Institute for Cardiovascular and Metabolic Research, School of Pharmacy, University of Reading, UK
Platelets are small circulating blood cells that play a pivotal role in the regulation of haemostasis by preventing excessive bleeding through blood clotting. In appropriate activation of platelets under pathological conditions leads to the formation of blood clots (thrombosis) within the blood vessels resulting in life-threatening conditions such as heart attacks and strokes. Moreover, due to their high number in the circulation, platelets play a significant role in the progression of inflammatory responses. Although currently used anti platelet drugs help save lives, they are associated with severe side effects such as bleeding and they are ineffective in some patients. This emphasizes the need for the development of safer and more effective therapeutic strategies for the prevention and treatment of cardiovascular diseases. Platelets possess formyl peptide receptors (FPRs), which were originally found on leukocytes where they play indispensable roles in the regulation of host defense and inflammatory responses. The expression of these receptors has been previously reported in platelets and their stimulation induces chemotaxis and calcium release in platelets. Together, these results suggest that the FPRs play a role in the regulation of platelet activation. However, the detailed characterization of these receptors in the regulation of platelet function has not been established yet. Therefore, in this study, we aim to determine the role of FPRs in the regulation of thrombosis and haemostasis using a range of in vitro and in vivo platelet functional assays. Furthermore, the regulatory mechanisms that control the functions of FPRs in platelets will also be established.
Our preliminary data support our hypothesis that FPRs play significant role in the modulatioof platelet function. We confirmed the expression of FPR1 in human platelets by transcriptomics and immunoblot analyses. In addition, the flow cytometry analysis indicates that the level of FPR1 is increased upon activation of platelets with an agonist. This suggests the presence of FPR1 in platelet granules. FPR1 agonists such as fMLF induced platelet activation in a dose-dependent manner and this was inhibited when FPR1 antagonists were used. In addition, a selective FPR1 antagonist, cyclosporin H inhibited platelet activation induced by agonists such as CRP-XL and this suggests that FPR1 mediated inverse signaling may impact on the regulation of platelet functions. Together these data demonstrate potential roles for FPRs in the regulation of platelet activation and therefore in thrombosis, and haemostasis.
Biography:
Maryam Salamah is a postgraduate researcher at the University of Reading working under the supervision of Dr Sakthivel Vaiyapuri. Her research currently focuses on the effects of formyl peptide receptors in the regulation of platelet function. She received her masterʼs degree in Biomedical Sciences from the University of Brighton, in which she had the chance to work at the Royal Sussex County Hospital to establish a technique for hematological diagnostic tests. She also obtained a certificate in Medical Laboratory Technology from King Abdulaziz University, where she had the chance to work as a laboratory technologist at various laboratories in King Abdulaziz University Hospital and King Faisal Hospital & Research Centre (KFH&RC).
1College of Science, University of Salahaddin-Erbil, Iraq
2Dipartimento di Chimica, Università di Pavia, Italy
3C.I.St.R.E., Università di Pavia, Italy
Genus Iris (Iredaceae) comprises over 300 species; 12 of them are present in Iraq. Iris persica has been used in Kurdish traditional medicine for the treatment of wound inflammation and tumor. However, Chemical and biological aspects of I. persica have not yet been investigated. The present study reports the first investigation on the isolation and characterization of bioactive compounds from flowers, bulbs and rhizomes of I. persica that has been collected from Kurdistan Region-Iraq and cytotoxicity effect of the isolated compounds against six human cancer cell lines were evaluated. Dry flowers, bulbs and rhizomes of I. persica were exhaustively extracted by maceration at room temperature, solvents of increasing polarity: hexane, methanol, methanol/water 70:30. Chlorophylls were removed from the methanolic extracts of flowers by filtration on a C-18 reversed phase column. Subsequently, the methanolic extracts of the flowers, bulbs and rhizomes were separately fractionated by repetitive preparative MPLC, on C-18 reversed phase, affording four compounds as the major products: tectorigenin (1), embinin (2), isovitexin (3) and trans-resveratrol-3-O-β-D-glucopyronoside (4). The structures of the compounds were identified on the basis of spectroscopic analyses and comparison with literature data.
