Research Article
Use of Topical Amphotericin-B to Minimise Nephrotoxicity in Sinonasal Mucormycosis
Dept. of Otorhinolaryngology Head and Neck Surgery, Jawaharlal Nehru Medical College, Kaher, Belagavi ,India
*Corresponding author: Chenchulakshmi Vasudevan, Dept. of Otorhinolaryngology Head and Neck Surgery, Jawaharlal Nehru Medical College, Kaher, Belagavi, India, Tel: 9731459325, E-mail: lakshmi9526@gmail.com
Received: May 16, 2022 Accepted: June 29, 2022 Published: July 14, 2022
Citation: : Nayak P, Vasudevan C, Harugop A. Use of Topical Amphotericin-B to Minimise Nephrotoxicity in Sinonasal Mucormycosis. Madridge J Otorhinolaryngol. 2022; 5(1): 81-85. doi: 10.18689/mjol-1000117
Copyright: © 2022 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
In the light of the global pandemic of SARS COV2 and the sudden surge of ROM-Rhinoorbital mucormycosis, immediate management and prognosis of the disease has become a prime matter of discussion among otorhinolaryngologists. The aggressive nature of the disease and the poor immunity of the host make it a herculean task to achieve remission.
Along-side the surgical debridement and administration of intravenous amphotericin, the local and topical administration of the drug to directly tackle the fungal spores has been a debatable yet enticing option as it acts directly at the site of the fungal invasion and bypasses renal clearance and its graver and feared consequences.
In our study we have used a three-pronged approach involving, surgical debridement, intra venous administration as well topical application of Amphotericin B.
Introduction
In the light of the global pandemic of SARS COV2 and the sudden surge of ROM-Rhinoorbital mucormycosis, immediate management and prognosis of the disease has become a prime matter of discussion among otorhinolaryngologists. The aggressive nature of the disease and the poor immunity of the host make it a herculean task to achieve remission.
Mucormycosis is an aggressive and frequently fatal fungal infection; the causative organism is a common inhabitant of the human upper airway. Rhizopus and other members of the order Mucorales usually cause disease in immunocompetent hosts. Diabetics are the most commonly afflicted group, and rhinocerebral mucormycosis is the most common anatomic subclass of disease. Clinical infection usually progresses rapidly, causing death within days of presentation [1,2]. This fungus is angioinvasive and causes thrombosis of vessels, with consequent black necrosis of nasal and sinus tissue [3]. Despite tremendous progress in the treatment of rhinocerebral mucormycosis, it still has a high mortality rate especially in immunocompromised patients. However, conventional treatment can itself cause substantial morbidity.
Along-side the surgical debridement and administration of intravenous amphotericin, the local and topical administration of the drug to directly tackle the fungal spores has been a debatable yet enticing option as it acts directly at the site of the fungal invasion and bypasses renal clearance and its graver and feared consequences.
In our study, at a tertiary care centre, we have used a three-pronged approach involving, surgical debridement, intra venous administration as well topical application of amphotericin B.
Material and Methods
A Retrospective, comparativestudy was done at our tertiary care centre among patients of sinonasal mucormycosis with respect to use of topical amphotericin as an addition to their treatment. Thirty patients diagnosed with sinonasal mucormycosis were included in the study. All the patients were subjected to CT scans of the paranasal sinus and orbit following which they were planned for surgical debridement. The patients with normal renal parameters were administered with injection Amphotericin B preoperatively and all patients received the injection post operatively for a period of minimum 2 weeks up to 4 weeks depending upon when KOH mounts were negative, and also the general condition of the patient.
Intra operatively, following the debridement of the unhealthy mucosa and removal of the necrotic bone, the surrounding healthy tissue was locally injected with Amphotericin B (50mg of the drug was injected at multiple sites of surrounding healthy tissue). Amphotericin B was also used for irrigation of the paranasal sinuses and Amphotericin-B-soaked packs were then placed for nasal packing that were removed on post-operative day 1, after 24 hours.
