Research Article
Sitagliptin Attenuates the Pharmacokinetics and Biodistribution of Hydrochlorothiazide in Rats
Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Nigeria Nsukka, Nigeria
*Corresponding author: Mbah J. Chika, Department of Pharmaceutical and Medicinal Chemistry, Faculty of Pharmaceutical Sciences, University of Nigeria, Enugu State, Nigeria, E-mail: chika.mbah@unn.edu.ng
Received: July 12, 2022 Accepted: August 08, 2022 Published: August 25, 2022
Citation: Chika MJ, Ngozi NJ, Ruth EG. Sitagliptin Attenuates the Pharmacokinetics and Biodistribution of Hydrochlorothiazide in Rats. Madridge J Pharm Res. 2022; 4(1): 60-66. doi: 10.18689/mjpr-1000110
Copyright: © 2022 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Context: Hydrochlorothiazide (HCTZ), is a thiazide diuretic indicated for the treatment of mild to moderate hypertension. Sitagliptin is the first dipeptidyl peptidase-4 (DPP-4) inhibitor used clinically in the treatment of type 2 diabetes mellitus. Objective: The present study was aimed at investigating the effect of sitagliptin on the biodistribution and pharmacokinetics parameters of hydrochlorothiazide in rats using a validated high performance liquid chromatography method.
Methodology: A simple, accurate and selective HPLC method with UV detection at 269 nm was developed and validated for simultaneous quantification of HCTZ and sitagliptin in rat plasma and tissues. Plasma samples were deproteinized with acetonitrile/methanol mixture (4:1) while the rat tissues were homogenized with the same solvent mixture. Chromatographic separation was performed on a C18 analytical column (250 mm×4.6 mm, 5 µm) using a simple isocratic mobile phase composed of acetonitrile and 0.01M potassium dihydrogen phosphate (70:30, v/v) at flow rate of 1.0 ml/min. The method was fully validated as per the US Food and Drug Administration Agency (FDA) and European Medicine Agency (EMA) guidelines.
Results: Calibration curves were linear over concentration range from 5-30 µg/ml in plasma and in tissues respectively for all analyses. The limit of detection and lower limit of quantization of the method were 0.0082µg/ml and 0.078 ng/mL respectively for HCTZ. Intra and inter-batch precision of analysis was < 1.5 % for plasma and < 4.0 % for tissues, accuracy ranged from 93.20 % to 98.60 % for plasma and 94.34 % to 98.67 % for tissues. The order of distribution of HCTZ in rat tissues was pancreas (0.69µg/g) > kidney (0.55µg/g) > liver (0.51µg/g) > heart (0.37µg/g). Minimal matrix effects were observed, and stability of stock standard solutions was within acceptability limits.
Conclusion: The validated method was considered sufficiently sensitive and was successfully applied for determination of biodistribution and pharmacokinetics parameters of HCTZ in rat. The administration of sitagliptin in rats leads to decrease in the pharmacokinetic behavior of HCTZ, and a lesser accumulation being observed in various tissues.
Abbreviations: HCTZ: Hydrochlorothiazide; Cmax: Maximum plasma concentration; Tmax: Time to reach maximum plasma concentration; Cltotal: Total clearance; Ke : Elimination-rate constant; t ½,: Elimination half-life; Vss: Steady-state volume distribution; MRT: Mean residence time; AUC0–24h: Area under the plasma concentration–time curve from 0 to 24 h; AUC0–∞: Area under the plasma concentration–time curve from 0 to infinity.
Keywords: HPLC, hydrochlorothiazide, Sitagliptin, Rat plasma and tissues.
Introduction
Biodistribution defines the amount and distribution pattern of a drug in different organs and tissues, during or after any clinical diagnostic or therapeutic application. Knowledge of biodistribution and pharmacokinetics of a drug will help to enhance the expected functionality of a drug in any selected region or organ of the body [1].
Diuretics are a major class of drugs utilized to reduce sodium and water accumulation in patients with heart failure or other edema related diseases. Hydrochlorothiazide (Figure 1), chemically defined as 6-Chloro-7-sulfamyl-3, 4-dihydro-1, 2, 4-benzothiadiazine 1, 1- dioxide, is a thiazide diuretic clinically used for the treatment of mild to moderate hypertension. As a thiazide diuretic, one of its mechanisms of action is by inhibiting sodium re-absorption in the distal convoluted tubule, leading to dieresis within 2 hours of its oral administration and indirectly provides an initial reduction in plasma volume that attenuates with long-term treatment [2,3,4,5].
