Mini-Review Article
Challenges on the Development of MRI-Compatible Neurosurgical Robotic Systems
Assistant Professor, Department of Mechanical and Automation Engineering, The Chinese University of Hong Kong, Hong Kong
*Corresponding author: Shing Shin Cheng, Assistant Professor, Department of Mechanical and Automation Engineering, The Chinese University of Hong Kong, Hong Kong, E-mail: sscheng@cuhk.edu.hk
Received: November 26, 2018 Accepted: February 20, 2019 Published: February 27, 2019
Citation: Cheng SS. Challenges on the Development of MRI-Compatible Neurosurgical Robotic Systems. Int J Robot Res Appl Autom. 2019; 1(1): 2-5. doi: 10.18689/ijra-1000102
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
True intraoperative magnetic resonance imaging (MRI)-guided neurosurgical robotic procedure could be achieved with a robust neurosurgical robotic system that not only satisfies the high magnetic field and geometric restriction imposed by the MR environment but also the high safety and accuracy standard in a neurosurgical procedure. This paper identifies three key challenges in the development of MRI-compatible neurosurgical robotic system, discusses the limitation of current approaches, and provides insights towards possible methods based on the latest research outcomes. Challenges that have been identified include materials for various neurosurgical robot components, actuators that provide sufficient resolution and suitable for head-mounted system, and sensors that can be integrated into a flexible millimeter-scale neurosurgical robotic instrument for shape and force sensing. Advancement in 3-dimensional (3D) printing technology, implementation of hybrid, Bowden cable-based, and magnetic actuation, and better integration of fiber optic sensors will be critical in overcoming the challenges and accelerating clinical translation of MRI-compatible robotic systems in neurosurgery.
Keywords: Magnetic Resonance Imaging; 3-Dimensional (3D) Printing Technology; Neurosurgical Robotic System.
Introduction
Magnetic resonance imaging (MRI) has been used for imaging human brain during pre-, intra-, and post-operative surgical procedures, besides its critical role in the diagnosis and prognosis of diseases. Its high spatial resolution, soft tissue contrast, and the absence of ionizing radiation set it apart as the imaging modality of choice for neurosurgeons [1]. In the past decade, MRI-guided neurosurgical robotic systems have become an increasingly important research subject in the medical robotics community [2]. The smooth integration of MRI and robots will ensure safe and precise straight needle targeting and flexible instrument manipulation in the human brain, thus reducing surgical risk factors and morbidity rate in the highly risky neurosurgical procedures. However, there are many challenges in the development of an MRI-compatible neurosurgical robotic system due to the many restrictions the MR environment poses and the high safety and accuracy standard demanded in a neurosurgical procedure [3]. An MRI-compatible robotic system has to pose no hazardous effects, does not affect significantly the MR image quality and does not have their operation affected by the magnetic field and MR scanning [1]. Neurosurgical procedures such as microsurgery, laser ablation, biopsy, and deep brain stimulation, often require submillimeter accuracy with the help of stereotactic framed and frameless systems [3]. This main contribution of this paper is that it identifies three key challenges from the hardware development perspectives in terms of materials, actuators, and sensors, and critically presented the promising solutions to overcome them.
