Research Article
Role of Virtual Colonoscopy for Diagnosis of Colorectal Tumours
1Department of General and Operative Surgery, Faculty of Medicine, Medical University "Prof. Paraskev Stoyanov" of Varna, Bulgaria
2Department of Anesthesiology, Emergency and Intensive Medicine, Faculty of Medicine, Medical University "Prof. Paraskev Stoyanov" of Varna, Bulgaria
3Department of Radiology, St. Marina University Hospital of Varna, Varna, Bulgaria
*Corresponding author: Krasimir Ivanov, Department of General and Operative Surgery, Medical University "Prof. Paraskev Stoyanov" of Varna, 55 Main Drinov Street, Varna 9002, Bulgaria, E-mail: kdi@abv.bg
Received: September 22, 2017 Accepted: November 1, 2017 Published: January 1, 2018
Citation: Ivanov KI, Ignatov V, Petrov D, et al. Role of Virtual Colonoscopy for Diagnosis of Colorectal Tumours. Madridge J Surg. 2018; 1(1): 1-5. doi: 10.18689/mjs-1000101
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Nowadays colorectal cancer (CRC) is the first most common neoplasm in men and the second most common one in women worldwide. Recently, virtual colonoscopy (VC) (or computed tomographic colonography, CTC) has proved to be a sufficiently sensitive and accurate method for CRC screening and diagnosis. The purpose of this retrospective investigation was to compare the diagnostic capacities of VC and optical colonoscopy (OC) in patients with colorectal neoplasms. Our study covered a total of 120 patients, 61 males and 59 females with colorectal lesions who underwent both VC and OC in St. Marina University Hospital of Varna between January, 2009 and December, 2015. We analyzed the indications for VC, its diagnostic value concerning tumour type, size, and localization. These indications included the following: a finishing procedure for viewing the colon; CRC staging and variability in anatomy and comorbidity, colonic postpolypectomy screening as well as non-invasive diagnostic modality. In 115 patients (in 95, 83% of the cases), VC detected colorectal lesions. A colon polyp was diagnosed in 94 patients (in 78, 33%) but a CRC - in 26 ones (in 21, 67% of the cases). VC specificity and sensitivity was 94% and 98%, respectively. The results of OC and VC were comparable (p>0.05). VC proved to be an accurate diagnostic method for CRC and colon polyps. It could be successfully applied in recognizing the two pathologies relative to the lesion size (OR=1.209, 95% CI 1.115-1.312). Because of its high specificity and sensitivity, VC should find a broader application as a significant tool for CRC diagnosis, staging and screening. VC is non-invasive and painless diagnostic procedure. It is useful as complementary option to OC and in cases with contraindications for OC as well.
Keywords: Colorectal cancer; Colon polyp; Virtual colonoscopy; Optical colonoscopy.
Abbreviations used: CI: Confidence Interval; CRC: Colorectal Cancer; CTC: computed Tomographic Colonography; OC: Optical Colonoscopy; OR: Odds Ratio; ROC: Receiver Operating Characteristic; VC: Virtual Colonoscopy.
Introduction
Nowadays colorectal cancer (CRC) is the first most common neoplasm in men and the second most common one in women worldwide [1]. CRC incidence and mortality rates vary up to 10-fold worldwide, with distinct gradients across human development levels, pointing towards widening disparities and an increasing burden in countries in transition [2]. Generally, CRC incidence and mortality rates are still rising rapidly in many low-income and middle-income countries.
Recently, virtual colonoscopy (VC) (or computed tomographic colonography, CTC) proved to be a sufficiently sensitive and accurate method for CRC screening and diagnosis [3, 4]. VC is developed in 1994 by Vining et al [5]. It is a new method of imaging the colon in which thin-section helical CT is used to generate high-resolution, two-dimensional axial images. Three-dimensional endoluminal images of the colon, simulating those obtained with conventional colonoscopy, are then reconstructed off-line [6]. This technique is an attractive alternative to existing screening tests for CRC, since it is relatively safe and minimally invasive (Fenlon). It allows the staging of CRC patients. A combination of early detection and adenoma removal remains the best method for reduction of CRC incidence and mortality rates [7].