Diabetes mellitus is a metabolic disease characterized by an increase in plasma blood glucose. Sitagliptin is a highly selective dipeptidyl peptidase-4 (DPP-4) inhibitor that blocks DPP-4 (enzyme) activity. It is used alone or in combination with metformin or simvastatin to treat diabetes mellitus [6].
As hypertension is one of the risk factors in diabetes mellitus, there exists the tendency that patients suffering from type 2 diabetes mellitus might also be associated with hypertension. This clinical disease state might necessitate concurrent administration of HCTZ and sitagliptin. Such concurrent administration of both drugs requires monitoring of their plasma concentration levels that will invariably provide information on their tissue distribution. HCTZ biodistribution to the tissues might also provide information on its ameliorative or detrimental roles on those tissues. Although, HCTZ is old in clinical applications, literature review has shown little or no information on its biodistribution in rats. Furthermore, the influence of sitagliptin on the biodistribution and pharmacokinetics of HCTZ has not been reported.
Therefore, the objectives of the present study were to (i) investigate the tissues penetration ability of HCTZ, and (ii) evaluate the effect of sitagliptin on the biodistribution and pharmacokinetic properties of HCTZ.
Materials and Methods
Materials and Reagents: Hydrochlorothiazide and sitagliptin were obtained from Getz Pharma Limited (Pakistan) and Juvise Pharmaceuticals; (France) respectively. HPLC grade of acetonitrile and methanol were purchased from Fisher Scientific (USA). Other chemicals and reagents used were of analytical grade.
Instrumentation and Chromatographic Conditions
Instrumentation: High performance liquid chromatography (Shimadzu Corporation, Kyoto Japan (Elite Lachrom) was used in the study. The chromatograph consists of SIL-10AD autosampler and a Model SPD-10AVP UV-detector. A glass vacuum filtration apparatus (Alltech Associates, IL, USA) was employed for the filtration of the mobile phase, using 0.45 µm filters obtained from Fisher Scientific, USA. Further degassing of the mobile phase was carried out by sonication in ultrasonic bath. A vortex mixer and a REMI C24 refrigerated centrifuge were used.
Chromatographic conditions: The method consists of C18 analytical column (250 mm×4.6 mm, 5 µm) with a simple isocratic mobile phase composed of acetonitrile and 0.01M potassium dihydrogen phosphate (70:30 % v/v) The method was validated in accordance with the United States Food and Drugs Administration guidelines for bioanalytical method as well as EMA. European Medicines Agency, C.H.M.P. Committee for Medicinal Products for Human Use, 2009. Detection was done using ultraviolet detector set at 269 nm. The analysis was accomplished within 5 min. A flow rate of 1 ml/min and elution was carried out at a temperature of 25°C.
Calibration standards and Quality control (QC) samples
The standard stock solution of hydrochlorothiazide (50 µg/ml) and working solution of internal standard (5 µg/ml) were prepared by dissolving requisite amount in methanol. Working solutions were obtained by diluting the stock solution with methanol. Calibration standard solutions of HCTZ were prepared by spiking the appropriate amount of the working solutions containing internal standard (5 µg/ml). into 100 µl drug-free rat plasma or tissue homogenates. Sitagliptin acted as internal standard. Precipitation of matrix was by adding 300 ul ice-cold acetonitrile-methanol mixture (4:1). The final concentrations of calibration standard samples were 5, 10, 15, 20, 25 and 30 µg/ml. Quality control (QC) standards were separately prepared in rat blank plasma and different tissue homogenates in the same way at low (5 µg/ ml), middle (15 µg/ml) and high (30 µg/ml) concentrations. All solutions were stored at 4 °C for further use.