Previous Work
The research and development work on MRI-compatible neurosurgical robots has been steadily increasing in the past decade [4,5]. The first MRI-compatible neurosurgical robot with six degrees-of-freedom (DoFs) was developed to perform needle insertion in 1995 [6]. NeuroArm (IMRIS Inc., USA), the first MRI-compatible neurosurgical robotic system for microsurgery, consists of two robotic arms powered by piezoelectric motors and is connected to a workstation that has a haptics-enabled human-machine interface [7]. It is compatible with an intraoperative MRI (iMRI) suite with a movable ceiling-mounted magnet. Many research groups have focused on developing robotic systems that can fit into the bore of a clinical MRI scanner to achieve true intraoperative MRI guidance. Fischerʼs group at Worcester Polytechnic Institute developed a stereotactic neurosurgical robot that is kinematically equivalent to the Leksell stereotactic frame to perform electrode placement for deep brain stimulation [8] and an MRI-compatible version of the concentric tube robot for applications including neurosurgery that requires a curved trajectory [9]. Websterʼs group developed a five degree-of-freedom (DoF) pneumatically actuated active cannula for deep brain ablation procedures [10]. Desaiʼs group at Georgia Institute of Technology developed different versions of neurosurgical robots called Minimally Invasive Neurosurgical Robotic System (MINIR-II) for cauterization and aspiration of deep brain tumors [11,12], as shown in figure 1a. Kwokʼs group developed a hydraulically actuated robot for bilateral stereotactic electrode placement [13] while Stoianoviciʼs group developed a pneumatic stepper motors (PneuStep)- actuated remote center of motion (RCM) needle-guide robot [14]. To date, there have been a few commercialized products in MRI-guided neurosurgical interventions. The ClearPoint® System (MRI Interventions, Inc., USA) has been developed to perform MRI-guided stereotactic neurosurgery, including brain tumor biopsy and electrode insertion [15]. Two skull-mounted laser systems [15] have been developed to perform MR-guided laser interstitial thermal therapy (MRgLITT) in neurosurgery, including the NeuroBlate® System (Monteris Medical, Inc., USA) and the Visualase® Thermal Therapy System (Medtronic, Inc., USA).
Discussion on Key Challenges and Potential Solutions
Material restriction
Restriction in the material and manufacturing choices is one of the major challenges in the development of an MRI-compatible neurosurgical robotic system, which usually consists of modules such as the head frame, transmission, and robotic end effector. The skull mounted head frame has to be lightweight and compact [16], so as to not increase patientʼs discomfort and to fit inside the bore of MRI, while offering sufficient stiffness and stability as a stationary or positioning platform for surgical instruments. The transmission module is needed to connect the actuators to the surgical instrument on the head frame, which normally does not house actuators due to weight restriction. It can become rather complicated in its moving mechanism and requires both stiffness and flexibility when used to transmit force for a multi-DoF robot, such as the MINIR-II robot, that also translates in and out of the brain [12], as shown in figure 1b. This robotic end effector, which would be the closest to the imaging region of interest (ROI), must not cause distortion to MR images showing the surgical site.
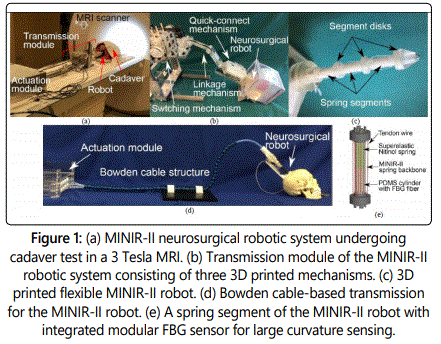
The materials used for the three modules of a neurosurgical robotic system have to be non-ferromagnetic materials while catering to different stiffness, weight, and size required for different modules. Plastic 3-dimensional (3D) printing techniques have provided a convenient way to prototype these MRI-compatible components. Fused deposition modeling (FDM) and selective laser sintering (SLS) [17] allow the development of customized components with complex shapes and geometries using materials with different mechanical properties, such as impact strength, flexural modulus, tensile strength, and high temperature resistance, and are suitable for constructing the head frame and transmission modules. The more high-resolution printing techniques, including stereolithography and multi-jet printing, allow development of dimensionally accurate robotic end effector [18], such as the MINIR-II robot shown in figure 1c, potentially with a combination of rigid and soft materials. The unique combination of strength, stiffness, electrical conductivity, and polished surface finish also makes non-magnetic metal 3D printing attractive in developing both macro- and micro-scale components for both structural and mechanical purposes. As 3D printing technology matures, the development of patient specific robotic instrument developed from the combination of plastic and metal 3D printing will improve accuracy and safety, uniquely critical in a neurosurgical procedure.
