International Conference on Medicinal and Pharmaceutical Chemistry
December 5-7, 2016 | Dubai, UAE
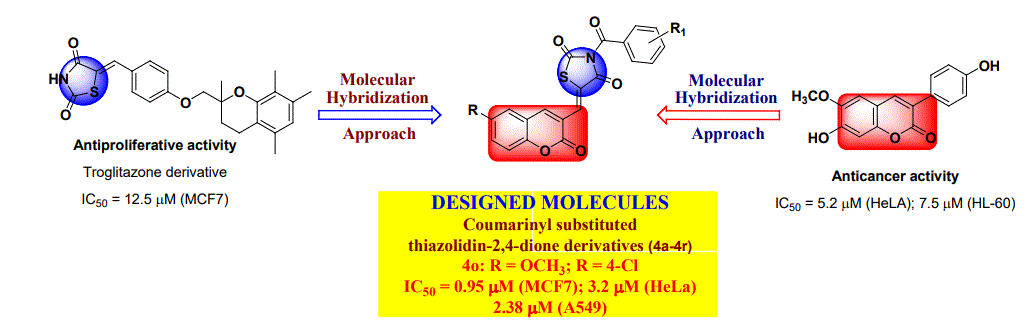
Design, synthesis and cytotoxicity evaluation of novel series of coumarinyl substituted thiazolidin-2,4-dione analogs as promising anticancer agents
1Department of Pharmaceutical Chemistry, K.L.E. University College of Pharmacy, India
2KLE University, India
3College of Health Sciences, University of KwaZulu-Natal, South Africa
In this research work, a series of eighteen novel coumarinyl substituted thiazolidin-2,4-dione analogs (4a-4r) have been designed by molecular hybridization approach, synthesized and their structures were established on the basis of FTIR, 1 H NMR, 13C NMR and elemental (CHN) analysis. These title compounds were screened for their cytotoxicity using MTT assay methodology against five different mammalian cancer cell lines viz. hormone dependant breast adenocarcinoma (MCF7), cervical carcinoma (HeLa), colorectal carcinoma (HT29), lung cancer (A549), prostate adeno carcinoma (PC3). The cytotoxicity screening studies revealed that MCF-7, HeLa and A549 cancer cell lines were sensitive to all the tested compounds. Though the compounds showed varying degrees of cytotoxicity in the tested cell lines, most significant effect was observed for compounds 4i (1.06, 2.4 and 3.06 µM) and 4o (0.95, 3.2 and 2.38 µM) against MCF7, HeLa and A549 cell lines respectively. In conclusion, the anticancer results of these promising leads strongly encouraged us for additional lead optimization with the aim of developing more potential anticancer agents.