The interest in VC has been renewed after a publication in the New England Journal of Medicine in 2015 [6-8]. This method warrants an almost 100% diagnostic success in detecting CRC and colon polyps. The patients with extensive and long-standing colitis have increased CRC risk. Differentiation between inflammatory stenosis in ulcerative colitis and CRC is the domain of endoscopy with biopsy while CTC is used as an adjunct in these patients in whom the colon can't be endoscopically accessed [9]. On the other hand, VC does not require any intravenous administration of sedatives, analgesia, or recovery time [10] when evaluating the colon proximally to obstructive lesions as well as the extracolonic abdominal and pelvic organs. Between 1.5% and 9.0% of CRC patients have a second synchronous cancer, and 27%-55% have multiple coexistent adenomatous polyps. Recently, there is a rising interest in the diagnosis and management of the synchronous CRC [11-15]. Failure to identify a synchronous cancer before surgery results in curative resection failure and is associated with the added morbidity and mortality of a second surgical procedure as well as with an invasive, potentially metastasizing cancer in the remaining colon [16]. CTC is well-tolerated and more acceptable to patients than OC and improves CRC screening compliance [7].
The purpose of the present study is to retrospectively analyze the results of VC and OC applications in the diagnosis of CR tumours and to reveal the particular role of VC in this respect.
Materials and Methods
Our study covered a total of 120 patients, 61 males and 59 females with colorectal lesions who underwent both VC and OC in St. Marina University Hospital of Varna between January, 2009 and December, 2015. We analyzed the indications for VC, its diagnostic value concerning tumour type, size, and localization. VC indications included the following: a finishing procedure for viewing the colon; CRC staging and variability in anatomy and comorbidity, colonic postpolypectomy screening as well as non-invasive diagnostic modality.
VC was performed when the following symptoms were present: abdominal pain, rectorhagia, anemia, constipation syndrome as well as if there was evidence of inherited predisposition. The contraindications for VC included active colon inflammation, e.g. diverticulitis, active stage of inflammatory bowel disease, toxic megacolon, acute abdominal pain, hernia acreta, as well as recent colorectal, abdominal or pelvic surgery.
Anatomical colon classification into six parts such as cecum, ascending colon and hepatic flexure, transverse colon and splenic flexure, descending colon, sigmoid colon, and rectum was used for the description of pathological lesions. The localization and morphology of the pathological findings were characterized. VC and OC specificity and sensitivity rates for colorectal lesions were comparatively calculated.
Preliminry patient's preparation consisted in three enemas and oral laxative administration. A routine OC protocol was made use of. VC protocol included the following: 12 hours prior to the imaging test, 20 mL of contrast matter was given p.o.; 4 hours before the test the patient drank iodine contrast (Ultravist®, Bayer) dissolved in 2 L of water. At VC initiation, gas was insufflated through the anus. First scanning was on abdominal position and the second one on the back with i.v. injection of contrast (3 flow 100 mL 60 sec later on). VC was performed with a 128 slash Dual Energy Siemens SOMATOM scanner. The PC software made two- and three-diomensional image reconstructions of the pathological sections (Fig. 1 and Fig. 2). Every examined study participant filled-in a questionnaire to assess his impressions with both methods - of VC and OC.
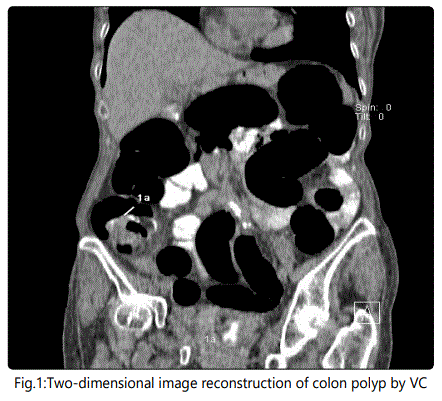
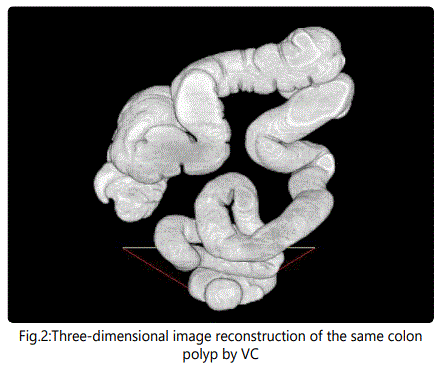
Data were statistically analyzed using SPSS software ver. 23. Chi-squared test was used to comparatively evaluate the correlations between OC and VC in CR tumor diagnosis and discomfort from VC and OC as well. Independent samples test was applied for the comparative evaluation of VC values in CRC patients. VC specificity and sensitivity for discrimination between colon polyp and cancer by tumor size were assessed with receiver operating curve (ROC) analysis at a cut-off value of 3 cm. Diagnostic accuracy of tumor size was determined by obtaining the largest possible area under the curve (AUC). Odds ratios (ORs) with 95% confidence intervals (CIs) for caterogical outcomes were calculated using logistic regression model. Two-tailed p-values (<0.05) were considered significant.