Actuator restriction
The actuators in an MRI-guided neurosurgical robotic system have to not only be MRI-compatible, but also provide sub-millimeter accuracy [19] due to the critical functions associated to various brain regions. Electric actuators, such as ultrasonic and piezoelectric motors [2], can provide high accuracy but it is found that their drivers have to be customized [8] and placed far away from the MR bore to allow proper functioning of the motors [12]. Shape memory alloy (SMA) has also been used in an MRI-compatible neurosurgical robot [18,20] but low actuation frequency, hysteretic performance, and control precision make it difficult to be implemented clinically in a neurosurgical procedure in the foreseeable future. Pneumatic motors [21] and hydraulic motors are other actuator choices for surgical tasks that require significant forces but the concerns about fluid leakage, cavitation, long transmission lines, and non-linear mechanical characteristics need to be addressed [21]. While all these MRI-compatible actuators are limited in their own ways, hybrid actuation, Bowden cable-based actuation, and MR-based actuation could potentially improve the pessimistic outlook of clinical translation of MRI-compatible neurosurgical robots. Hybrid actuation entailing multiple actuation mechanisms is described by Guo et al. [13], where a conventional motor is placed outside the MRI room to initiate the actuation process in a master piston and pressurized hydraulics is used as the transmission mechanism that connects the master and the slave systems. PneuStep is also a hydrid motor that is developed using pneumatics as the source of actuation and behaves like a stepper motor [14] and has been tested to be MRI safe. It is still relatively complex to manufacture due to the dozens of components and materials involved [22]. Bowden cable system with the reliable electric actuators placed a distance away is one of the most feasible actuation mechanisms to develop MRI-compatible neurosurgical robotic system, especially those based on skull-mounted head frame. The Bowden Cable could be in the form of PEEK capillary tubing [16], flat wire coil, and Loc-Line hose with Teflon tubing, which has been attempted for the MINIR-II robotic system [23] as shown in figure 1d. The concept has been successfully implemented in commercial systems [15], albeit using manual control instead of motors at the proximal end of the Bowden cables. The ideal actuation method is to remove the regular actuators entirely from the MR room while using MRI itself to simultaneously localize (sense) and propel (actuate) tetherless millimeter robots [24] in the brain [25]. The limited access to clinical MRI scanner and proprietary pulse sequence source codes of MRI manufacturers are existing hurdles that have to overcome through close interdisciplinary collaboration between academic and industrial researchers.
Sensor restriction
Flexible robots are particularly useful for neurosurgery, compared to other MRI-guided procedures such as prostate, abdominal, and breast biopsy [5], to allow bending outside the line of sight and avoid critical significant manipulation of brain tissues. Force sensing and shape sensing are therefore important in neurosurgery to ensure that the flexible robot is closely monitored in terms of its positioning and interaction relative to its surrounding. This is mainly due to the unavailability of dynamic MR imaging at sufficiently high spatial and temporal resolution [26]. Currently, MRI-compatible trackers that can be placed on robotic instruments are very limited. EndoScout® (Robin Medical, Inc, USA) system is an MRI-safe 6-DoF electromagnetic sensor that can be integrated with the MRI to sense the position and orientation of its sensor based on the native gradient fields of the MR scanner [27]. Its relatively large footprint makes it difficult for integration into the interventional instruments for neurosurgery. Megrez® Navigation system (Symbow Medical Technology Co., China) is an MRI-compatible optical tracking system based on the Polaris Spectra (Northern Digital Inc., Canada) infrared camera [28]. These tracking systems could only be used for pose tracking at discrete points of a flexible robot. Fiber optic sensor remains the MRI-compatible sensor that would provide both shape and force information for a neurosurgical flexible robot and must be integrated in a clinically ready MRI-guided neurosurgical robot [29]. The intensity modulated fiber optic sensors lacks sensitivity and resolution besides having a relatively large fiber for integration into millimeter and submillimeter instruments in the brain [29]. Both wavelength-modulated Fiber Bragg grating (FBG) sensor and phase-modulated Fabry-Perot (F-P) interferometer sensor are reliable sensors for actively measuring shape and force changes [29]. Besides the high cost associated with the optical source, mounting the fibers on a flexible robot has proven to be challenging, especially for large bending curvature [30]. Recently, a reusable modular FBG bending sensor consisting of micro NiTi spring and PDMS tube with embedded fiber, as shown in figure 1e, has been developed for a single segment of the MINIR-II robot to measure large angular deflection [31]. The complex fabrication process and repeatability issue still need to be addressed for its robust integration into a neurosurgical robot.