One group consisted of seventeen patients including those admitted in the general wards and those with a COVID negative status, and stepdown icu patients who received topical amphotericin alongside the mainstay of treatment, while the remaining 13 patients only received IV Amphotericin B. Following post-operative nasal pack removal, saline nasal douching was begun 3 times a day and after each nasal douching amphotericin drops were instilled locally. Repeat DNE was done on Post operative day 7 for all patients and the repeat KOH mounts were taken to look for fungal elements. Further DNEs and repeat debridements were planned as per the need based on the KOH mount reports, clinical the improvement and the general condition.
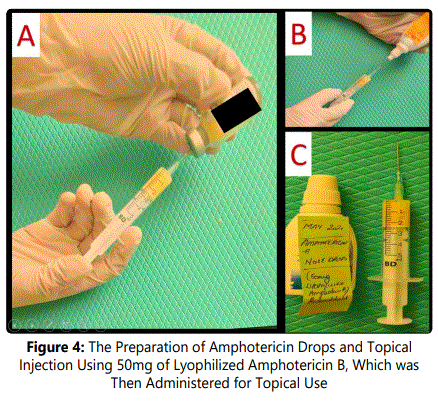
The nasal drops of Amphotericin B were prepared using one reconstituted vial of lyophilized Amphotericin B, which contained 50mg of the drug that was added to 20ml sterile water and used for nasal topical application, this was done 3 times a day, before each such administration, a saline nasal wash was done to ensure removal of crusts and discharge to allow the drug to act on the Sino nasal mucosa and bone that was involved. Shown in Figure 4 is the preparation and administration of Amphotericin B for topical use.
The patients were clearly instructed about performingsaline nasal douching before using these drops, in order to ensure the availability of the amphotericin at the affected site. The nasal wash was done using a bottle with a long nozzle that would enter the nasal cavity and say “kha” to ensure the close of the nasopharynx, and then they performed the nasal douching and the application of the drops under supervision following which self-administration was allowed. After this we observed all patients carefully to look for any adverse effects of this form of administration. The need for multiple surgical debridements, the clinical improvement and the renal parameters and general condition of the patients were then assessed.
Results
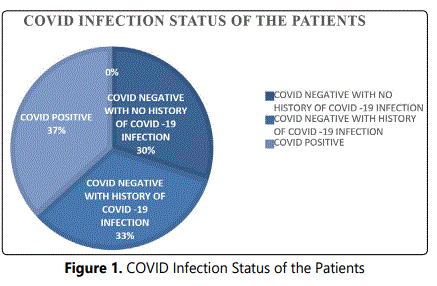
All the 30 patients included in the series were diagnosed to have Sino-nasal mucormycosis based on clinical, radiological features of CT and MRI scans of paranasal sinuses and orbits which and also confirmed by the histopathological report of the tissue samples. In all cases the mean duration between onset of symptoms and clinical diagnosis was 3 days and the mean duration between diagnosis and the primary surgical debridement was within 24hrs of presenting to the hospital. Eleven out of the 30 patients, accounting to 36.7% were positive for COVID-19 infection and were operated as well as isolated in separate operation theatres and wards. Among the remaining 19 patients, seven patients gave history COVID infection as well as steroid treatment in past 2-6 months. 80% of the patients were above the age of 50 years. COVID-19 infection was the most common predisposing factor among all patients, followed by diabetes mellitus. Table 1 shows the different forms of mucormycosis in our study group, and table 2 shows the diabetic status of the patients which played a major role in the pathogenesis of the disease.
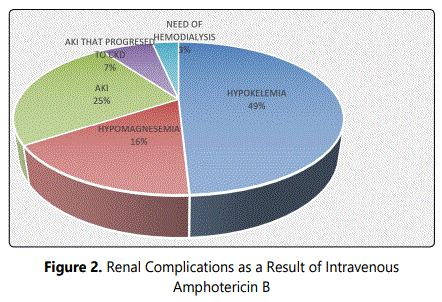
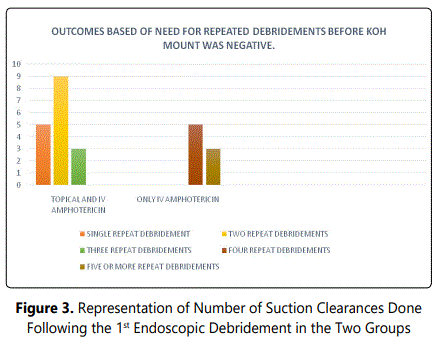
The pie charts in figures 1, 2, 3 show the COVID status and the renal complications and finally the number of surgical debridement done respectively.


