Research Article
Dorzolamide in situ Gel Forming System: Characterization and Evaluation for Glaucoma Treatment
1Department of Pharmaceutics, Delhi Institute of Pharmaceutical Sciences and Research, (Formerly College of Pharmacy), University of Delhi, Pushp Vihar, Sector III, New Delhi-110017, India
2Amneal Pharmaceuticals, Piscataway, NJ 08854, USA
*Corresponding author: Sameer Sachdeva, Research Scientist, Amneal Pharmaceuticals, Piscataway NJ 08854, USA, Tel: 3123153194, E-mail: samsach14@gmail.com
Received: May 2, 2017 Accepted: June 23, 2017 Published: June 30, 2017
Citation: Kataria P, Katara R, Sahoo PK, Sachdeva S. Dorzolamide in situ Gel Forming System: Characterization and Evaluation for Glaucoma Treatment. Madridge J Pharm Res. 2017; 1(1): 13-21. doi: 10.18689/mjpr-1000103
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The objective of the present study was to develop an efficient in situ gel forming system of Dorzolamide for the treatment of glaucoma. The in situ gel was formulated using alginate and hydroxyl propyl methyl cellulose (HPMC) polymer combination. The developed formulation was characterized for various in vitro parameters e.g. drug content, pH, isotonicity, surface tension, rheological behavior, drug release profile and transcorneal permeation. The rheological behavior of all formulations was not affected by the incorporation of the drug. The bioadhesive strength of the developed formulation was found to be 0.311 Nmm. The optimized formulation was stable and non-irritant to rabbit eyes. In vitro drug release was found to be 91.27 ± 0.61 % over a period of 10 hours. The best-fit kinetic model (Korsmeyer model) for the optimized formulation DIG 8 suggests that the drug release via Fickian-diffusion mechanism. A maximum mean difference of 27.4% in lowering intra ocular pressure was observed between control and treated eyes with optimized formulation after water loading (p<0.01). The in situ alginate/HPMC gel forming system could be a potential approach for Dorzolamide formulation to enhance ocular bioavailability for the treatment of glaucoma, ultimately improving the patient compliance.
Keywords: In Situ Gel; Dorzolamide; Alginate; Intraocular pressure; Ocular delivery.
Introduction
Medications for ocular diseases are often administered topically to attain a higher concentration at the local site and to avoid any systemic side effects. Ocular diseases like conjunctivitis, uveitis and glaucoma are generally treated with solutions, suspension and semisolids like ointments and gels [1-3]. Conventional liquid ophthalmic formulations results in poor bioavailability and decreased therapeutic response due to high tear turn over and constant lachrymal drainage in the eye [4].
The constant tear drainage sweeps away a large portion of the applied drug solution via nasolachrymal duct to the gastro-intestinal tract from where it may be absorbed systemically, causing untoward side effects [2].Moreover, conventional dosing shows an initial peak level of the drug, which may be usually several folds higher than the required therapeutic level, imparting the reason for toxicity again.
Other preparations like ointments, suspensions, inserts and aqueous gels are capable to extend the pre-corneal residence time and slow down the drug elimination form eye. Although these ocular systems offer better retention over the conventional liquid dosage form but due to drawbacks like blurred vision caused by ointments or lack of patient compliance associated with inserts; they have not been widely accepted [5,6].
From the patient's view point, ideal dosage form is one which could sustain the drug release and retain the formulation with the corneal surface without causing the discomfort. Enhancement of local bioavailability, reduced dosing frequency and better patient compliance can be achieved if the precorneal residence time of a drug could be extended by selection of appropriate vehicle. This can be achieved by using in situ gel forming system for ocular delivery. These systems have the tendency to exhibit the sol to gel phase transition by the change in a specific microenvironment or physicochemical parameter in the cul-de-sac. The key advantage of in situ gel formulation is the possibility of administering accurate and reproducible doses promoting pre-corneal retention.
Depending on the method employed for the the sol-gel phase transition on the ocular surface, three types of systems have been used; pH-induced, [4,7,8] temperature-sensitive [9-15] and ion-triggered systems including alginate and gellan gum [16-19] and carbopol/pluronic [20].
Glaucoma is a progressive optic neuropathy affecting millions of people in the world and is a major cause for irreversible blindness. One of the main risk factors involved in the progression of such disease is elevated intraocular pressure (IOP). Elevated IOP can result in retinal ganglion cell damage and optic nerve atrophy leading to irreversible loss of vision. It is believed that glaucoma is a result of an imbalance between aqueous humor secretion and its drainage within the ocular chamber [21].