Conclusion
Researchers are facing limitation in choices, technical challenges and integration difficulty in the areas of material, actuator, and sensor to satisfy the stringent requirements of the MR environment and neurosurgery. Plastic and metal 3D printing, hybrid, Bowden cable-based and MR actuation, and fiber optics sensing have proven to be some of the promising technologies to resolve the aforementioned challenges. Collaboration among engineers, MR radiologists, and neurosurgeons must be formed to ensure the clinical relevance of the robotic system. MR manufacturers and industrial partners are encouraged to get involved in and support this research field to improve access to the MR facility and resolve integration problems. While many other factors, including operational costs and market demand, regulatory and ethical issues, MR-based robot registration and navigation issues, and computational cost for real-time image-based control, will shape the future of MRI-guided neurosurgical robotics, overcoming the key challenges described will prove to be the first step towards realistically achieving clinical translation of MRI-guided robots in neurosurgery.
References
- Roger G, Burdet E, Chinzei K. MRI-compatible robotics. IEEE Eng Med Biol Mag. 2008; 27(3): 12-14.
- Hata N, Pedro M, Gregory F. Robotics in MRI-guided interventions. Top Magn Reson Imaging. 2018; 27(1): 19-23. doi: 10.1097/RMR.0000000000000159
- Smith JA, Jivraj J, Wong R, Yang V. 30 Years of neurosurgical robots: Review and trends for manipulators and associated navigational systems. Ann Biomed Eng. 2016; 44(4): 836-846. doi: 10.1007/s10439-015-1475-4
- Ahmed SI, Javed G, Mubeen B, et al. Robotics in neurosurgery: A literature review. J Pak Med Assoc. 2018; 68(2): 258-263.
- Monfaredi R, Cleary K, Sharma K. MRI Robots for Needle-Based Interventions: Systems and Technology. Ann Biomed Eng. 2018; 46(10): 1479-1497. doi: 10.1007/s10439-018-2075-x
- Masamune K, Kobayashi E, Masutani Y, et al. Development of an MRIcompatible needle insertion manipulator for stereotactic neurosurgery. J Image Guid Surg. 1995; 1(4): 242-248.
- Sutherland GR, Wolfsberger S, Lama S, Zarei-nia K. The evolution of neuroArm. Neurosurgery 2013; 72: 27-32. doi: 10.1227/NEU.0b013e318270da19.
- Li G, Su H, Cole GA, et al. Robotic system for MRI-guided stereotactic neurosurgery. IEEE Trans Biomed Eng. 2015; 62(4): 1077-1088. doi: 10.1109/TBME.2014.2367233
- Su H, Cardona DC, Shang W, et al. A MRI-guided concentric tube continuum robot with piezoelectric actuation: a feasibility study. IEEE Int Conf Robot Autom. 2012; 1939-1945.
- Comber DB, Barth EJ, Webster RJ. Design and control of a magnetic resonance compatible precision pneumatic active cannula robot. J Med Devices. 2014; 8(1): 011003. doi: 10.1115/1.4024832
- Ho M, Kim Y, Cheng SS, Gullapalli R, Desai JP. Design, development, and evaluation of an MRI-guided SMA spring-actuated neurosurgical robot. Int J Rob Res. 2015; 34(8):1147-1163. doi: 10.1177/0278364915579069
- Wang X, Cheng SS, Desai JP. Design, Analysis, and Evaluation of a Remotely Actuated MRI-Compatible Neurosurgical Robot. IEEE Robot Autom Lett. 2018; 3(2): 2144-2151. doi: 10.1109/LRA.2018.2809447
- Guo Z, Dong Z, Lee KH, et al. Compact design of a hydraulic driving robot for intra-operative MRI-guided bilateral stereotactic neurosurgery. IEEE Robot Autom Lett. 2018; 3(3): 2515-2522. doi: 10.1109/LRA.2018.2814637
- Jun C, Lim S, Wolinsky JP, et al. MR safe robot assisted needle access of the brain: preclinical study. J Med Robot Res. 2018; 3(1): 1850003.