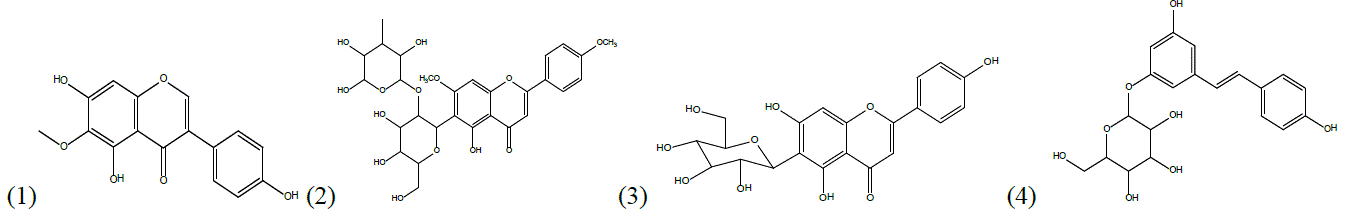
The cytotoxic activity was measured against six human cancer cell lines, the effects of two isolated compounds, Tectorigenin (1) and embinin (2), on the proliferation of tumor cells were evaluated in comparison with the well-known antitumor drug cisdiamminedichloroplatinum(II) (cisplatin) by MTT assays. In particular, MCF7 and SkBr3 breast, endometrial Ishikawa, ovarian BG-1, mesothelioma IST-MES1 and lung A549 cancer cells were treated for 48h with increasing concentrations of tested compounds. Compound P2 showed a stronger inhibitory activity than cisplatin in five cell lines from total of the six cell lines; MCF7, SkBr3, Ishikawa, BG-1, IST-MES1 and A549, embinin IC50 ± S.D of 6 (±3), 4(±1), 10(±3), 8(±2), 7(±2) and 9(±1) µM. while cisplatin (standard) IC50 ± S.D of 17(±4), 10(±2), 10(±3), 12(±3), 12(±2) and 13(±2)µM respectively.
In conclusion, this is first study on phytochemical study of I. persica as well as cytotoxic acitivity of embinin isolated flowers of I.persica, this study confirms that Embinin could be considered as a natural anticancer. At the same time, the present study confirms the traditional use of Iris persica L. in the treatment of tumor.
Biography:
Faiq H. S. Hussain was born 1953 at Sulaimani city, Kurdistan region, Iraq. He successfully awarded B.Sc. in Chemistry 1976, M.Sc. in Organic chemistry 1978 and Ph.D. in Organic chemistry at Manchester university-UK 1985. He is senior of Organic chemistry at Chemistry department, University of Salahaddin-Erbil. He was Head of Chemistry Department 1992-1998 at college of science, Salahaddin university-Erbil. He has 30 publications in international and local journals. He supervised 21 master students and 8 Ph.D. students in his career. Head of Research group of PROKURUP project, University of Pavia-Italy, 2011 to date with Prof. Giovanni Vidari at department of Organic chemistry.
1Pusan National University, Republic of Korea
2Dankook University, Republic of Korea Oxo-bile acids are useful cholic acid derivatives with promising properties and have bothmedical and pharmaceutical applications. They are one of the most important intermediatesfor the synthesis of ursodeoxycholic acid (UDCA), which is currently the only product approved by the US FDA for the treatment of primary sclerosing cholangitis. However, large quantitiesof UDCA cannot be obtained from natural sources. For this reason, commercial UDCA is produced by the chemical manipulation of plentiful primary bile acids suchas cholic acid and chenodeoxycholic acid. In these chemical transformations, criticaloxidation steps are required steps for the practical syntheses of oxo-bile acidintermediates. In many cases, toxic heavymetal-based oxidizing agents have been used in spite of their toxicity. From the greenchemistry standpoint, the utilization of less harmful agents is always desirable andindeed there have been efforts to develop more eco-friendly conditions for the synthesisof oxo-bile acid derivatives. In this connection, we describe the applicationof a cerium-catalyzed oxidation to secondaryalcohol groups of bile acids in the hope of developing a more versatile and greenermethod for the preparation of various oxo-bile acids.
Biography:
Hwayoung Yun did his undergraduate work at Seoul National University. He have intensively experienced the synthesis of complex small molecules through the studies on total synthesis of various natural products such as macrolides, alkaloids, iridoids, macrolactams and polypeptides. A range of academic training and an in-depth research experience have provided he with considerable expertise in biomedical disciplines including synthetic organic, medicinal chemistry and chemical biology. His ultimate research interests are the discovery of bioactive small molecules as selective regulators of intracellular signaling pathways and investigation of their biological mode of action.