Dorzolamide hydrochloride {4-(Ethyl amino)-5, 6-dihydro6-methyl-4H-thieno [2, 3b] thiopyran-2- sulphonamide 7, 7-dioxide mono hydrochloride} is a carbonic anhydrase inhibitor which is soluble in water. Dorzolamide hydrochloride lowers IOP via blocking active secretion of sodium and bicarbonate ions from the ciliary body to the aqueous humor [22]. Little work has been done on evaluating Dorzolamide hydrochloride in vivo ocular antihypertensive activity. The prepared formulations were characterized and evaluated for their in vitro as well as in vivo performance. The objective of the present investigation was to develop alginate and hydroxyl propyl methyl cellulose (HPMC) based in situ gel forming system of Dorzolamide for the treatment of glaucoma.
Materials and Methods
Materials
Dorzolamide hydrochloride was provided as a gift sample by Hetero Drugs Ltd. (Hyderabad, India). Sodium alginate was purchased from Sigma Aldrich (USA). HPMC (Methocel E50LV) was kindly gifted by Ranbaxy Laboratories (Gurgaon, India). All other reagents were of analytical grade and were used as received.
Methods
Preparation of in situ Gel of Dorzolamide hydrochloride
Aqueous solutions of varying concentrations of alginate and HPMC were prepared [5]. Polymers were accurately weighed and dissolved in distilled water in 100 mL beaker under stirring. Dorzolamide (2%) was dissolved in distilled water (25 mL) containing Benzalkonium chloride (0.01%v/v) as preservative and mannitol as the tonicity modifier. The drug solution was then mixed with polymer solution and volume was made up to 100 mL. Finally, the pH of the polymer solutions were adjusted to 5.6 using HCl (0.1 N) and NaOH (0.5 M).
Drug Polymer Interaction Study
The drug excipient interaction studies were carried out before preparing the formulation. The solution of alginate, HPMC and Dorzolamide were prepared individually and in combination and were autoclaved. The UV spectra were taken before and after autoclaving using double beam UV-spectrophotometer (Hitachi, India) at 254 nm. The DSC and FT-IR spectra of individual polymer or in combination were also performed to identify the drug-excipient interaction.
Differential Scanning Calorimerty
Endothermic peak of the drug was observed using DSC scan. In the sample pan, sample (drug, alginate, HPMC, alginate/HPMC physical mixture) was placed on one side and on the other side, blank pan was placed as a reference. The sample was heated between 40-300°C at the rate of 10°C/minute using DSC (Shimadzu TA-60, Japan). Nitrogen gas was introduced at the flow rate of 50 mL/minute maintaining a pressure of 2 bars.
Fourier Transform Infrared Spectrum Analysis
FT-IR spectral analysis were carried out using FT-IR spectrophotometer (Perkin Elmer, BX2, India). The drug (5 mg) and samples (drug, alginate, HPMC, alginate/HPMC physical mixture) were weighed and thoroughly triturated with 95 mg of KBr. A pellet prepared out of the mixture was introduced into the instrument in pellet sampling compartment. The spectrum was recorded between 400- 4000 cm-1.
Physicochemical Evaluation of Dorzolamide Ophthalmic In Situ Gel Formulation
Clarity, pH, gelation studies, surface tension and drug content were evaluated. The general appearance of the formulation was observed by visual inspection in front of black and white background. The pH was measured using a pH meter (Hanna instruments). Isotonicity testing was done by placing the mixture of blood and diluted prepared formulation using Neubauer's counting chamber [23] and observing the RBCs under the microscope. The drug content was determined by diluting 1 mL of the formulation up to 100 mL with simulated tear fluid and analyzed using UV-Visible spectrophotometer at 254 nm. The gelling capacity was determined by placing 100 μL of the formulation in a vial containing 2 mL of freshly prepared simulated tear fluid. The system was equilibrated at 35°C throughout the experimentation. Gelation was visually assessed by recording the time for gel formation and the time for the formed gel to deform. The surface tension of the prepared instilled solutions was determined using stalagmometer.
Rheological studies
The viscosities of prepared formulations were measured by using Rheometer (Rheolac QC, India). The prepared solution was allowed to form in situ gel in the simulated tear fluid and then the viscosity was measured. The viscosity of samples was measured at different angular velocities. A typical run comprised of angular velocity ranging from 10 to 100 rpm with equal wait for each rpm. After waiting 6 seconds at 10 rpm, the velocity was increased to 100 rpm with a same hold time at each speed. Then the hierarchy of angular velocity was reversed (100-10 rpm) with similar waiting period. The average of two readings was then used to calculate the viscosity. To evaluate viscosity change after instillation and mixing with simulated tear fluid, rheological measurements were taken after diluting the formulations with simulated tear fluid. The viscosities of the formulations were also measured after addition of the drug.