Sample Preparation
The general procedure of preparing for HCTZ analysis in plasma and tissue homogenates involved direct precipitation of matrix by adding ice-cold acetonitrile-methanol mixture (4:1). In the analysis, 100 µl of plasma or tissue homogenate samples in glass centrifuge tubes were vortex for 5 min. Following centrifugation at 10,000 rpm for 5 min, the organic solvent (upper layer) was transferred to a clean tube and evaporated to dryness under a stream of nitrogen at 40 °C. An aliquot (20 µl) of supernatant obtained by reconstituting the residue in 100 µl of methanol, centrifuged at 10,000 rpm for 5 min was injected onto the HPLC system. Since the density of exoplanets is a key parameter for our study, to expand our dataset as far as possible, we have also downloaded available data from the EU exoplanet database of [2]. For convenience, we use the Jupiter density (ρjup) as the density unit. We have also calculated the parameter of gravity in units of Jupiter surface gravity (gjup= 24.79m/s^2), i.e.,g =Mjup/Rjup^2.
Method Validation
The bioanalytical method was validated for linearity, accuracy, precision, specificity, selectivity, recovery, matrix effect and stability [7,8].
Calibration curves, lower limit of quantification and carry-over effect: Calibration standard solutions were prepared as described above in triplicate and analyzed on three consecutive days. Linear calibration curves were obtained by plotting the peak area ratio of HCTZ versus the concentration of HCTZ. Regression parameters of the slope, intercept and correlation coefficient were calculated by linear least-squares regression. The lower limit of quantification (LLOQ) was determined from the analyte peak signal and baseline noise level by analyzing five replicates of spiked samples at the relevant concentrations. We defined it as the lowest concentration of HCTZ in a sample giving a peak area with a signal-to-noise ratio higher than 10. Carry-over effects were studied by injecting blank samples following the calibration standard at the highest concentration. In accordance with official guidelines, carry over in the blank should not exceed 20% of the LLOQ.
Accuracy and precision: Intra-day accuracy and precision were determined by analyzing three QC samples at low (LQC), medium (MQC) and high (HQC) levels with six assay per concentration at the same day, whilst the inter-day precision and accuracy were evaluated over three consecutive days. We determined accuracy by comparing the calculated concentration to the added concentration, using calibration curves. The intra-day and inter-day precisions were assessed by calculating the relative standard deviation (RSD).
The accepted criteria for the data were that the mean value of accuracy should be within 15% of the actual value and precision (RSD) determined at each concentration level should not exceed 15% of the coefficient of variation (CV). However, not more than 20% is acceptable at the lower limit of quantification (LLOQ) of the mean value or of the CV respectively.
Specificity and selectivity: We assessed specificity and selectivity of the method by comparing chromatograms obtained by analyzing blank plasma and tissue homogenate samples, blank samples spiked with standard HCTZ and rat samples after intravenous administration of HCTZ. According to the validation guidelines, absence of interfering components is accepted where the response is less than 20% of the LLOQ for the analyte.
Extraction recovery: The extraction recovery of HCTZ from rat plasma was determined at low, medium and high levels of QC samples in five replicates. Recoveries were evaluated by comparing the mean observed peak areas in the QC samples extracted from blank rat plasma or blank tissue homogenate samples with mean peak areas of HCTZ spiked in post-extracted matrix. Percentage extraction recovery was calculated using the formula:

Matrix effect: The matrix effect (ME) was determined by comparing the peak area of HCTZ obtained from the spiked-after-protein precipitation samples with the standard solution of HCTZ in the mobile phase at low, medium and high concentration levels. The percentage matrix effect was calculated with the following formula.

The extraction recovery and matrix effect for internal standard were also done at a particular concentration (5 µg/ml). Stability study: The stability of HCTZ in plasma and tissue homogenates was determined by analyzing QC samples under different storage conditions. Assessment of short-term stability was by analyzing QC samples kept at ambient temperature (25°C) for 8 h and refrigeration conditions (4°C) for 8 h. The freeze-thaw stability of HCTZ was determined over three freeze-thaw cycles within 3 days. In each freezethaw cycle, the spiked plasma and tissue homogenate samples were frozen at -20°C for 24 h and thawed at room temperature. The samples were refrozen when completely thawed for 12–24 h under the same conditions. After three cycles, the percent loss of the HCTZ was determined by comparing the concentrations with those obtained before freezing. Long-term stability was studied by samples determinations following a period of 30 days of storage at −20 °C. The results compared with those obtained from the freshly prepared samples. We considered a percent deviation of ± 15% from the nominal concentration and precision of less than 15% to be acceptable as stable sample under the storage condition tested.