Results
In 115 patients (in 95,83% of the cases), VC was positive for colorectal lesions. A colon polyp was diagnosed in 94 patients (in 78,33%) but a CRC - in 26 patients (in 21,67% of the cases).
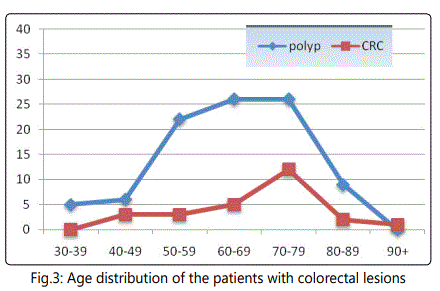
CRC occurred most commonly in the age group between 70 and 89 years but colon polyps did in the age groups between 60 and 79 years. Most patients were between 60 and 90 years old (Fig. 3). There was no statistically significant difference between the patients' groups of 30-50 years and above 80 years concerning the presence of colon polyp and CRC. The number of colon polyp patients was reliably greater than that of CRC ones (p<0.05) (Fig. 3).
The main complaints of patients with colorectal lesions were rectorhagia (in 25%), anemia (in 55, 5%), and abdominal pain (in 30% of the cases).
The diagnostic capacity for additional pathological findings is a particular benefit of VC as demonstrated on Table1.
Patient's preferences of these diagnostic methods based on discomfort level during the procedures were indicated on Figure 4.
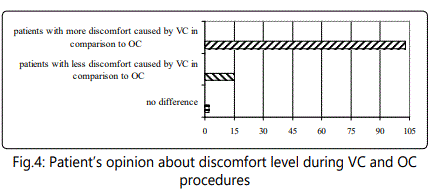
The analysis of the questionnaire about patient's attitude to both methods showed that 85.83% of patients reported less discomfort while 12, 5% reported more discomfort caused by VC as compared to OC (Fig. 4). This difference was statistically significant (t=7.97, p<0.001).
Based on VC results, the therapeutic strategy was modified in 10% of our patients. Surgery was done in any CRC patients as CRC localization was proved in all of them. There was coincidence between intraoperative tumour localization, on the one hand, and OC and VC descriptions in 97% and in 96% of the cases, respectively. Data obtained by OC and VC were comparable (t=4.2, p<0.05).
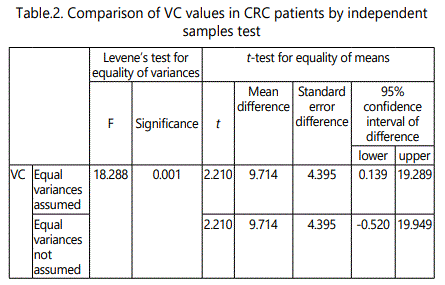
The statistically significant comparisons of VC values in CRC patients were demonstrated on Table 2.
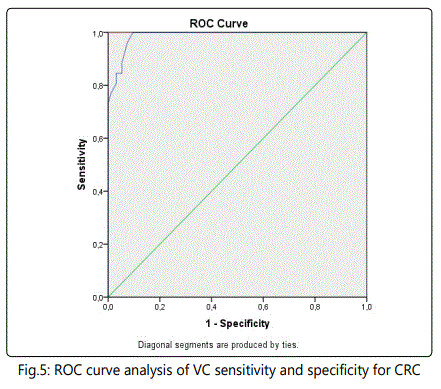
The results of ROC curve analysis indicated that usage of tumor size at appropriate cut-off values discriminated the patients with colon polyp and CRC (AUC=0.98, 95% CI: 0.97- 1.0, p<0,05) with sensitivity of 88.5% and specificity of 94.7% (Fig. 5). The blue curve presented the results from the comparison between CRC and colon polyp data as diagnosed by VC. It was close to 1.0 which represented these statistically significant high sensitivity and specificity rates for these pathologies. Tumor size was a positive predictive marker for malignancy. This method could be successfully used in recognizing the two pathologies relative to their lesion size (OR=1.209, 95% CI: 1.115-1.312). Therefore, VC was an accurate differential diagnostic method for CRC and colon polyps.