In vitro drug release studies
A 2 mL aliquot of the formulation was taken in the dialysis tube (Sigma Chemicals), which was suspended in a beaker at 37 ± 0.5°C containing 100 mL simulated tear fluid (pH 7.4) under continuous stirring. Aliquots of medium were withdrawn at different time intervals and equal volumes of fresh media were added to replace the withdrawn samples. Withdrawn samples were filtered, diluted appropriately, and were analyzed for the drug content by UV-Visible spectrophotometer at 254 nm. Cumulative percent drug released was then calculated.
Permeation Studies
In vitro drug permeation studies were carried out by placing in situ gel formulations on freshly excised goat cornea, fixed between donor and receptor compartment of an all glass modified Franz diffusion cell having surface area of 3.91 cm2 . Due to limited viability of cornea the study was carried out for only 4 hours. In order to simulate the tear flow, the donor compartment was infused with simulated tear fluid, pH 7.4, at a flow rate of 20 μL/minute throughout the study. The percentage of drug drainage from the formulation was determined using UV-spectrophotometer at regular interval by collecting the drained sample. Similarly, 1 mL sample was withdrawn at regular interval from receptor compartment (containing 15 mL simulated tear fluid, pH 7.4, under stirring at 37°C) and permeated amount of drug was determined. The volume was replenished with the same amount of fresh medium each time to maintain the sink condition.
At the end of the experiment; each cornea (freed from adhering sclera) was weighed, soaked in 1 mL methanol, dried overnight at 90°C, and reweighed. From the difference in weights, corneal hydration was calculated. Similarly, the permeation studies were also carried out by placing the marketed formulation of Dorzolamide to compare the release profile with the optimized formulation.
Bio-adhesion study
The adhesion study of formulation DIG 8 after gel formation was measured using the apparatus as illustrated by Agrwal and Mishra [24]. The apparatus consisted of two circular aluminium discs (3 cm diameter). One disc was allowed to hang on an iron stand fastened with a hook provided on the back side of the disc. The other disc was connected to a pre-weighed lightweight plastic bag using a hook attached on its back. The in situ gel was placed between the two discs, which were then kept under constant pressure for 5 minutes (preload time) to initiate adhesion bond. Afterwards, water was added to the plastic bag through an intravenous infusion at a set rate of 1 drop/minute until the two adhered discs detached from each other. The water collected in the bag was weighed. The weights of collected water were converted into force required for detachment.
Ocular Irritation Study
The study was carried out in compliance with guidelines compiled by CPCSEA (Committee for the purpose of control and supervision of experiments on animals), Ministry of culture, Government of India. All the study protocols were approved by local Institutional Animals Ethics Committee (IAEC). Animals were housed under standard laboratory conditions with 12 hours cycles of light and dark (light from 6 a.m. to 6 p.m.); given a standard pellet diet, vegetables and water ad libitum.
In vivo ocular irritation studies for optimized formulation were performed on 6 New Zealand white rabbits, each weighing 1.5-2.0 kg. The sterile optimized formulation DIG 8 (50 μL) was applied into the left eye of the rabbit. The right eye, treated with blank polymer solution, served as control. To prevent loss of material, the upper and lower lids were gently held together for 5 seconds. The formulations were instilled thrice a day for a period of 21 days, and the rabbits were observed periodically for redness, swelling, watering of the eye. Irritation score was determined according to Draize technique [25].
Pharmacodynamic Studies
The IOP lowering activity of the optimized formulation (Formulation DIG 8) was carried out in normotensive and hypertensive rabbit (water loading) model using Non-Contact Tonometer (NCT) Nidek-3000, Japan. In water loading model, IOP elevation was achieved in unanaesthetized rabbits. [26] The animals were subjected to forced ingestion of water (70 mL/kg of water) through an intragastric infant feeding tube leading to rise in IOP lasting for 2 hours.
Normotensive rabbits were randomly divided into 2 groups comprised of 3 rabbits each. On the day of experiment baseline IOP was estimated. The rabbits in first group were instilled with 50 μL of the formulation in one of the randomly chosen eye while the other eye was treated with 50 μL of the vehicle (solution without drug). Second group received 50 μL of marketed formulation (DORZOX eye drops) in one of the eye while the other eye received an equal volume of saline as in group 1. The IOP was measured at regular intervals for a period of 8 hours.
For evaluation in water loading model, rabbits were divided into two groups of 3 animals each. The baseline IOP was estimated after overnight fasting. The first group was instilled with 50 μL of marketed formulation (DORZOX eye drops) in one of the randomly chosen eye and 50 μL of saline in contralateral eye. The second group received 50 μL of formulation DIG 8 in one of the randomly selected eye and same volume of vehicle in the other eye. The rabbits were administered with water (70 mL/kg) through an intragastric infant feeding tube 1 hour after the drug/vehicle instillation. This was followed by IOP measurements at an interval of 15 minutes for period of 120 minutes. The change in IOP (ΔIOP) was determined according to the following equation:
ΔIOP = IOPTreated eye- IOPControl eye
ΔIOP is reported as the mean ± SD (mmHg) for n=3 at various time intervals.