Animals: Male Sprague-Dawley rats (weight 190± 10 g,) were bought from the Faculty of Veterinary Medicine of University Nigeria. Animal welfare and experimental procedures were strictly in accordance with the related ethics regulations of the Veterinary Medicine Faculty and complied with the ‘Principles of Laboratory Animal Care’. All animals were kept in an environmentally controlled breeding room (temperature maintained at about 25 ± 2 °C and for at least one week before starting the experiments. They were fed with standard laboratory food and water ad libitum. Prior each experimental study, the rats were fasted for 12 h with free access to water.
Pharmacokinetic and tissue distribution studies: In order to evaluate the applicability of the present investigation, the validated method was used to analyze plasma and different tissue samples after intravenous administration of HCTZ at 2 mg/kg/day in rats.
In the pharmacokinetic study, twelve rats were randomly assigned to two groups (n = 6 per group). The group I received HCTZ alone while group II received combination of HCTZ and sitagliptin. The drugs were given by intravenous injection through the tail vein at the dose of 2 mg/kg. Blood samples (0.5 ml) of rats administered with HCTZ were collected in heparinized tubes from the orbital sinus at time intervals of 0.0 (pre-dose) 0.5, 1, 2, 4, 6, 8, 12, 24 h post-dosing. The blood samples were centrifuged at 12,000 rpm for 10 min to obtain plasma. The separated plasma samples were stored at −70 °C before bioanalysis. The same procedure was applicable when HCTZ was co-administered with sitagliptin.
The tissue distribution study was carried out using thirty rats that were divided into five groups (n = 6 per group) randomly and HCTZ was administered intravenously through the tail vein at a dose of 2 mg/kg. The rat was under anesthesia with diethylether prior to blood collection and the animal being sacrificed at fixed times (0.5, 1.0, 2.0, 4.0 and 6.0 h). Different tissue samples (heart, kidney, liver and pancreas) were removed for the analysis of HCTZ contents. The tissue samples were rinsed in saline and blotted dry with filter paper, and then weighed for wet weight. Each weighed tissue was homogenized in ice-cold physiological saline solution (500 mg/ml) using a glass tissue homogenizer. Acetonitrile (10 ml), methanol (10 ml) and tissue homogenate (10 ml) were mixed. The suspension was stored at 4°C for 18 hours to extract each of the drugs and then centrifuged (15,000 rpm, 10 min). The supernatant was available to be injected into the chromatograph or stored at −70 °C until bioanalysis was performed using the described procedure. The same procedure was applicable when HCTZ was co-administered with sitagliptin.
Pharmacokinetic analysis: The concentration of HCTZ in plasma and tissues was determined at each time interval. The pharmacokinetic parameters, namely maximum concentration (Cmax), area under the plasma-concentration-time curve (AUC) from zero to last measurable plasma sample time and to infinity, total clearance (Cltot), terminal elimination rate constant (Kel), volume of distribution at steady-state (Vss), the apparent plasma or tissue elimination half-life (t1/2), and mean residence time (MRT) were determined by the noncompartmental method. The area under the plasma concentration-time curve was calculated using the logarithm linear trapezoidal method [9,10].
Statistical analysis: The concentration of HCTZ was obtained by making reference to the calibration curve. Comparisons between groups were performed by one-way analysis of variance. (ANOVA). Data collected were expressed as the mean ± standard deviation. Statistical differences were evaluated using t-test. A p -value of 0.05 or less was considered significant.