Discussion
Nowadays OC is considered the gold standard for diagnosis of colorectal pathology because of the possible biopsy examination of colon damage. However, its application is restricted in case of obstructive lesions and absent patient's tolerance. As an invasive procedure, it requires sedation and takes a longer time [17]. Despite its efficacy and diagnostic value, small polyps <1 cm are missed in one third of the cases. The entire colon can't be examined in 10% of the cases, which along with the risk of bleeding and colon perforation (in 0.1- 0.3% of the cases) necessitates better diagnostic methods for colorectal lesions.
The new and already approved method of VC provides minimal invasiveness and structural assessment of the entire colon [9] as well as fast imaging of the entire colon, no need of sedation, and a low risk of procedure-related complications. This technique is performed in symptomatic patients suspected of colorectal pathology and in patients with incomplete or contraindicated OC. VC can be applied in conjunction with a full OC to confirm diagnosis and staging with a low risk of complications compared to OC alone. Postprocedure perforation in VC and OC amounts only to 0.03% and 0.009%, respectively [7]. VC is preferred in comorbid patients, too. In a systematic review and meta-analysis involving 49 studies and providing data about 11151 patients of which there are 414 CRC patients (3.71% of the cases), VC sensitivity for CRC is 96.1%. In a subgroup of 25 trials involving 9223 patients, OC sensitivity for CRC is 94.7 % [10]. Therefore, VC is a highly sensitive diagnostic examination for CRC. VC sensitivity (of 98%) in our own study correlates with that in this meta-analysis.
VC capacity to detect a lesion increases with the size of this lesion. For lesions <5 mm, VC sensitivity is up to about 50%. Many smaller lesions of the colon (<5 mm in size) are considered clinically insignificant. However, VC seems to be a relaible method for colorectal lesion screening and assessing. Besides it is preferred by the majority of the patients.
A randomized, controlled CRC screening study shows that sensitivity of fecal occult blood test, VC and OC is 64%, 77% and 80%, respectively [18]. Unwanted side effects such as vagal reactions are very rare, indeed.
CTC allows the accurate assessment of both colonic and extracolonic pathologies as a useful diagnostic tool in patients for whom complete OC is not achievable [19]. CTC is a valuable diagnostic tool for examining the entire colon and a good alternative compared to other CRC screening tests because of its high sensitivity values [20, 21], especially in colorectal lesions over 1 cm. [20, 22] CTC combined with OC provides a more accurate preoperative determination of CRC localization and invasion depth than OC alone [23].
Conclusion
Our results and literature data available convincingly demonstrate that because of its non-invasiveness, high specificity and sensitivity, VC can play a significant role in CRC diagnosis, staging and screening as well. This method is painless and thus it does not require any sedation. It is useful as complementary option to OC and in cases with contraindications for OC as well.