Stability Studies
To observe the effect of storage conditions on efficiency of optimized formulation, the stability study was carried out by keeping the sample at varying temperatures and humidity conditions. The optimized formulation was sterilized in an autoclave at 121°C for 15 minutes and kept for stability studies at accelerated temperature (40°C ± 2°C/75% R.H.), room temperature (25°C ± 2°C/60% R.H.) and refrigerated condition (4 ± 1°C) as per ICH guidelines. The samples were evaluated for various parameters i.e. appearance/clarity, pH, gelling capacity, drug content at regular interval for a period of 6 months. The logarithm of percentage drug remaining were calculated and plotted against time in days. The degradation rate constant was determined using slope of this curve.
Statistical Analysis
The data were subjected to multiple regression analysis using GraphPad Prism Software 3.0. The statistical methods involving analysis of variance (ANOVA) and t-test were applied to analyze the data. A p value < 0.05 was considered as indicative of significance.
Results and discussion
Drug-excipient Interaction Study
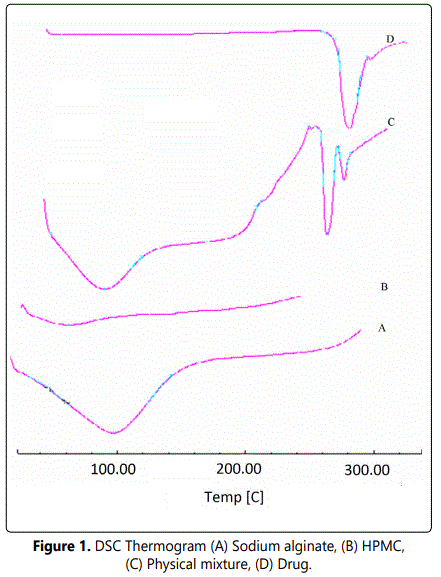
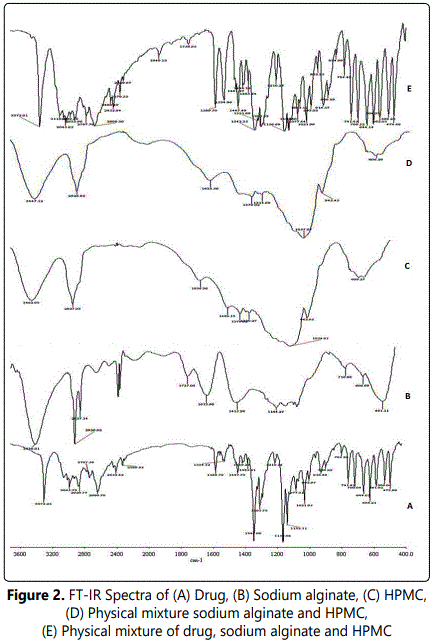
Studies were carried out to ascertain any kind of interaction of the drug with the excipients used in the formulation. For this purpose formulation and polymers were analyzed by UV, DSC and FT-IR. No interaction of the excipients with drug was found in UV analysis which is confirmed by the absence of shift in the λmax of the drug. The absence of drug-excipient interactions were also confirmed by DSC and FT-IR study. In DSC, Dorzolamide endotherm was observed at 276°C and no shift in the endotherm of Dorzolamide was observed in the physical mixture (drug + polymer) as shown in figure 1. Resemblance of spectra of Dorzolamide as such and in physical mixture displays the absence of any significant interaction in FTIR studies as shown in figure 2.
Physicochemical Evaluation of Dorzolamide Ophthalmic In Situ Gel Formulation
Physicochemical characteristics such as appearance, drug content and pH of the formulations were studied and found to be satisfactory as shown in the table 1. The formulations were found to be clear at room temperature and no foreign particulate matters were observed. A slight haziness was observed after autoclaving which could be due to precipitation of HPMC at elevated temperature and it disappeared after cooling.
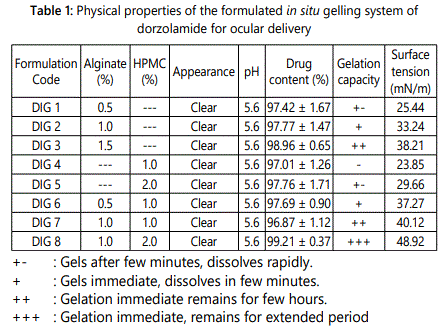
An important aspect of ophthalmic in situ gel forming formulation is that it should have all the characteristics such as pH, surface tension and isotonicity similar to the microenvironment of lachrymal fluid. The selection of formulation pH was based on higher solubility and greater stability of Dorzolamide at pH of 5.6 [22]. The drug was found to be uniformly distributed in formulation. Formulation DIG 7 and DIG 8 were found to have the surface tension value of 40.12 mN/m and 48.92 mN/m which is close to lachrymal secretion as shown in table 1. This conferred better spreading characteristics of the formulation onto the ocular surface.