Novosibirsk State University, Russia
The development of efficient and convenient systems for the delivery of nucleic acid-based drugs into cells is an urgent task. Despite many efforts in this field, this problem cannot be considered as completely solved. A promising way is the use of various nanoparticles. We developed methods of immobilizing DNA fragments to titanium dioxide nanoparticles with the formation of TiO2~DNA nanocomposites. Three forms of TiO2 nanoparticles (amorphous, anatase, and brookite) were used for the construction of nanocomposites. Two approaches to the preparation of nanocomposites are described: 1) noncovalent sorption of oligonucleotide-polylysine conjugates onto TiO2 nanoparticles, and 2) the electrostatic binding of oligonucleotides to TiO2 nanoparticles precovered with polylysine. Both methods provide an efficient and strong immobilization of DNA fragments onto nanoparticles that lead to nanocomposites with a capacity of up to 60 nmol/mg for an oligonucleotide. Different types of oligonucleotides and their analogs can be immobilized on TiO2 nanoparticles, including single and double stranded DNA and RNA fragments of different length, oligo-2ʼ-O-methyl-ribonucleotides, phosphorothioate oligonucleotides, and recently described 2ʼ-O-methylribonucleotides with charge-neutral phosphoryl guanidine groups, as well as peptide nucleic acids. It was shown that oligonucleotides in nanocomposites retain their ability to complementary interactions. Independently of the form of nanoparticles and the immobilization method, the nanocomposites were demonstrated by confocal microscopy to penetrate into eukaryotic cells without any transfection agents and physical impact. It should be emphasized that a significant part of nanocomposites was internalized in cell nuclei. The proposed nanocomposites can be considered as an efficient approach to deliver oligonucleotides and their analogs into cells.
“The work was supported by the grant of Russian Scientific Foundation no. 16-15-10073.”
Biography:
Marina N. Repkova was born in Kronshtadt, Russia. She received the MS degree in chemistry from Novosibirsk State University, Russia, in 1979 and the Ph.D. degree in bioorganic chemistry from Novosibirsk Institute of Bioorganic Chemistry in 1999 (Russia). From 1979 to present, she has been Postgraduate Student, then Research Scientist, with the Laboratory of Nucleic Acids Chemistry from Novosibirsk Institute of Bioorganic Chemistry (then Institute of Chemical Biology and Fundamental Medicine) of Siberian Branch of Russian Academy of Sciences. She is the author of more than 100 articles and more than 10 patents. Her research interests include the synthesis of Oligonucleotides and their derivatives and the synthesis of nucleic acid-based nanocomposites and their delivery into cells. Currently she works at the Novosibirsk State University.
Department of chemistry, School of advanced sciences, VIT University, India
Aim: The present study was undertaken to evaluate in vitro antidiabetic activity of crude and partially purified fractions of Pithecellobium dulce fruit.
Method: The fruits of P. dulce were extracted with methanol and 20 % ethanol (Hydro alcoholic extract). The methanolic extract was partitioned successively with petroleum ether (MPE), chloroform (MCE) and ethyl acetate (MEE). The hydroalcoholicextract was purified by ion exchange chromatography to yield three water soluble fractions PDP-1, PDP-2, and PDP-3. Methanolic and hydroalcoholic extracts were assessed for total phenolic, flavonoid and carbohydrate content using Folin-Ciocalteuʼs reagent, aluminium chloride and phenol-sulphuric acid method respectively. Then the extracts were further quantified with respect to alphaamylase and alpha-glucosidase inhibitory activity.
Result: IC50 values of themethanolic and hydroalcoholic extract against alpha-amylasewere found to be 587 µg/ml and 575.07 µg/ml respectively, alpha-glucosidase inhibition was 535.09µg/ml for methanolic extract and 462.46 µg/ml for hydroalcoholic extract which was similar to effect displayed by the positive control acarbose. Furthermore, PDP-2 shows potent activity when compared to all the other purified fractions.
Conclusion: The result obtained indicate that the crude extract and partially purified fractions are possessed asignificant level of activity. However, these effects need to be confirmed using in-vivo models and clinical trials for its effective utilisation as therapeutic agents.
Keywords: P. dulce, alpha amylase, alpha-glucosidase, antidiabetic.
