more sensitive and specific. Blood was collected from each animal before slaughtering and allowed to clot for separation of serum. Serum samples were stored at -20°C until examination.
Preparation of antigen
Two hydatid cyst fluid (HCF) antigens (antigens A and B) were found to be the most immunogenic antigens in HCF [13] [14]. The two antigens were precipitated together from HCF. This was done by adding 2M phosphotungstic acid and 2M magnesium chloride solutions to clarified HCF while continuously stirring the mixture. The precipitate formed was suspended in physiological saline [15]. This antigens' solution was used to coat microtitre plates for indirect ELISA which was performed on 200 selected camels sera [16]. ELISA test was done as described by [17] as manufacture directions
Results and Discussion
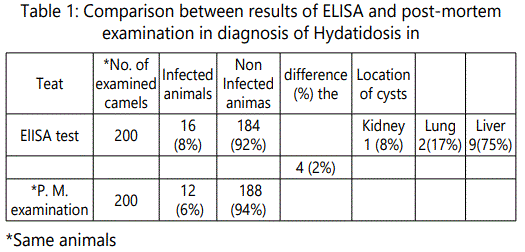
Hydatidosis is an important parasitic zoonosis and the disease has been recorded in almost all parts of the world during execution of veterinary inspection in slaughter houses [18]. The results were evident that out of 200 slaughtered camels examined visually and manually by palpation and incision, 12 (6%) were found harboring hydatid cysts in, livers, lungs and kidneys. Of the total 12 infected, 9 (75%) had hydatid cysts in the liver, 2 (17%) in the lung, and 1 (8%) found in the kidney. It is indicated that livers and lungs are the most commonly affected organs with hydatid cysts due to the reason that they are the first large capillary fields encountered by the blood born onchosphers. The results of this study revealed that liver is the most commonly affected organ which might be due to the reflection of the route of parasite entry and seem to support the hypothesis of hepatic portal distribution of onchosphers leading firstly to liver infection [19]. The distribution of cysts in different organs based on their sizes. The infection rate increases as the age of animal increases. It was found that aged animals may gain access of infection due to longer exposure than young ones. It was also reported by [20] that, the number of infected eggs ingested by intermediate host is determined by the level of contamination and infectivity of the eggs. Furthermore, the number of eggs that develop into hydatid cysts is controlled by the immune system of the host. The findings of this study revealed that low number of the slaughtered camels were infected with hydatid cysts. The argument behind the lowest infection rate was the unique raising system and the feeding management of camel as well as the weak relationship between camel and stray dogs may explain these findings [21]. Confirmed to our results, [22] could not detect hydatid cyst from any of the examined slaughtered camels. Also, some authors recorded nearly similar percentage of hydatid cysts as, [23] (8.8%) [24] who revealed that the overall annual prevalence rates of camel infection were 5.5% (1992), 6.1% (1993), 6.7% (1994), 8.2% (1995) and 4.3% (1996), [25] (4.9%), [26] (9%) and [27] (5.5%). On the other side, the obtained results were lower than that recorded by [28] (20,4%), [29] who recorded 50% in Northern of Guinea zone and 55.5% in Sudan zone, [29] (40%), [30] (16%), [31] (20.7%).
By using HCF Antigen A and B [12] (32.9%) and [32] (26%). The high (20est percentage of infection which is mainly attributed to the old age at the animal was slaughtered and examined for the presence of hydatid cysts. It was found that the environmental conditions such as suitable condition for survival of the eggs of E. granulosus and the presence of large number of stray dogs around the raising area of animals are the main factors governing the prevalence rate of hydatidosis in ruminants [21]. In addition, out of the total 12(6%) cysts collected, 5(41,7%) were fertile an16d 7(58.3%) were sterile cysts. The occurrence of fertile cysts was higher in liver 4(33.3%) than in lung 1(8.3%) while the sterile cysts found more frequently in liver 5(41.7%) than in lung 1(8.3%) and kidney 1(8.3%). As far as this study is concerned, the results of fertility were lower than that obtained by [29] (94.5%), [23] (66.7%) and [31] (58.2%). While [30] cited that 6.3% barber fertile hydatid cyst which seem to be lower than our results. Variation in fertility could be attributed to strain differences in traits such as host and organ preference, development rate, infectivity, pathogenesis and antigenicity and drug resistance [20].
Diagnosis of hydatidosis is still problematic [33]. Serological tests such as immunoelectrophoresis, double diffusion in agar, or indirect hemagglutination are being replaced by more sensitive assay methods such as enzyme-linked immunosorbent assay (ELISA) [34]. ELISA was, highly specific (90 %) for camels natural CE infection [12] and its sensitivity was found to be 98% [16] Concerning the pre-slaughtered camels, the finding outlined in Table"1" showed that the percentage of hydatidosis by ELISA technique was (8%). This trend is lower than that recorded by (35) (26.6%) in local camels, 32% in imported camels) and EL-Baz (1994) (40%). The incidence of infection was higher by indirect ELISA technique (8%) in comparison with the data of P.M examination (6%). It is clearly evident in Table" 1" that 4(2%) animals out of 188 (94%) non infected camels gave positive results serologically by ELISA. This was agreed with [36] as the difference between sero-diagnosis and PM diagnosis of hydatidosis may be related to presence of small sized hydatid cyst which could not be demonstrated by visual examination or presence of infections in other parts of the body did not accurately investigated. The main problems for the serodiagnosis of Cystic echinococcosis are often the unsatisfactory performance of the available tests and the difficulties associated with the standardization of antigenic preparations and techniques [37]. To overcome these drawbacks, highly sensitive and specific antigens and antigenic components derived from different developmental stages of E. granulosus must be available [38] [39] [40].
In conclusion: The results of this study indicated that hydatidosis is of great public health and economic significance in the governorate of Assuit. Detection of hydatid cysts in the slaughtered camels abattoirs and its improper disposal will act as a source of infection to final host (mainly dog) and transmission to human beings. Accordingly, conducting public campaign is urgently required to control this disease through destruction of stray dogs, prohibiting illegal slaughter of animals outside abattoirs, proper disposal of infected organs, fencing of slaughterhouses and increase awareness of the people on the epidemiology of the disease. Identification of infected animals during their life could facilitate slaughtering them under special control measures which ensure total condemnations of their infected tissues and eliminate the random arrival of the cysts to dog, the matter which play the role to minimize the infection in dogs and wide spread of the diseases. Moreover it is necessary to mention that, control measures of hydatidosis must be carried on parallel to that of human beings, also against the definitive host and the other intermediate ones. It can be also concluded that ELISA should be considered not as an alternative but as a useful addition to the range of immunodiagnostic tests available for serodiagnosis of hydatid
References