Isotonicity testing of the optimized formulation exhibited no change in the shape of red blood cells (bulging or shrinkage) which endorsed the isotonic nature of developed formulation (data not shown). The prepared in situ gel formed a translucent matrix on addition to the simulated tear fluid warmed to 37± 2°C. The gelling capacity was expressed as the time taken by the gel to remain intact in tear fluid before erosion takes place which is shown in table 1. Minimum gelling capacity was exhibited by formulations containing HPMC (DIG 4 and DIG 5). This may be attributed to failure of the polymer to bind with cations [5]. In contrast, formulations based on combination of sodium alginate and HPMC (DIG 7 and DIG 8) exhibited the maximum gelling capacity as compared with formulations having only one polymer (DIG 2-DIG 5). As the formulation is exposed to simulated tear fluid, gelation occurs due to ionic cross-linking of the alginate chains by the divalent cation forming the egg-box model [19]. The formation of a stable gel was obtained immediately which is in accordance to the short gelation time of the delivery system upon instillation in the eye.
Rheological Studies
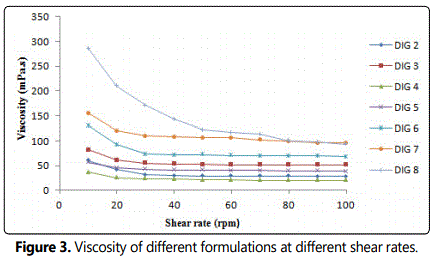
The formulation should have an optimum viscosity that will allow easy instillation into the eye and undergo a rapid sol-gel transition. The viscosity of polymer solutions at different concentrations were measured at varying shear rates for the relevance of administration by dropping. The formulation containing 1% alginate did not show sufficient viscosity to withstand the shear force generated during blinking for prolonged time. However, 2% alginate solution might pose a problem in vision and instillation into the eye due to higher consistency. Therefore, a feasible approach to decrease the amount of alginate required for gelation by incorporating HPMC in the preparation was used.
A concentration of 1.0% alginate and 2.0% HPMC was selected as it showed satisfactory attributes of viscosity and gelling capacity. The formulations (with and without drug) exhibited pseudoplastic rheology as evidenced by thixotropic behavior and a decrease in the viscosity with increase in angular velocity. This rheological behavior could be beneficial as the blinking rate is inversely proportional to the viscosity of the formulation. Therefore, the prepared formulation will have minimum effect on normal physiology of the eye.
The viscosity of all the prepared formulations was in the order DIG 8 >DIG 7 >DIG 6≈ DIG 5 >DIG 3>DIG 4 as shown in figure 3.
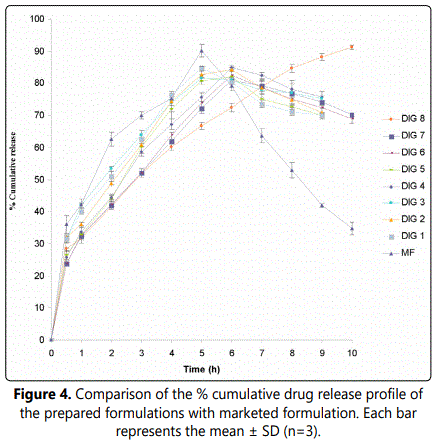
In Vitro Release Studies
The comparative release profile of drug from in situ gel and marketed formulation (DORZOX eye drops) is shown in Figure 4. Almost 92% of the entire drug released into the medium after 5 hours from marketed formulation referred as MF (DORZOX eye drops) and the release rate declined thereafter. The formulation DIG 8 exhibited significantly sustained release pattern as 31.63% of the drug was released after 1 hour and approximately 72.0% of Dorzolamide released into the medium after 6 hour with continued release. Initially fast release could be due to the drug adsorbed on the surface of prehydrated swollen matrix of polymers. When this formulation comes in contact with simulated tear fluid, it converts into transparent gel which releases the drug in a sustained manner via diffusion [20]. It was observed that HPMC and alginate alone could not retard the release rate sufficiently for extended period of time while alginate along with HPMC could sustain the release for longer time. The drug release was statistically significant from the formulation DIG 8 as compared with alginate (DIG2) and HPMC (DIG 5) with p value of <0.0001, =0.0018 and <0.0001 at first, sixth and ninth hour of the release period, respectively. The drug release was statistically significant in case of drug release from DIG 8 was compared with the marketed formulation with p value of <0.0001, = 0.0010 and <0.0001 at first, sixth and tenth hours, respectively.