Rajiv Gandhi Centre for Biotechnology, India
Hepatocellular Carcinoma (HCC) is the fifth commonest malignancy worldwide. There is an obvious critical need to develop improved methods for chemoprevention and treatment of HCC as the current strategies are ineffective. Naturally occurring phytochemicals and dietary compounds have emerged as promising molecules with chemopreventive and chemotherapeutic potential in different types of cancers. Recently, autophagy has emerged as a potential target in cancer chemotherapy, but its precise role being controversial. The study intends to investigate the role of autophagy in cell death induced by selected anticancer lead molecules (resveratrol, silibinin, glycopentalone) from plants in HCC cells. The anticancer agents were validated for their cytotoxic activity in HCC cell lines HepG2 and Hep3B. Chromatin compaction was characterized by Hoechst staining and cell cycle arrest by FACS analysis, which affirmed the activation of apoptosis. Our studies demonstrated that the selected anticancer agents induced autophagy together with apoptosis, dose and time dependently. Activation of autophagy was evidenced by fluorescence microscopic detection of autophagic vacuoles, formation of acidic vesicular organelles (AVOs), immunoblotting patterns for conversion of LC3-I to LC3-II, immunofluorescence analysis of recruitment of LC3-II to the autophagosomes and autophagic flux analysis using Bafilomycin A1. Also, the pathways regulating autophagy were analysed which revealed the involvement of PI3/Akt/mTOR pathway. The antagonistic roles of autophagy and apoptosis in response to exposure of HCC cells with these agents were evident through morphological and autophagy inhibition studies. Combining autophagy modulators with plant derived anticancer lead molecules would be an exciting modality for combating the worries of liver cancer.
Biography:
Greeshma Tom is pursuing PhD in Biotechnology under the guidance of Dr. Asha V. V., Scientist EII, Plant based bioactives and disease biology Lab, Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, India. She works on the evaluation of various cell death pathways activated during chemotherapy against liver cancer. Prior to doing PhD, she completed her graduate and post graduate degrees from Bangalore University and M.G University, India. She was awarded Research Fellowship by CSIR (Council of Scientific and Industrial Research) in 2012. She has published in peer reviewed journals with her colleagues at RGCB and presented papers in international conferences.
Rajiv Gandhi Centre for Biotechnology, India
Although inflammatory barrier is a critical defensive mechanism, exaggerated responses(chronic inflammation) of innate inflammatory cells, eg. macrophages, dendritic cells etc, produce serious damage leading to cancer, atherosclerosis, arthritis, diabetes and septic shock. Since inflammation involves multiple proinflammatory mediators and pathways that lead to a wide range of changes in pathology, it is difficult to target the desired area when treating inflammation. The current treatment involves use of non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids which exert effect mainly through blockage of cyclooxygenases (COX). Of the two isoforms of cyclooxygenases (PGE2 poducing enzyme-COX), COX-1 is the constitutively expressed form required for physiologic functions and COX-2 is induced during inflammatiom. Selective COX-2 inhibition can avoid most of the toxic effects of NSAIDs like gastric erosion, inhibition of platelet aggregation etc. to an extent. Macrophages, the major inflammatory cell type, release cytokines, ROS, growth factors, chemokines, TNF-α, interleuckin-1 and MCP-1, PGE2 in response to activation signals (LPS, TNF-α) through the canonical NF kb pathway activation. So NF-κB and the signalling pathways have become a focal point for intense drug discovery. Also inactivation of MAPKs might inhibit inflammatory mediators (NO and PGE2), making them potential targets for anti-inflammatory therapeutics. Tinospora cordifolia is well known for its anti-inflammatory potential in several invivo models, but the underlying molecular mechanism or the phytochemisry remains unknown. The isolated fraction inhibits the production of TNF-α, PGE2 and NO through cox-2 selective inhibition of p65 translocation and p38 phosphorylation. Characterization of this particular fraction will be beneficial in the treatment of chronic inflammatory condition without extensive side effects.
Biography:
Sheena Philip is a Ph. D student under Dr. Asha V. V, Scientist E-II, in Plant based bio-actives and disease division at Rajiv Gandhi Centre for Biotechnology, Thiruvananthapuram, Kerala, India. Her research work mainly focus on the isolation of bio-active molecules from herbals directed against chronic inflammatory conditions. Prior to joining as a Ph. D student, she has qualified for CSIR fellowship and she also holds a masterʼs degree and Bachelorʼs degree in Biochemistry from the University of Calicut. She has published in peer reviewed journals and presented papers in international conferences.