Results
Linearity of Calibration Curve and Lower Limit of Quantification
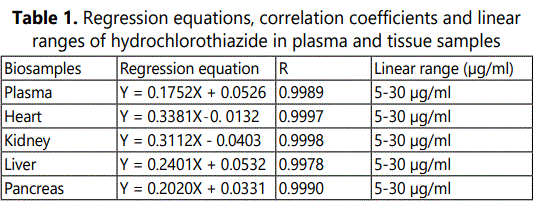
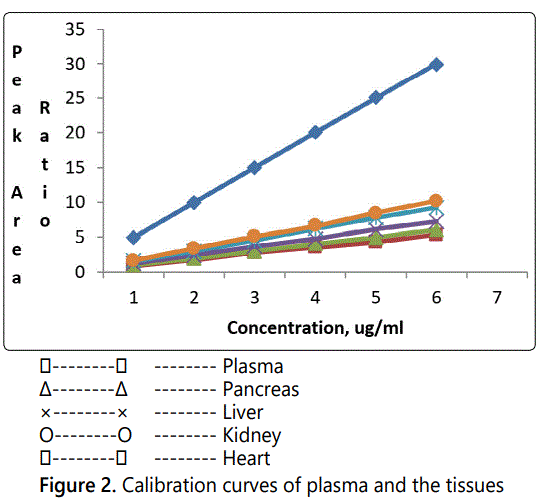
Calibration curves of HCTZ were obtained over a concentration range from 5–30 µg/ml for plasma and the tissues. The regression equations for the calibration curves of HCTZ in plasma and tissues are given in Table 1. Figure 2 presents the calibration curves of plasma and the tissues.
In the validation process, quality control (QC) samples at 5, 15, 30, µg/ml spiked in plasma, heart, kidney, liver and pancreas respectively, were determined for three days in sets of five replicates. The calibration curves for QC samples were linear with the correlation coefficients not less than 0.9960. The lower limit of quantification (LLOQ) was evaluated by analyzing five replicates of spiked samples at the concentrations lower than 0.08µg/ml. The lower limit of quantification for HCTZ was the lowest concentrations of calibration curve with signal-to-noise ratio > 10. The limit of detection (LOD), calculated using a signal to noise ratio of 3-to-1 was 0.0082µg/ml for plasma and tissues.
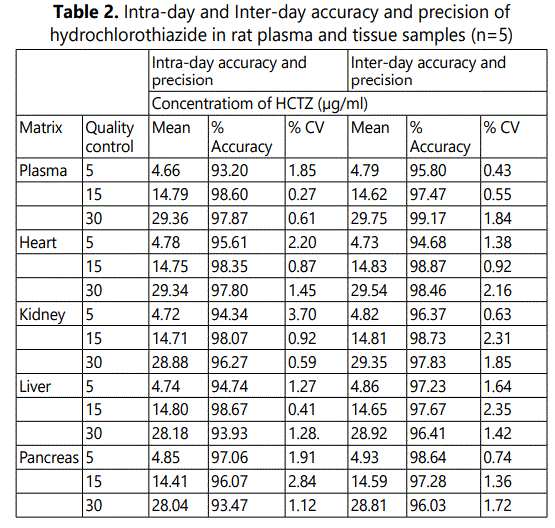
Precision and Accuracy
The precision and accuracy of the method are given in Table 2. All the samples were spiked with HCTZ at three concentration levels. The accuracy was within the range of 93.20% to 98.60 %.for the plasma and 94.34% to 98.67% for the tissues. The precision of both intra-day and inter-day analyses was below 4.0 % for both plasma and tissues. Since the accuracy and precision were within official specifications [( ± 15% ) and (<15%) respectively], we considered accuracy and precision of the method acceptable.
The selectivity and specificity results demonstrated the lack of chromatographic interference from endogenous plasma or tissue components at the retention time of the HCTZ and internal standard. The results of the carryover analysis in each analytical run showed that the accuracy and the precision of the proposed method were not affected because there was no enhancement in the response observed in the double blank, after subsequent injection of the highest calibration standard at the retention time of HCTZ
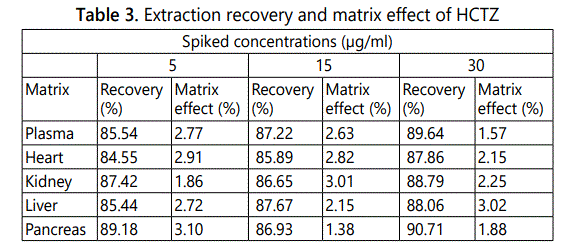
Extraction recovery and matrix effect: Mean extraction recovery and matrix effect were at LQC, MQC and HQC levels. The percent recovery ranged from 84.55% to 90.71%. The matrix effect ranged from 1.38% to 3.10%. The data are presented in Table 3.