The release data were subjected to different kinetic models (zero-order, first-order, and Higuchi diffusion model) to determine the mechanism of drug release from the prepared in situ gel formulation. The release constant was calculated from the slope of the appropriate plots and the regression coefficient was determined. It was found that the in vitro drug release was best explained by Higuchi equation as the plots showed the highest linearity (r2 =0.9932). The data were also fitted according to well known Korsmeyer equation. A linear relationship with a regression coefficient of 0.9951 and predicted n value (0.4719) expressed the predominant drug release mechanism to be Fickian diffusion [27].
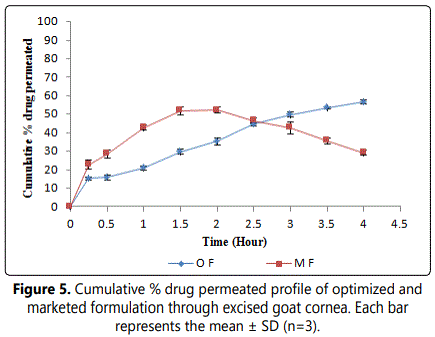
Transcorneal Permeation Study
To mimic the real life condition, permeation studies were conducted with freshly excised goat cornea and the results are depicted in figure 5. Considering the viability of cornea the experiment was conducted for 4 hours and the drug permeation from formulation ranged between 49.0-50.0%. The corneal hydration level with each experiment was found to be below 80% indicating that cornea is kept intact while applying the formulation.
Ideally, an in situ gelling delivery system should be a free flowing liquid to allow easy instillation into the eye as eye drops. However, it has been reported that a prolonged precorneal residence of the formulation containing the in situ gelling polymer, alginic acid, is not only because of its ability to gel in the eye but also due to its mucoadhesive properties [28]. Thus such vehicles will not be susceptible to immediate drainage from the eye surface and will have prolonged residence time in contrast to conventional ophthalmic formulation i.e. solutions. Now if we compare the drug drainage rate through excised goat cornea we noticed that percentage drug drained was higher in marketed formulation (DORZOX eye drops) than optimized formulation. The earlier studies on in situ gelling system reinforce the fact that these formulations cleared at a slow rate and remained at corneal surface for longer time duration [29]. These results suggest that under in vivo condition quite a substantial quantity of drug from marketed formulation may be drained into the nasolachrymal duct and gain entry into systemic circulation causing adverse effect.
Bioadhesive strength
The bioadhesive strength of the developed formulation was found to be 0.311 Nmm. The combination of alginate and HPMC polymers resulted into the highest bioadhesive strength among the prepared formulations. Various mechanisms have been proposed to explain the in vitro bioadhesion phenomena. These included electrical double layers, electrostatic attractions, hydrogen bonding, Van derwaals force, hydrophobic bonding, wetting, diffusion interpenetration, physical entanglements and surface free energy [30]. Both alginate and HPMC being polyanionic hydrophilic polymers containing carboxylic groups adhere to mucosal surface as they attract water from the mucus gel layer adherent to the ocular surface. Incorporation of HPMC increased the surface charge density of the formulation and hence, enhanced the mucoadhesion tendency. Moreover, the carboxylic groups in HPMC can form hydrogen bonds with tissue, imparting better bioadhesion. The possible mechanism behind the slower drainage rate of the drug from in situ gelling formulation may be due to bioadhesive nature of the polymers which is in agreement with previously published data [29].
Ocular Irritation Test
The tolerability of the optimized formulation was performed as per the ocular irritation test described by Draize et al. For the polymer system (with and without drug) the average irritation scores were zero. No ocular damage (in terms of swelling, redness and watering of the eye) or abnormal clinical signs to the cornea, iris or conjunctiva were visible. The results of the ocular irritation studies indicated that the optimized formulation showed an excellent ocular tolerance.
Pharmacodynamic Evaluation
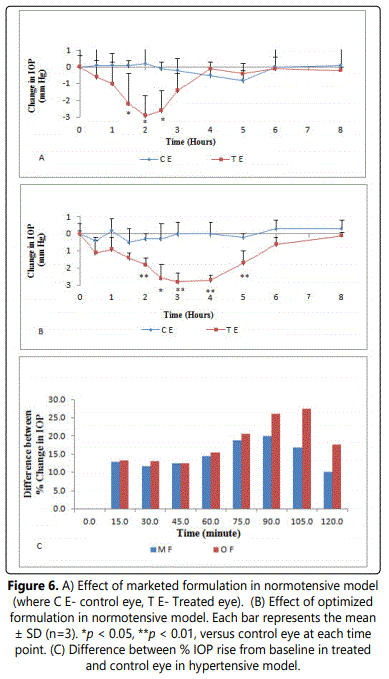
The pharmacodynamic study was carried out to examine and compare the effect of the optimized and the marketed formulation (DORZOX eye drops- referred as MF) in the in vivo IOP lowering efficacy. The effects of both the formulations were studied in normotensive and water loading model. In normotensive rabbits which were treated with optimized formulation (DIG 8- referred as OF), the IOP declined gradually and the mean percent IOP reduction in drug treated eye was 23.1% from baseline as compared to 1.3% in vehicle treated control eye as displayed in figure 6. The maximum mean IOP reduction in normotensive rabbit eyes treated with marketed formulation (MF) was 18.3% from baseline as compared to 2% in vehicle treated control eye as shown in figure 6.