1 Department of Biochemistry and Molecular Biology, Monash University, Australia
2Monash Institute of Pharmaceutical Sciences, Monash University, Australia
3Harry Perkins Institute of Medical Research, QEII Medical Centre, Australia
Chemokine receptors are G-protein coupled receptors (GPCRs) that regulate the movement of leukocytes during inflammation. CCR2, a major chemokine receptor on monocytes and macrophages, binds to several CC chemokine ligands and plays a key role in atherosclerosis, obesity and type 2 diabetes. The major ligands of human CCR2 include monocyte chemoattractant protein 1 (CCL2) and MCP-3 (CCL7). Here we show that MCP-1 and -3 have distinct potencies and efficacies of signalling at CCR2 and we identify structural features of the chemokines and receptor contributing to the differences. First, using a series of chemokine chimeras, constructedby swapping the three main receptor recognition regions between MCP-1 and MCP-3, we have identified structural elements of MCP chemokines responsible for differences in receptor activation. We found that the chemokine N-terminus plays a major role towards full versus partial agonism. The affinities of the chemokine chimeras to the CCR2 also confirmed that the N-terminus makes a significant contribution to receptor binding by these two chemokines. Second, using a series of CCR2 mutants, we have identified elements of CCR2 that interact preferentially with the chemokines. The affinity of chemokine binding and the potency of ERK-1/2 phosphorylation by MCP-1 and MCP-3was determined for each receptor mutant. Four of the mutants, Y120F, R206A, I263A/N266A and Y259F displayed differential effects on the affinity of MCP-1 relative to MCP-3. These mutated residues are clustered together in the transmembrane region of the receptor. We conclude that this region of the receptor plays a major role in distinguishing between the two cognate chemokines, apparently by differential interactions with the N-terminal regions of the chemokines. Our investigation has yielded significant new information on chemokine receptor binding and signalling, which will guide future drug development.
Biography:
My name is Zil e Huma and I am a final year PhD scholar in the Department of Biochemistry and Molecular Biology at Monash University, Australia. In addition, I am also working as a teaching associate at the same department. My area of interest revolves around understanding the signalling mechanisms of GPCRs. Chemokines and their receptors have always been a target of interest because of their role in inflammatory diseases. I am interested to investigate different chemokines which cause differential signalling at the same receptor. This study will help understand the chemokine-receptor interactions to develop novel therapeutic agents.
1University of Tartu, Institute of Technology, Estonia
2Department of Molecular Biology, Umeå University, Sweden
3Laboratory for Molecular Infection Medicine Sweden (MIMS), Umeå University, Sweden
4Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences v.v.i., Czech Republic
5Graduate School of Science and Engineering, Saitama University, Japan
Bacteria employ an array of systems to sense their environment and respond to various stimuli. One of such systems is mediated via changes in the intracellular levels of alarmone nucleotides guanosine tetraphosphate (ppGpp) and pentaphosphate (pppGpp), collectively referred to as (p)ppGpp. The nucleotides are synthesized by RelA/SpoT Homologue (RSH) enzymes via an in-line nucleophilic attack of the 3ʼ-OH group of GDP (or GTP) on the β-phosphate of ATP. (p)ppGpp is a pleotropic intracellular effector targeting numerous molecular targets. It regulates transcription via direct interaction with two allosteric sites of Escherichia coli RNAP; suppresses translation via binding to the GTP-binding pocket of ribosome-associated GTPases, DNA replication via binding to the active site of DNA-dependent RNA polymerase primase DnaG, and nucleotide biosynthesis via direct competition with nucleotide substrates of several enzymes involved in synthesis of GTP and ATP. In addition, (p)ppGpp activates its own production via interaction with ribosome-dependent Escherichia coli RSH RelA.
An acute increase in (p)ppGpp concentration – referred to as ‘the stringent responseʼ – orchestrates a survival program leading to increased virulence and antibiotic tolerance. Due to the central role of the (p)ppGpp in regulation of bacterial virulence and recently proposed connection to formation of antibiotic-tolerant persister cells, (p)ppGpp-mediadted signaling constitutes a promising target for development of novel antibacterials.
Although ppGpp itself is an activator of the ribosome-associated ppGppsynthetaseRelA, several ppGpp mimics have been developed as RelA inhibitors. However promising, the currently available ppGpp mimics are relatively inefficient, with IC50 in the sub-mM range. In an attempt to identify a potent and specific inhibitor of RelA capable of abrogating (p)ppGpp production in live bacterial cells, we have synthesized and tested a targeted nucleotide library using a biochemical test system comprised of purified Escherichia coli components.
Biography:
My research interests are chemistry and biological properties of modified nucleic acid components. Currently I am working on several projects: 1. Stringent response modulators, 2. Lipophosphonoxins – novel antibacterial compounds, 3. Phosphonate azanucleotide inhibitors of HGXPRT as potential antimalarial and antibacterial agents.
1992-1996: Department of Organic Technology, UCT, Prague
1996-2000: Institute of Organic Chemistry and Biochemistry CAS; Ph. D. study
2001-2002: Department of Chemistry, University of Sheffield
2002-2004: Institute of Organic Chemistry and Biochemistry, CAS
2004- 2004: Center for Drug Design, University of Minnesota, USA
2005-Now: Institute of Organic Chemistry and Biochemistry CAS
Acknowledgments
This work was supported by the Czech Science Foundation grant number 15-11711S.
1Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences v.v.i., Czech Republic
2Charles University, Faculty of Science, Department of Analytical Chemistry, Czech Republic
4University of Tartu, Institute of Technology, Estonia
5Department of Molecular Biology, Umeå University, Sweden
6Laboratory for Molecular Infection Medicine Sweden (MIMS), Umeå University, Sweden
7Graduate School of Science and Engineering, Saitama University, Japan
Various bacterial responses to environmental stimuli lead into changes in intracellular concentration of small molecules (nucleotides, nucleosides and their derivatives). Robust, sensitive and simple method for characterizationof thesechanges is therefore necessary. E.g. in the case of stringent response –a potential novel drug target - intracellular levels of alarmone nucleotides guanosine tetraphosphate (ppGpp) and pentaphosphate (pppGpp), need to be determined.
There are two key steps in the process of determining nucleotide levels in bacterial biomass (complex samples with strong matrice effect):1) extraction method with quantitative and reproducible yield, and 2) good analytical method capable todistinguish and quantify individual nucleotides.
To characterize nucleotide pools in bacteriawe have chosen LC-MS in HILIC mode of separation that exhibits many advantages over commonly used ion pair (IP) LC coupled with UV-VIS detector. Moreover, the ballast mass from bacteria are well separated from majority of analytes and do not disturb the analysis.
Biography:
After the period of pesticide analysis in certified laboratories, I switched to medicinal chemistry and continue working with liquid chromatography and mass spectrometry techniques. After several years, I have started PhD studies. Modern analytical chemistry is my hobby and could be often quite challenging.
2003-2008: Faculty of Food and Biochemical Technology, UCT, Prague
2008: National Veterinary Institute, Prague
2009-2010: Institute of Organic Chemistry and Biochemistry, CAS
2010-now: Institute of Organic Chemistry and Biochemistry, CAS; Charles University, Department of Analytical Chemistry, Prague; Ph.D. study
Acknowledgments
This work was supported by the Czech Science Foundation grant number 15-11711S (DR) and internal IOCB Pragueproject 9330/33.
Novosibirsk State University, Russia
Recent advances in nanomaterials made possible to tackle difficult problems, in particular, the application of titanium dioxide nanoparticles in biological tasks. The conditions required for biological experiments (neutral pH values and water-soluble state of nanoparticles) often lead to aggregation of nanoparticles and changes in their morphology. Low aggregation stability of extremely small nanoparticles and their toxic properties make it necessary to devise special methods for synthesis of nanoparticles aimed at improving their dispersion stability and biosafety. We designed TiO2 nanoparticles for using in nanocomposites with oligonucleotides and found ways for diminishing the toxicity of TiO2-NPs and increasing the dispersion stability of the sols in physiological media by optimizing the conditions of chemical treatment of TiO2 sols. The effect of the nature of singly charged electrolyte cation (Li+, Na+, K+, NH4+) used for neutralization of the initially acid TiO2 sols, a method of electrolyte introduction into sols, chemical nature of dialyzing solution, and surface modification of nanoparticles with isopropyl glycidyl ether (IGE) on the size of TiO2 particles at pH 6.5-7.5 and their cytotoxic properties was studied. Investigation of TiO2 sols dispersion by a set of physicochemical methods showed that chemical nature of the electrolyte cation affects sol agglomeration, which increases in a series Li+ > Na+ > NH4+, and the use of phosphate buffer for sol dialysis facilitates the formation of more uniform disperse systems. It was shown that the surface modification of TiO2 nanoparticles with IGE and using phosphate buffer diminishes its toxic effect on the cells and prevents undesirable interaction of TiO2 with cell components. TiO2 nanoparticles showed their potency as the basis of nanocomposites bearing DNA fragments for interaction with nucleic acid targets in cells.
The work was supported by the grant of Russian Scientific Foundation no. 16-15-10073.
Biography:
Elena V. Bessudnova was born in Sovetsk, Russia. She received the MS degree in chemistry from Southern Federal University, Russia, in 2007 and the Ph.D. degree in physical chemistry from Kemerovo State University, Russia, in 2014. From 2010 to present, she has been Postgraduate Student, then Research Scientist, with the Environmental Catalysis Laboratory from Boreskov Institute of Catalysis, Siberian Branch of Russian Academy of Sciences. She is author of more than 10 articles. Her research interests include the synthesis and design of nanoparticles and their study physico-chemical properties. Currently she works at the Novosibirsk State University.
