International Conference on Medicinal and Pharmaceutical Chemistry
December 5-7, 2016 | Dubai, UAE
The Journey of 1,5-disubsitituted tetrazoles with the cyclooxygenase enzymes
1Faculty of Science, American University of Madaba, Jordan
2Faculty of Health Sciences, American University of Madaba, Jordan
3Department of Chemistry, The Hashemite University, Jordan
4Department of Oncology, University of Alberta, Canada
Throughout five years of research, we reported 29 examples of novel 1,5-diaryl tetrazole derivatives (typical azoles) as cyclooxygenase-2 (COX-2) inhibitors. We investigated two modes of connectivity in regards to the pharmacophore unit position and type. The inhibitory potency of the typical active azoles ranged between 1.2->100 µM toward COX-2 enzyme. The molecular docking studies of the most potent candidates showed that they are well seated inside the active site of both enzymes COX-1 and COX-2. Our recent structure-activity relationship study of a novel set of 1,5-disubstituted tetrazoles, together with the molecular docking results, proved the importance of the introduced structural modifications of the typical azoles. These modifications included using linkers with different lengths at one of the phenyl group (OH, CH2 OH, CH2CH2OH) and inserting a methylene (CH2) unit between the central motif and the other phenyl group which holds the pharmacophore unit. Our bioassay screening data showed that the azole which has the CH2 spacer, the longest linker (ethyl alcohol), and the methylsulfonyl (CH3SO2) unit has the best inhibition potency for both enzymes IC50: COX-1 = > 200 µM, COX-2 = 3 µM. Further, the molecular docking studies illustrated that this azole, our lead compound, is well seated and capable of forming strong hydrogen bonds with certain amino acid residues inside the active site of the COX-2 enzyme. In the case of the COX-1 enzyme, however, the pharmacophore unit is drafted away from the active site resulting in extremely weak or no inhibition for the house keeping enzyme COX-1.
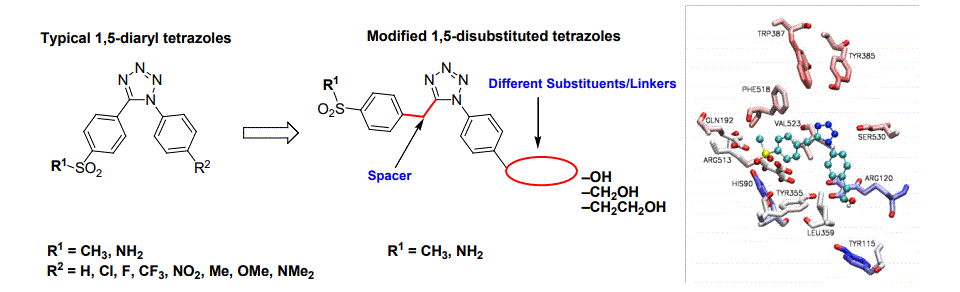
In summary, our lead compound is capable of targeting only the COX-2 enzyme, while preserving the COX-1 enzyme untouched, safe, and active.
Biography:
Since 2011, Dr. Jawabrah Al-Hourani, Baker (JB) has been employed at the American University of Madaba (AUM) in Jordan. He was awarded an early promotion to Associate Professor in 2015 based on his scientific achievements at AUM. He obtained his PhD in organic chemistry in 2005 from the Chemnitz University of Technology in Germany funded by the German Academic Exchange Service (DAAD). Between 2007–2011, he did his postdoctoral research as part of two different multidisciplinary research teams at the University of Alberta, Edmonton, Alberta, Canada, the first being at the National Institute for Nanotechnology, and the second at the Cross-Cancer Institute. Dr. JB has established his own research lab at AUM to pursue his scientific journey in diverse fields of organic chemistry, with a special focus on the synthesis of novel probes for cancer imaging and treatment. He is passionate about translational cancer research and drug design and discovery.