The optimized formulation significantly extended the duration of IOP lowering effect of the drug (5 hour), instead of marketed formulation (3 hour). The reason for extended duration of pharmacodynamic effect could be the enhanced precorneal residence time of the optimized formulation. This could lead to a reduction in the number of Dorzolamide application, contributing to improved patient compliance and reduced side effects.
The unilateral treatment in hypertensive model with DIG 8 and DORZOX eye drops resulted in significant protection against the rise in IOP in response to water loading. The rise in IOP with DIG 8 treated eyes was significantly less when compared with the vehicle treated eyes from 15 minutes to 105 minutes after water loading (p<0.01). A maximum mean difference of 27.4% in IOP rise was observed between control and treated eyes in DIG 8 treated group at 105 minutes after water loading (p<0.01) with a statistically significant difference persisting throughout the experimental period.
In DORZOX treated group a maximum mean difference of 20% in IOP rise was observed between control and treated eyes at 90 minutes after water loading (p<0.001) as shown in figure 6. Though, a significantly higher protection against the rise in IOP was observed in marketed formulation treated group during first 30 minutes post water loading as compared to DIG 8 treated group (p<0.01). But a significantly sustained and delayed peak IOP lowering effect for up to 105 minutes (water loaded model) was found in case of optimized formulation. Similar patterns of IOP lowering by the formulations were observed in normotensive and water loading model. However, the marketed formulation demonstrated a rapid onset of action at earlier stage but these effects remain only for a shorter period of time as compared with optimized formulation.
These findings were also confirmed by earlier studies performed on pilocarpine in situ gel formulation on normotensive model [19]. The formulation so prepared may avoid the repeated administration of the dosage form along with reduced side effects and improved patient compliance. We presume that the optimized formulation perhaps favour the better ocular bioavailability than marketed formulation by conferring a better therapeutic effect because of longer precorneal residence time
Stability Studies
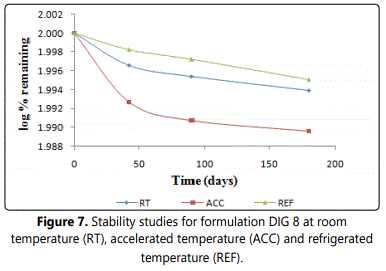
Optimized formulation (DIG 8) was subjected to the accelerated stability studies for six months as per the ICH guidelines. Degradation rate constants were found to be 3.83 × 10-4, 1.64 × 10-4 and 1.09 × 10-4 days-1 for accelerated, room and refrigerated condition respectively as shown in figure 7. Least amount of degradation was observed when the formulation was stored in a stored in a refrigerated condition. Overall percentage drug degradation would be less than 5%, hence tentative shelf life of 2 years may be assigned to the formulation.
Conclusions
In conclusion, we suggest that in situ gel-forming ophthalmic solutions could be considered as potential approach as Dorzolamide formulation. This in situ gel forming formulation of Dorzolamide was able to enhance bioavailability and significantly lower the IOP through its sustained drug release and longer precorneal residence time as compared with the marketed formulation. The in situ gel formulation appears to be well adhered and non-irritating to the rabbit eye. We believe that Dorzolamide in situ formulation could be a better approach in the treatment of glaucoma than conventional aqueous eye drops.
Conflict of Interest
Authors declare that they have no conflict of interest.
Animal studies
All institutional and national guidelines for the care and use of laboratory animals were followed.
Acknowledgments
Authors are thankful to the AICTE, Delhi for providing financial assistance to Puneet Kataria.