Umm Al-Qura university, Saudi Arabia
Objectives: The aim of this study is to compare the efficacy of the anti-angiotensin drug, ramipril and irbesartan on the vascular protection of kidneys of streptozotocin (STZ)-induced diabetic rats (DR).
Methods: 110 male albino rats were divided into 7 main groups. Group-1 (10 normal control rats; NC). Group-2 (10 rats) was injected intra-peritoneally with STZ (Diabetic Rats; DR). Group-3 (10 DR) is controlled by insulin. Groups 4 to 7 (20 DR), each is subdivided into two subgroups that received either low or high dose of ramipril or irbesartan with or without insulin. Two months post treatment, rat-tail blood was collected to measure: Fasting blood sugar, HbA1c, total serum proteins, albumin and lipid profiles. Urine was collected to measure albuminuria. Kidneys were isolated for histopathological study.
Results: Biochemically, both ramipril and irbesartan (without insulin) lowered albumin concentration in urine samples especially at high doses. Histopatholog- ically, there is no beneficial response of both drugs without insulin. Combination of insulin to- gether with either drug has beneficial effects biochemically and histopathologically at high doses. Low dose irbesartan only has renoprotective effect in DR treated with insulin. The other biochem- ical parameters showed negligible response to both drugs.
Conclusion: Low dose irbesartan and high doses of both drugs have renoprotective effect in DR treated with insulin.
Biography:
Abdulelah A. Thigah was born on December 19, 1992 and live in mecca, graduated from Umm Al-Qura university with bachelorʼs degree in medicine and surgery. interested in clinical researches and health promotion programs, looking forward to provide the best care and awareness to the community and to be one of the well known characters in his speciality as well as a great role model.
Umm Al-Qura university, Makkah, Saudi Arabia
Objective: A potentially important role of IKKβ in mediating insulin resistance and the ability of salicylates to inhibit IKKβ activity is suggested. We decided to examine the role of different doses of aspirin (low, moderate and high) in experimentally induced diabetic rats.
Materials and Methods: Diabetes Mellitus (DM) in rats were induced by administration of nicotinamide (NAD), 15 min prior to the single dose of streptozotocin STZ i.p. Ninety male albino rats were used in this study. They were divided into 6 main groups. The first was served as control, which receives no medications. The second group was diabetic induced rats as mentioned above. The third group was controlled by insulin after induction of D.M. Groups from the fourth to the six consist of 20 diabetic induced rats and further subdivided into rats taking either aspirin alone in different doses (low, moderate or high) or aspirin and insulin.
Results: Different doses of aspirin showed that moderate and to a greater extent high dose aspirin administration to diabetic rats have greater impact on fasting blood glucose levels whether treated with insulin or not. Again, HBA1c % in diabetic rats treated with insulin and receiving high dose aspirin was lower than diabetic rats treated with insulin only or even taking low dose aspirin.
Conclusion: The study concluded that the inflammatory pathways hold a substantial part in insulin resistance in type 2 DM.
Biography:
Abdullah A. Alshamrani was born on February 25, 1992 in Jeddah-Saudi Arabia. Graduated from Umm Al-Qura (UQU) University in Makkah-Saudi Arabia with a bachelorʼs degree in medicine and surgery on June 2016. He currently work as a medical intern.. Interested in internal medicine and health awareness campaigns. Hoping to be a good doctor one day.
Abdullah is currently resides in Jeddah-Saudi Arabia
1Department of Pharmaceutical Chemistry, Jinnah University for Women; Pakistan
2Baqai Institute of Reproduction and Developmental Sciences, Member Pakistan Society of Anderology and Sexual Medicine, Pakistan
Background: Every single couple out of ten are in search of medical care because of infertility. Men older than 40 years and Women older than 30years are at an increased risk of infertility. Presence of Infertility can be both in males and females. The estimation of male infertility is frequently underestimated or delayed. They may also experience the history of testicular, prostate, or sexual problems. WHO guidelines reported that the man with a sperm count of <20 millions/ml is considered as 0oligospermia and nearly 100 million man around the world are surviving with erectile dysfunction. Symptomatic approaches steadily reducing the magnitude of couples classified as having idiopathic infertility. This is than perceived that no simple tests can conclude the probability of pregnancy in congregation in which the man is an infertile partner.
Methods: A study has been designed and conducted to figure out existence, problems, and causes associated with infertility in men and treatment options. For this purpose, 33 patients (n=33) has been selected and investigated from different private and government health care hospitals.