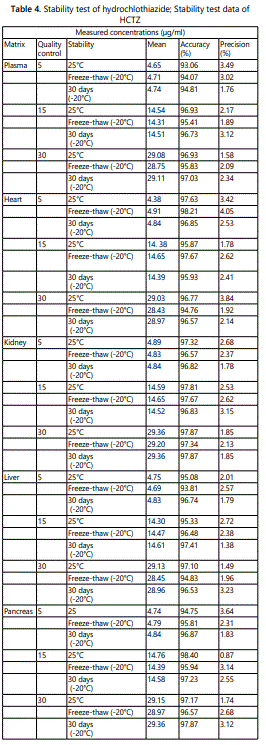
Stability: The stability tests were designed to cover the anticipated conditions that the samples may experience. The stability for HCTZ was investigated at LQC, MQC and HQC levels. The results presented in Table 4, showed that HCTZ complied with a percent deviation of ± 15% from the nominal concentration and precision of less than 15% required for acceptance as stable sample under the storage condition. The accuracy was in the range 90% to 98% while the precision was in the range 1% to3%,. Freeze/thaw cycles results showed that HCTZ in plasma and tissue homogenate samples was stable in the processed samples.
In order to evaluate the suitability of the validated analytical method for biodistribution and pharmacokinetic studies, it was used for determination of plasma and tissue concentrations of HCTZ after intravenous administration of a single dose (2 mg/kg) to rats.
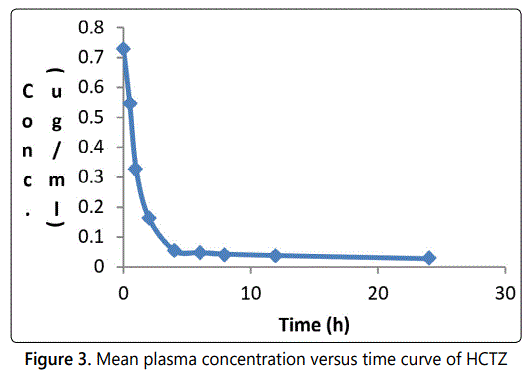
The mean plasma concentration–time profile for HCTZ in plasma after an intravenous administration is illustrated in Fig. 3. The graph shows that after intravenous administration, the plasma concentration of HCTZ first decreased rapidly and then more slowly. The pharmacokinetic parameters obtained from the mean plasma concentration versus time curve (Figure 3) are given in Table 5
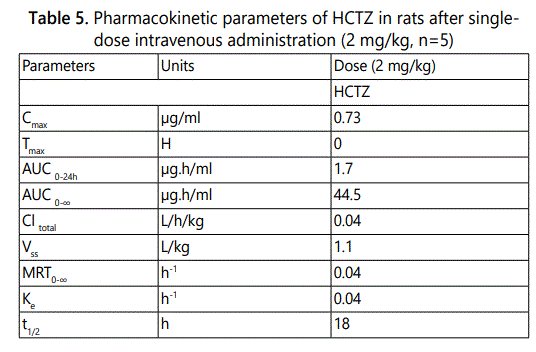
The results in Table 5 showed plasma maximum concentration (Cmax) of 0.73 µg/ml at Tmax of 0 h. The area under the curve at infinity (AUC0→∞) using the logarithm trapezoidal method was 44.5 µg × h/ml.