Conflict of Interest
The authors confirm that there is no conflict of interest regarding this manuscript.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127(12): 2893-2917. doi: 10.1002/ijc.25516
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017; 66(4): 683-691. doi: 10.1136/gutjnl-2015-310912
- Torres C, Szomstein S, Wexner SD. Virtual colonoscopy in colorectal cancer screening. Surg Innov. 2007; 14(1): 27-34. doi: 10.1177/1553350607299563
- Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection - systematic review and metaanalysis. Radiology. 2011; 259(2): 393-405. doi: 10.1148/radiol.11101887
- Fenlon HM, Nunes DP, Schroy PC 3rd, Barish MA, Clarke PD, Ferrucci JT. A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N Engl J Med. 1999; 341(20): 1496-1503. doi: 10.1056/ NEJM199911113412003
- Bellows C, Gagliardi G, Bacigalupo L. Review of computed tomographic colonography from a surgeon's perspective. J Gastrointest Liver Dis. 2015; 24(2): 215-223. doi: 10.15403/jgld.2014.1121.242.blws
- Singh K, Narula AK, Thukral CL, Singh NR, Singh A, Kaur H. Role of CT colonography in colonic lesions and its correlation with conventional colonoscopic findings. J Clin Diagn Res. 2015; 9(4): TC14-TC18. doi: 10.7860/JCDR/2015/12686.5853
- Patel JD, Chang KJ. The role of virtual colonoscopy in colorectal screening. Clin Imaging. 2016; 40(2): 315-320. doi: 10.1016/j.clinimag.2015.07.009
- Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003; 349(23): 2191-2200. doi: 10.1056/NEJMoa031618
- Arriba M, Sánchez R, Rueda D, Gómez L, García JL, Rodríguez Y, et al. Toward a molecular classification of synchronous colorectal cancer: clinical and molecular characterization. Clin Colorectal Cancer. 2017; 16(1): 31-37. doi: 10.1016/j.clcc.2016.07.014
- Bick BL, Ponugoti PL, Rex DK. High yield of synchronous lesions in referred patients with large lateral spreading colorectal tumors. Gastrointest Endosc. 2017; 85(1): 228-233. doi: 10.1016/j.gie.2016.06.035
- He W, Wei M, Yang X, Chen B, Wu Q, Zheng E, et al. Do inflammatory markers predict prognosis in patients with synchronous colorectal cancer? Medicine (Baltimore). 2017; 96(17): e6607. doi: 10.1097/MD.0000000000006607
- Kim WS, Lee HS, Lee JM, Kwak MS, Hwang SW, Park SH, et al. Fluoro-2- deoxy-D-glucose positron emission tomography/computed tomography for the detection of proximal synchronous lesions in patients with obstructive colorectal cancer. J Gastroenterol Hepatol. 2017; 32(2): 401- 408. doi: 10.1111/jgh.13486
- Yu J, Xu Q, Huang DY, Song JC, Li Y, Xu LL, et al. Prognostic aspects of dynamic contrast-enhanced magnetic resonance imaging in synchronous distant metastatic rectal cancer. Eur Radiol. 2017; 27(5): 1840-1847. doi: 10.1007/s00330-016-4532-y
- Fenlon HM, McAneny DB, Nunes DP, Clarke PD, Ferrucci JT. Occlusive colon carcinoma: virtual colonoscopy in the preoperative evaluation of the proximal colon. Radiology. 1999; 210(2): 423-428. doi: 10.1148/ radiology.210.2.r99fe21423
- Macari M, Bini EJ. CT colonography: where have we been and where are we going? Radiology. 2005; 237(3): 819-833. doi: 10.1148/radiol.2373041717
- You JJ, Liu Y, Kirby J, Vora P, Moayyedi P. Virtual colonoscopy, optical colonoscopy, or fecal occult blood testing for colorectal cancer screening: results of a pilot randomized controlled trial. Trials. 2015; 16: 296. doi: 10.1186/s13063-015-0826-7
- Maggialetti N, Capasso R, Pinto D, Carbone M, Laporta A, Schipani S, et al. Diagnostic value of computed tomography colonography (CTC) after incomplete optical colonoscopy. Int J Surg. 2016; 33 Suppl 1: S36-S44. doi: 10.1016/j.ijsu.2016.05.053
- Devir C, Kebapci M, Temel T, Ozakyol A. Comparison of 64-detector CT colonography and conventional colonoscopy in the detection of colorectal lesions. Iran J Radiol. 2016; 13(1): e19518. doi: 10.5812/iranjradiol.19518
- Kumar M, Cash BD. Screening and surveillance of colorectal cancer using CT colonography. Curr Treat Options Gastroenterol. 2017; 15(1): 168-183. doi: 10.1007/s11938-017-0121-7
- Yu HH, Huang HY, Jiang YS, Zhu C, Guo CG, Dai M, et al. Accuracy of CT colonography for the detection of colorectal neoplasm: a subgroup metaanalysis. Zhonghua Liu Xing Bing Xue Za Zhi. 2017; 38(6): 814-820. doi: 10.3760/cma.j.issn.0254-6450.2017.06.025
- Kanazawa H, Utano K, Kijima S, Sasaki T, Miyakura Y, Horie H, et al. Combined assessment using optical colonoscopy and computed tomographic colonography improves the determination of tumor location and invasion depth. Asian J Endosc Surg. 2017; 10(1): 28-34. doi: 10.1111/ ases.12313