- Willie JT, Tung JK, Gross RE. MRI-guided stereotactic laser ablation. Image Guided Neurosurgery. 2015; 375-403.
- Li C, King NKK, Ren H. A Skull-Mounted Robot with a Compact and Lightweight Parallel Mechanism for Positioning in Minimally Invasive Neurosurgery. Ann Biomed Eng. 2018; 46(10): 1465-1478. doi: 10.1007/s10439-018-2037-3
- Ventola CL. Medical applications for 3D printing: current and projected uses. P T. 2014; 39(10): 704-711.
- Kim Y, Cheng SS, Diakite M, Gullapalli RP, Simard JM, Desai JP. Toward the development of a flexible mesoscale MRI-compatible neurosurgical continuum robot. IEEE Trans Robot. 2017; 33(6): 1386-1397. doi: 10.1109/TRO.2017.2719035
- Bjartmarz H, Rehncrona S. Comparison of accuracy and precision between frame-based and frameless stereotactic navigation for deep brain stimulation electrode implantation. Stereotact Funct Neurosurg. 2007; 85(5): 235-242.
- Kim Y, Cheng SS, Desai JP. Active Stiffness Tuning of a Spring-Based Continuum Robot for MRI-Guided Neurosurgery. IEEE Trans Robot. 2018; 34(1): 18-28. doi: 10.1109/TRO.2017.2750692
- Yang B, Roys S, Tan UX, et al. Design, development, and evaluation of a master–slave surgical system for breast biopsy under continuous MRI. Int J Rob Res. 2014; 33(4): 616-630. doi: 10.1177/0278364913500365
- Groenhuis V, Stramigioli S. Rapid Prototyping High-Performance MR Safe Pneumatic Stepper Motors. IEEE ASME Trans Mechatron. 2018; 23(4): 1843-1853. doi: 10.1109/TMECH.2018.2840682
- Cheng SS, Wang X, Desai JP. Design and analysis of a remotely-actuated cable-driven neurosurgical robot. International Conference on Intelligent Robots and Systems (IROS). 2017. doi: 10.1109/IROS.2017.8205980
- Martel S. Beyond imaging: Macro-and microscale medical robots actuated by clinical MRI scanners. Sci Robot. 2017; 2(3): eaam8119. doi: 10.1126/scirobotics.aam8119
- Vartholomeos P, Bergeles C, Qin L, Dupont PE. An MRI-powered and controlled actuator technology for tetherless robotic interventions. Int J Rob Res. 2013; 32(13): 1536-1552. doi: 10.1177/0278364913500362
- Navkar NV, Deng Z, Shah DJ, Tsekos NV. A framework for integrating realtime MRI with robot control: application to simulated transapical cardiac interventions. IEEE Trans Biomed Eng. 2013; 60(4):1023-33. doi: 10.1109/TBME.2012.2230398.
- Franz AM, Haidegger T, Birkfellner W, Cleary K, Peters TM, Maier-Hein L. Electromagnetic tracking in medicine—a review of technology, validation, and applications. IEEE Trans Med Imaging. 2014; 33(8): 1702-25. doi: 10.1109/TMI.2014.2321777
- Gao W, Jiang B, Kacher DF, et al. Real-time probe tracking using EMoptical sensor for MRI-guided cryoablation. Int J Med Robot. 2018; 14(1). doi: 10.1002/rcs.1871
- Su H, Iordachita II, Tokuda J, et al. Fiber Optic Force Sensors for MRIGuided Interventions and Rehabilitation: A Review. IEEE Sens J. 2017; 17(7): 1952-1963. doi: 10.1109/JSEN.2017.2654489
- Sefati S, Alambeigi F, Iordachita I, Armand M, Murphy RJ, Armand M. FBGbased large deflection shape sensing of a continuum manipulator: Manufacturing optimization. 2016 IEEE SENSORS. 2016. doi: 10.1109/ICSENS.2016.7808910
- Rahman N, Deaton NJ, Sheng J, Cheng SS, Desai JP. Modular FBG Bending Sensor for Continuum Neurosurgical Robot. IEEE Robot Autom Lett. 2019; 4(2): 1424-1430. doi: 10.1109/LRA.2019.2896451