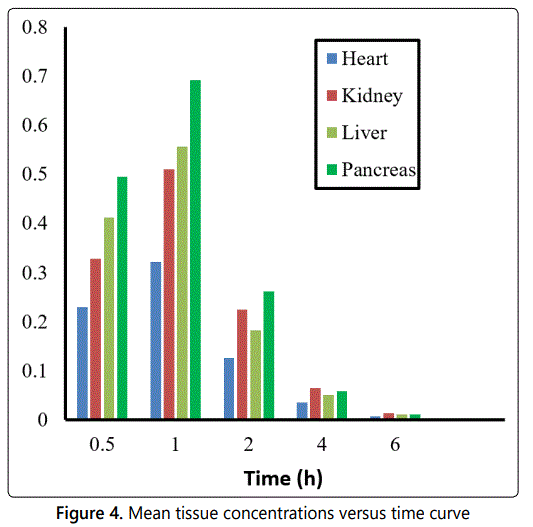
The validated method was also successfully utilized to determine the concentration of HCTZ in rat tissues namely heart, kidney, liver and pancreas following intravenous administration. Prior to sacrificing the rats and harvesting the tissues, blood samples taken from the rats were obtained at intervals of 0.5, 1.0, 2.0, 6.0 h and HCTZ concentrations determined as previously described. The plasma maximum concentrations obtained were 0.32 (RSD = 4.9 %), 0.57(RSD = 2.7 %), 0.53(RSD = 8.5 %), 0.69 (RSD = 2.5 %) as given in Table 6. The concentration levels of HCTZ obtained for the analyzed tissues namely heart, kidney, liver and pancreas were 0.37 (RSD = 4.3 %), 0.55 (RSD = 2.8 %), 0.51 (RSD = 3.4 %), and 0.69 (RSD = 1.1 %) µg/g respectively. The area under the curve at infinity AUC0–∞ for tissues namely heart, kidney, liver and pancreas was 0.74, 1.22, 1.14, 1.42 µg × h/ml respectively. The result data are presented in Table 6. The plot of mean tissue concentration of HCTZ versus time curve is given in Figure 4.
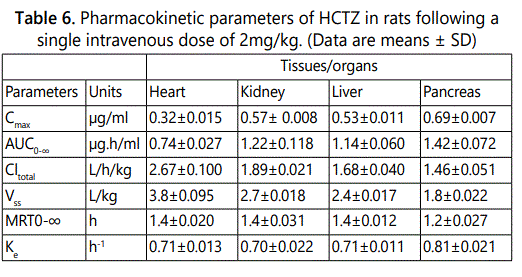
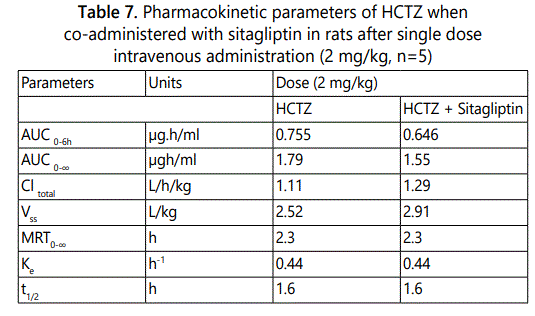
Furthermore, we investigated the effect of sitagliptin on pharmacokinetic and biodistribution of HCTZ after co-administration of both drugs at a single dose of 2 mg/kg per drug through the intravenous route. We utilized two tissues of interest namely kidney and pancreas. Both tissues tend to have higher accumulations of HCTZ when compared to the other tissues studied. Furthermore, they are tissues of interest in terms of the clinical applications of both drugs. Blood and tissue samples were obtained at intervals of 0.5, 1.0, 2.0, 6.0 h respectively. The pharmacokinetics results are given in Table 7. The study observed that at 4h after intravenous co-administration of both drugs to the rat, the Cmax of HCTZ in the kidney was 0.0286 µg/g (compared to 0.0651µg/g when HCTZ was given alone). Likewise, the Cmax of HCTZ in the pancreas was 0.0430 µg/g (compared to 0.0578 µg/g when HCTZ was given alone). Therefore, the obtained data indicated that sitagliptin decreased the maximum tissue concentrations of HCTZ in the kidney and pancreas respectively. The maximum plasma concentrations were similarly decreased.
Discussion
Theoretically, the rate of tissue uptake of a drug in nonsteady-state conditions can be calculated using equation 1:
dCT/dt = k/VT (fPCP - fTCT --------------- Equation 1, where CP and CT are the plasma and tissue concentrations respectively, fP and fT are the free fractions of the drug in plasma and tissue respectively, k is the intercompartmental transfer rate constant, VT is the distribution volume of the tissue compartment.
The equation assumes that drug transfer between plasma and tissue is due only to passive processes, and that only the free drug fraction can be transferred [11]. In order to obtain the partition coefficient of the drug in a specific tissue, Equation 1 can be simplified to equation 2:
dCT/dt = K (PCP - CT) --------------- Equation 2, where P is the partition coefficient of the drug in a specific tissue. It is defined as the tissue-to-plasma concentration ratio (CT/CP) at steady state or the free fraction ratio (fP/fT) of the drug, K is a constant defined as:
K = kfT/VfT
The mean correlation coefficients of the calibration curves of HCTZ were 0.9989, 0.9997, 0.9908, 0.9978 and 0.9990 for plasma, heart, kidney, liver and pancreas, respectively indicating the linearity of the curves.