References
- Jain SP, Sejal PS, Rajadhyaksha NS, Singh PS PP. In situ ophthalmic gel of ciprofloxacin hydrochloride for once a day sustained delivery. Drug Dev Ind Pharm. 2008; 34: 445-452. doi: 10.1080/03639040701831710
- Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for Dorzolamide hydrochloride. AAPS PharmSciTech. 2009; 10(3): 808-819. doi: 10.1208/s12249-009-9268-4
- Gaudana R, Jwala J, Sai HB, Mitra AK. Recent perspectives in ocular drug delivery. Pharmaceutical Research. 2009; 26(5): 1197-1216. doi:10.1007/s11095-008-9694-0
- Srividya B, Rita MC, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J Control Rel. 2001; 73(2-3): 205-211. doi: 10.1016/S0168-3659(01)00279-6
- Liu Z, Li J, Nie S, et al. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm. 2006; 315(1-2): 12-17. doi: 10.1016/j.ijpharm.2006.01.029
- Liu Y, Liu J, Zhang X, Zhang R, Huang Y, et al. In situ gelling gelrite/alginate formulations as vehicles for ophthalmic drug delivery. AAPS Pharm Sci Tech. 2010; 11(2): 610-620. doi: 10.1208/s12249-010-9413-0
- Gurny R, Boye T, Ibrahim H. Ocular therapy with nanoparticulate systems for controlled drug delivery. J Control Rel. 1985; 2: 353-361. doi: 10.1016/0168-3659(85)90057-4
- Desai SD, Blanchard J. in vitro evaluation of pluronic F127 based controlled release ocular delivery systems for pilocarpine. J Pharm Sci. 1998; 87(2): 226-230. doi: 10.1021/js970090e
- El-Kamel AH. In vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleate. Int J Pharm. 2002; 242(1): 47-55. doi: 10.1016/S0378-5173(02)00234-X
- Cho KY, Chung TW, Song HH, et al. Release of ciprofloxacin from chondroitin 6-sulfategraft- poloxamer hydrogel in vitro for ophthalmic drug delivery. Drug Dev Ind Pharm. 2005; 31(4-5): 455-463. doi: 10.1080/03639040500214688
- Qi H, Chen W, Huang C, et al. Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int J Pharm. 2007; 337(1-2): 178-187. doi: 10.1016/j.ijpharm.2006.12.038
- Vadnere M, Amidon G, Lendenbaum S, Haslam JL. Thermodynamic studies on the gel-sol transition of some pluronic polyols. Int J Pharm. 1984; 22(2-3): 207-218. doi: 10.1016/0378-5173(84)90022-X
- Rozier A, Mazuel C, Grove J, Plazonnet B. Gelrite®: a novel, ion-activated, in situ gelling polymer for ophthalmic vehicles. Effect on bioavailability of timolol. Int J Pharm. 1989; 57: 163-168. doi: 10.1016/0378-5173(89)90305-0
- Sanzgiri YD, Maschi S, Crescenzi V, et al. Gellan-based systems for ophthalmic sustained delivery of methyl prednisolone. J Control Rel. 1993; 26(3): 195-201. doi: 10.1016/0168-3659(93)90186-9
- Balasubramaniam J, Pandit JK. Ion-activated in situ gelling systems for sustained ophthalmic delivery of Ciprofloxacin hydrochloride. Drug Delivery. 2003; 10(3): 185-191. doi: 10.1080/713840402
- Cohen S, Lobel E, Trevgoda A, Peled Y. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Control Rel. 1997; 44: 201-208. doi: 10.1016/S0168-3659(96)01523-4
- Lin H, Sung KC. Carbopol/ pluronic phase change solutions for ophthalmic drug Delivery. J Control Rel. 2000; 69(3): 379-388. doi: 10.1016/S0168-3659(00)00329-1
- Afouna MI, Khedr A, Abdel-Naim AB, Al-Marzoqi A. Influence of various concentrations of terpene-4-ol enhancer and carbopol-934 mucoadhesive upon the in vitro ocular transport and the in vivo intraocular pressure lowering effects of dorzolamide ophthalmic formulations using albino rabbits. J Pharm Sci. 2010; 99: 119-127. doi: 10.1002/jps.21803
- Agrwal V, Mishra B. Design, development and biopharmaceutical properties of buccoadhesive compacts of pentazocine. Drug Dev Ind Pharm. 1990; 25: 701-709. doi: 10.1081/DDC-100102229
- Gupta SK, Saxena R, Agarwal R, et al. Estimation of intraocular pressure in rabbits using noncontact tonometer: A comparative evaluation with schiotz tonometer. Methods Find Exp Clin Pharmacol. 2007; 29(6): 405-409. doi: 10.1358/mf.2007.29.6.1119161
- Korsmeyer M, Gurny R, Doelker E, Buri P, Peppas N. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983; 15: 25-35. doi: 10.1016/0378-5173(83)90064-9
- Smart JD, Kellaway IW, Worthington HEC. An in vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. J Pharm Pharmacol. 1984; 36(5): 295-299. doi: 10.1111/j.2042-7158.1984.tb04377.x
- Gupta H, Jain S, Mathur R, et al. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Delivery. 2007; 14: 507-515. doi: 10.1080/10717540701606426
- Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: Two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002; 28(4): 353-369. doi: 10.1081/DDC-120002997