Results: Most of the patients are in the middle of their ages reported infertile. Primary infertility in infertile patients is 73% where as the presence of secondary infertility in the remaining respondent is 27%. Among all, 52% of the patients have co-morbid history of diabetes mellitus, 18% of them have issues about genitourinary trauma and infections, 30 patient have been identified problems related morphological count, 51% of them investigated abnormal volume of man semen. Evaluation of the study by Krugerʼs strict morphology test reveals that over 75% of the men having insignificant infertility issues whereas 25% of them were observed significant infertility issues.
Conclusion: It is concluded that male infertility is independent on the age factor whereas, excessive use of tobacco, alcohol, high fat food consumptions, obesity, heavy weight exercises, sedative life styles, contacts with chemical or toxins, stress or psychological disturbances might provoke issues of infertility in males.
Keywords: Infertility, semen analysis, Krugerʼs strict criteria
1Department of Phytochemistry, Medicinal and Aromatic Plants Research Institute, National Center for Research, Sudan
2Department of Pharmaceutics, University of Gezira, Sudan
3Department of Pharmaceutical Chemistry, University of Khartoum, Sudan
4Department of Pharmaceutics, University of Khartoum, Sudan
5Department of Pharmacognosy, University of Khartoum, Sudan
6Department of Pharmacology, University of Khartoum, Sudan
Objective: To investigate the pharmacokinetic and toxicity profiles and spectrum of biological activities of three phytochemicals isolated from Tarconanthus camphoratus L.
Methods: Several integrated web based in silico pharmacokinetic tools were used to estimate the druggability of Hispidulin, Nepetin and Parthenolide. Afterward, the structural based virtual screening for the three compoundsʼ potential targets was performed using PharmMapper online server. The molecular docking was conducted using Auto-Dock 4.0 software to study the binding interactions of these compounds with the targets predicted by PharmMapper server.
Results: The permeability properties for all compounds were found within the limit range stated for Lipinskiʼs rule of five. Only Parthenolide proved to be able to penetrate through blood brain barrier. Isopentenyl-diphosphate delta-isomerase (IPPI), uridine-cytidine kinase-2 (UCK-2) and the mitogenactivated protein kinase kinase-1 (MEK-1) were proposed as potential targets for Hispidulin, Nepetin and Parthenolide, respectively. Nepetin and Parthenolide were predicted to have anticancer activities. The activity of Nepetin appeared to be mediated through UCK-2 inhibition. On the other hand, inhibition of MEK-1 and enhancement of TP53 expression were predicted as the anticancer mechanisms of Parthenolide. The three compounds showed interesting interactions and satisfactory binding energies when docked into their relevant targets.
Conclusion: The ADMET profiles and biological activity spectra of Hispidulin, Nepetin and Parthenolide have been addressed. These compounds are proposed to have activities against a variety of human aliments such as tumors, muscular dystrophy, and diabetic cataracts.
Keywords: Tarconanthus camphoratus L., Hispidulin, Nepetin, Parthenolide, In silico pharmacokinetic, Molecular docking, PharmMapper server, and Auto-Dock 4.0 software
School of Health Sciences, University of KwaZulu-Natal, South Africa
The re-emerging Zika virus is an arthropod-borne virus that has been described to have explosive potential as a worldwide pandemic. The initial transmission of the virus was through a mosquito vector, however, evolving modes of transmission has allowed the spread of the disease over continents. The virus already been linked to irreversible chronic central nervous system (CNS) conditions. The concerns of the scientific and clinical community are the consequences of Zika viral mutations, thus suggesting the urgent need for viral inhibitors. There have been large strides in vaccine development against the virus but there are still no FDA approved drugs available. Rapid rational drug design and discovery research is fundamental in the production of potent inhibitors against the virus that will not just mask the virus, but destroy it completely. In silico drug design allows for this prompt screening of potential leads, thus decreasing the consumption of precious time and resources. This study demonstrates an optimized and proven screening technique in the discovery of two potential small molecule inhibitors of Zika virus Methyltransferase and RNA dependent RNA polymerase. This in silico “per-residue energy decomposition pharmacophore” virtual screening approach will be critical in aiding scientists in the discovery of not only effective inhibitors of Zika viral targets, but also a wide range of anti-viral agents.
Biography:
Pritika Ramharack is a PhD student under the supervision of Prof M.E. Soliman at the Department of Pharmaceutical Sciences, University of Kwa-ZuluNatal, South Africa. Her current research concentrates on the design of inhibitors against the Zika virus using in silico studies. She also has experience with molecular and cellular biology.