The validation method was performed in accordance with US Food and Drug Administration Agency and European Medicine Agency (EMA) guidelines on bioanalytical method validation. Linearity, lower limit of quantitation, inter-day and intra-day precision and accuracy, matrix effect, recovery and stability of the HCTZ were found to be satisfactory. The selectivity and specificity evaluation showed no interference from the endogenous substances or metabolites.
The validation results demonstrated that the method is accurate, reproducible, sensitive, selectivity and specific for determination of HCTZ in rat plasma and tissues. The order of maximum plasma concentration of HCTZ for the tissues was pancreas > kidney > liver > heart in the pharmacokinetic study. In the biodistribution investigation, the order of penetration of HCTZ into tissues was pancreas > kidney > liver > heart.
The pharmacokinetics of HCTZ when co-administered with sitagliptin in rats demonstrated that the co=administration did not significantly change the pharmacokinetic behavior of HCTZ both in plasma and in specific tissues. These changes involved minimal increase in the volume of distribution, total clearance, a decrease in the area under the curve of plasma concentrations versus time of the co-administered drugs in comparison with the HCTZ alone.
The tissue biodistribution of HCTZ showed a lesser accumulation of the HCTZ in the two tissues investigated when co-administered with sitagliptin. Sitagliptin gave 2.2-fold decrease in the concentration of HCTZ in kidney and 1.3-fold decrease in the concentration of HCTZ in pancreas respectively.
Conclusion
A sensitive and simple HPLC method for the determination of HCTZ in rat plasma and tissues was developed and validated. The HPLC method was successfully applied to biodistribution and pharmacokinetic study of HCTZ after intravenous administration of single dosage (2 mg/kg) to rats. Moreover, the method allowed for the determination of effect of sitagliptin on the biodistribution and pharmacokinetic characteristics of HCTZ involving the co-administration of single dose of each drug. In general, the results show that the analytical method is suitable for the determination of concentrations of HCTZ in biological matrices.
References
- Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010; 62(3): 284-304. doi: 10.1016/j.addr.2009.11.002
- Ali SS, Sharma PK, Garg VK, Singh AV, Mondal SC. The target-specific transporter and current status of diuretics as antihypertensive. Fundam Clin Pharmacol. 2012; 26(2): 175-179. doi: 10.1111/j.1472-8206.2011.01012.x
- Ecelbarger CA, Tiwari S. Sodium transporters in the distal nephron and disease implications. Curr Hypertens Rep. 2006; 8:158-165. doi: 10.1007/s11906-006-0013-z
- Barbhaiya RH, Craig WA, Corrick-West HP, Welling PG. Pharmacokinetics of hydrochlorothiazide in fasted and nonfasted subjects: a comparison of plasma level and urinary excretion methods. Journal of Pharmaceutical Sciences. 1982; 71(2): 245-248. doi: 10.1002/jps.2600710226
- Shah S, Khatri I, Freis ED. Mechanism of antihypertensive effect of thiazide diuretics. Am Heart J. 1978; 95: 611-618. doi: 10.1016/0002-8703(78)90303-4
- Gallwitz B. Sitaglipitin with metformin: Profile of a combination for treatment of type- 2 diabetes. Drugs Today. 2007; 43: 681-689. doi: 10.1358/dot.2007.43.10.1136901
- FDA. Guidance for Industry, Bioanalytical Method Validation. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). 2001.
- Yamaoka K, Nakagawa T, Uno T. Statistical moments in pharmacokinetics. J Pharmacokinet Biopharm. 1978; 6(6): 547-558. doi: 10.1007/BF01062109
- McNamara PJ, Fleishaker JC, Hayden TL. Mean residence time in peripheral tissue. J Pharmacokinet Biopharm. 1987; 15: 439-450. doi: 10.1007/BF01066523
- Karol MD, Veng-Pedersen P, Brashear RE. Diffusion and flow transfer of theophylline across the blood-brain barrier: pharmacokinetic analysis. J Pharmacokinet Biopharm. 1983; 11(3): 273-287. doi: 10.1007/BF01061868