Medical treatment was begun for 21 patients before surgery and post operatively for the remaining 9, in the form of injectable Amphotericin B. Following IV Amphotericin B, 33.3% of the patients developed features of nephrotoxicity after the 1st dose and 60% of them developed nephrotoxicity within the 1st three doses.
Adverse effects of the treatment using IV Amphotericin ranged from fever with chills within 1-2 hours of administering the drug due to which many a times we had to stop the IV infusion, followed by localized thrombophlebitis and need for daily removal of periphral IV lines and securing new ones. We even resorted to central venous lines for continued administration of the drug in 4 of our patients. Further electrolyte imbalance, such as hypokalemia and hypomagnesemia, hyponatremia, among which potassium loss was seen in almost all patients as the priliminary symptom of nephrotoxicity due to amphotericin, within the 1st dose for nine patients and the rest showed similar changes by 3rd dose. Potassium correction was a challenge due to the need for constant cardio-vascular monitoring, daily repeat of ECG, RFT and close monitoring of fluid intake and urinary output. Patients also complained of nausea, vomiting, and muscle cramps during and even after the daily infusion.
We found that 73.3% of the patients couldnʼt receive daily doses of the injectable Amphotericin B, beyond the 1st three doses due to altered renal parameters in the form of raised creatinine and hypokalaemia, which occurred as a consequence of the drug induced nephrotoxicity. In 5 patients injection Amphotericin B was completely withheld due to the severity of the AKI and 2 patients even required urgent haemodialysis.
Another difficulty in this route of administration was quite often wastage of the drug or reduction in its efficacy as a result of its photosensitivity in spite of dark coloured IV sets. This prolonged exposure was attributed to the fact that the infusions had to be stopped during episodes of chills and fever, or due to severe thrombophlebitis.
All the 17 patients who were in the general wards and COVID negative continued to receive topical Amphotericin B daily, whereas the remaining 13 patients in ICUS, those using oxygen masks and in COVID-19 isolation wards were not receiving topical treatment due to difficulty in nasal douching and the adequate administration of the drops Patient compliance was good for this topical use of the drug due to ease of topical Amphotericin B, unlike the difficulties faced with iv such as chills, skin eruptions and thrombosis, nausea and vomiting which we commonly came across in all the patients. The topical administration of the drug was continued throughout the hospital stay as it had no side effects even when the injectable dose was withheld.
The outcome of using topical amphotericin as a route of administration was assessed based on symptomatic relief of headache, facial pain, swelling and discolouration of the skin and reduced crusting. Outcome was assessed clinically by evaluation of the sinonasal mucosa and other DNE findings as well as by the reduced number of repeated surgical debridement and co-related with repeats of CTPNS.
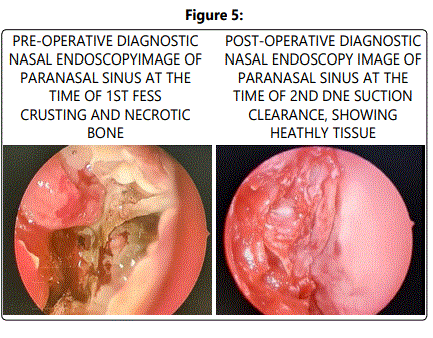
In the Figure 5, we show a comparison of preoperative endoscopic image taken at the time of the primary FESS and the endoscopic image at the time of the 2nd suction clearance, following repeated debridement in a patient with use of topical Amphotericin B along with IV amphotercin B. Need for repeat endoscopic debridement and suction clearance was lesser among the patients using topical Amphotericin alongside the injectable amphotericin. Repeat DNE and clearance rate was less, KOH was negative within the 2nd or 3rd DNE suction clearance amongst those using topical Amphotericin B. Among the topical using group only 3 patients needed 3 secondary debridements, whereas 9 patients needed 2 suction clearances and 5 of them needed only one suction clearance. Clinical and symptomatic improvement was noted among all these patients following this topical use of amphotericin. In the other group, of 13 patients who were not receiving the topical preparation a minimum of 4 to 5 repeated debridements were done, before achieving KOH negative samples, among them 5 patients due to deterioration of general condition repeat debridements could also not be done.
Discussion
Invasive fungal sinusitis has always posed a threat to the patients who succumb to it as well a challenge to treating surgeons. With the breakout of mucormycosis in the post COVID-19 era, with its high morbidity and mortality rate the question of novel methods to tackle the disease has again come into vogue.
Traditionally the management has been aggressive surgical debridement and use of long-term antifungal treatment, but this treatment itself had a high morbidity rate due the severe side effects of the drug right from electrolyte disturbances and local reactions at the site of injection and sometimes even anaphylaxis causing mortality [3].
B Saedi et al in their study titled “Endoscopic management of rhinocerebral mucormycosis with topical and intravenous Amphotericin B”, have concluded that the combination of endoscopic surgical debridement with the use of topical Amphotericin B usage, followed by intravenous Amphotericin B therapy, led to lower rates of morbidity compared with conventional treatment among their study subjects [4].
Rebekah et al. In their article which describes several novel methods to administer antifungal agents for all forms of fungal infections have spoken in great depth about the efficacy of using amphotericin aerosols as well and nasal sprays, though it was primarily begun to treat pulmonary aspergillosis and showed good outcome, in this relation the delivery of the drug to the nasal sinuses via sprays and nasal washes appears to be a reliable method, and just as several studies done then showed good outcome we too at our tertiary care centre procured good outcomes by such usage of topical amphotericin [7,8].
In the past the role of the intranasal delivery was assessed for treating pulmonary and upper respiratory aspergillosis and was found to show statistically significant results as see in the study titled Intranasal Amphotericin B Reduces the Frequency of Invasive Aspergillosis in Neutropenic Patients, by G. Mark Jeffery et al. Which shows us that the drug delivery method is efficient [9].
Intravenous injection of Amphotericin B even at its therapeutic dose has been known to cause grave drug reactions and the nephrotoxicity, this in turn leads to electrolyte imbalance. Hypokalaemia, hypomagnesemia is the most commonly noted effects of the drug, these imbalances have to corrected before the drug can be continued to be administered to the patients due the grave risks of the same [11,12,8]. Hence the continuity in the treatment and the dosing of the drug to achieve ideal drug delivery to the affected site is lost, when we purely depend on IV route, which is one of the biggest challenges offered by this route of drug administration, whereas the topical administration of the drug can be given without any discontinuity. None of the previously done studies on local and topical amphotericin have documented any reactions at the site or side-effects, neither have we reported any such side effect Sadeghi et al in their study have commented about how the use of amphotericin topically led to a lower incidence of postoperative complications following treatment by endoscopic sinus surgery [4] and they recommend use of topical amphotericin as a good treatment modality in mucormycosis management, which was similar to the results obtained in our study.
Conclusion
Based on the outcome of the patients in our series we observed that, by the use of topical Amphotericin B directly at the site of sinonasal involvement of mucormycosis we achieved better clearance of the infection, as the drug reduced the load of the fungal spores and acted locally, but due to there being a significant bias between the two groups with respect to general condition, and IV Amphotericin it is not possible to definitely conclude the same.
We wish to meanwhile highlight other feature of this form of drug administration, such as the patient compliance which was seen to be good due to the method being feasible and technically simple. This form of drug administration didnʼt require monitoring and could be performed easily even after discharge from the hospital, unlike intravenous administration which needs careful monitoring and compulsory and prolonged hospital stay, this helped to continue the action of the drug for a longer duration. It also seemed to have reduced the number of repeated surgical debridements. No side effects were noted upon such use of the drug thus we were able to continue daily administration through this route irrespective of the complication being faced in the IV route.
This is one of the first studies to our knowledge to assess the effect of topical amphotericin in the present COVID-19 associated mucormycosis outbreak, we also faced difficulty in the use of the topical preparation adequately for those in ICUs and isolation wards due to constrains in availability of manpower and other resources. We recommend that further studies with larger sample sizes and detailed statistical analysis would be required to ascertain the efficacy of this form of topical preparation of Amphotericin B as a component in the pre-existing treatment of mucormycosis.
Compliance with Ethical Standards
All authors have read and approved the final manuscript.
Funding: N/A (no funding).
Data Availability: Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.
Declaration
Conflict of Interests: The authors declare that they have no conflict of interests.
Ethical Approval: Institutional ethical committee approval was obtained for the conduction of this study. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent for Participation: The participants had full mental capacity to understand and comprehend the study, also the objectives and the methodology were clearly explained to all the participants following which informed and written consent was obtained for participation from all the participants.
Consent of Publication: Written and informed consent for publication was obtained from the participants as per the guidelines of the institution and the journal prerequisites.
Acknowledgements: NA
References
- Odessey E, Cohn A, Beaman K, Schechter L. Invasive mucormycosis of the maxillary sinus: extensive destruction with an indolent presentation. Surgical infections. 2008; 9(1): 91-8. doi: 10.1089/sur.2006.039
- Gale GR, Welch AM. Studies of opportunistic fungi. I. Inhibition of Rhizopus oryzae by human serum. American Journal of Medical Sciences. 1961; 241(5): 604-12.
- Bergstrom L, Hemenway WG, Barnhart RA. Rhinocerebral and otologic mucormycosis. Ann OtolRhinolLaryngol. 1970; 79(1): 70-81. doi: 10.1177/000348947007900107
- Saedi B, Sadeghi M, Seilani P. Endoscopic management of rhinocerebral mucormycosis with topical and intravenous amphotericin B. The Journal of Laryngology & Otology. 2011; 125(8): 807-810. doi: 10.1017/S0022215111001289
- Ferguson BJ. Mucormycosis of the nose and paranasal sinuses. Otolaryngologic Clinics of North America. 2000; 33(2): 349-65. doi: 10.1016/s0030-6665(00)80010-9
- Alobid I, Bernal M, Calvo C, Vilaseca I, Berenguer J, Alós L. Treatment of rhinocerebral mucormycosis by combination of endoscopic sinus debridement and amphotericin B. American journal of rhinology. 2001; 15(5): 327-331.
- Ricchetti A, Landis BN, Giger R, Zheng C, Lacroix JS. Effect of local antifungal treatment on nasal polyposis. Oto-Rhino-Laryngologia Nova. 2002; 12(2): 48-51. doi: 10.1159/000070911
- Head K, Sharp S, Chong LY, Hopkins C, Philpott C. Topical and systemic antifungal therapy for chronic rhinosinusitis. Cochrane Database of Systematic Reviews. 2018; (9). doi: 10.1002/14651858.CD012453.pub2
- Jeffery GM, Beard ME, Ikram RB, Chua J, Allen JR, Heaton DC, et al. Intranasal amphotericin B reduces the frequency of invasive aspergillosis in neutropenic patients. The American journal of medicine. 1991; 90(6): 685-92.
- Jiang RS, Hsu CY. Endoscopic sinus surgery for rhinocerebral mucormycosis. American Journal of Rhinology. 1999; 13(2): 105-10. doi: 10.2500/105065899782106751
- Safdar A, Ma J, Saliba F, Dupont B, Wingard JR, et al. Drug-induced nephrotoxicity caused by amphotericin B lipid complex and liposomal amphotericin B: a review and meta-analysis. Medicine (Baltimore). 2010; 89(4): 236-244. doi: 10.1097/MD.0b013e3181e9441b
- Wade RL, Chaudhari P, Natoli JL, Taylor RJ, Nathanson BH, Horn DL. Nephrotoxicity and other adverse events among inpatients receiving liposomal amphotericin B or amphotericin B lipid complex. Diagn Microbiol Infect Dis. 2013; 76(3): 361-367. doi: 10.1016/j.diagmicrobio.2013.04.001


