Research Article
Bone Anchored Hearing in Children with Aural Atresia: A Comparison of outcomes with Transcutaneous Magnetic Surgical and Non-surgical Options
Short Running Head: Outcomes of Magnetic Bone Anchored Hearing Aids in Pediatric Aural Atresia
1Department of Otolaryngology Head and Neck Surgery, Icahn School of Medicine at Mount Sinai, Mount Sinai Health System, New York, USA
2Ear Institute, New York Eye and Ear of Mount Sinai, Mount Sinai Health System, New York, USA
*Corresponding author: Maura K Cosetti, Department of Otolaryngology Head and Neck Surgery, Ear Institute, New York Eye and Ear of Mount Sinai, 380 2nd Ave 9th Floor, New York 10010, USA, Tel: 212-979-4542, E-mail: Maura.Cosetti@mountsinai.org
Received: August 9, 2018 Accepted: September 1, 2018 Published September 5, 2018
Citation: Worrall DM, Chen S, Tepper R, Wanna G, Cosetti MK. Bone Anchored Hearing in Children with Aural Atresia: A comparison of outcomes with Transcutaneous Magnetic Surgical and non-surgical options. Madridge J Otorhinolaryngol. 2018; 3(1): 64-69. doi: 10.18689/mjol-1000113
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Objective: To compare audiologic outcomes and skin related complications in children with aural atresia treated with non-surgical softband or transcutaneous magnet-based implantable bone anchored hearing devices (mBAHD).
Methods: Retrospective cohort study at a tertiary referral center of pediatric patients with conductive hearing loss from congenital aural atresia. The Cochlear™ Baha Attract system (Cochlear Americas, Centennial, CO) mBAHD (n=11) was compared to the nonsurgical softband BAHD (n=11) in terms of aided and unaided hearing thresholds, aided word recognition scores (WRS), and device related complications.
Results: Age, length of follow-up (mean=28.2 months, range: 13-43 months), and bone conduction thresholds for the atretic ear were comparable between groups (p>0.05). There were no surgical complications. The mean aided gain in the speech reception threshold (SRT) was similar between groups (p=0.55). However, the mean (SD) aided unilateral WRS was higher in the soft band group: 83.9% versus 71.2% in the mBAHD group (p=0.023). Skin complication rate was 0% in the soft band cohort and 54.5% mBAHD cohort (p=0.004) with four cases of persistent pain and erythema, and two cases of pain alone limiting device use. Interventions included decreasing magnet strength, adding soft pads, topical and oral antibiotics, and reducing device usage.
Conclusions: mBAHD are effective for auditory rehabilitation in pediatric congenital aural atresia with equivalent aided gain in SRT to soft band devices. Slightly improved aided WRS was seen in the soft band cohort. Despite lack of an external abutment, skin complications with mBAHD are common and may be mitigated by prompt evaluation and use of the lowest feasible magnet strength.
Keywords: Aural Atresia; mBHAD; Surgical; Non Surgical Options; Bone Anchored Hearing; Age and Length.
Introduction
Incomplete canalization of the temporal bone during development can cause congenital aural atresia, characterized by a complete absence of the external auditory canal (EAC), with frequently associated abnormalities of the ossicular chain and a maximal conductive hearing loss. Congenital aural atresia occurs in 1 in 10,000 births, typically occurring unilaterally and has a greater incidence in males [1]. Although often sporadic, congenital aural atresia can be associated with other craniofacial abnormalities, notably those seen in Treacher-Collins and Goldenhar syndromes. Early interventions to rehabilitate hearing are critical to speech and language acquisition. Options for hearing rehabilitation in aural atresia include surgical reconstruction of the EAC (atresiaplasty) and/or use bone anchored hearing devices.
Atresiaplasty success is predicated upon favorable anatomy, surgical expertise, appropriate patient and family expectations, and close post-operative follow-up. While atresiaplasty attempts to approximate normal anatomy, hearing outcomes can range from excellent to fair and issues of restenosis and postoperative otitis externa abound [2,3]. As a result, there has been a shift to the early use of bone-anchored hearing devices (BAHD) in children with congenital aural atresia.
While atresiaplasty can be employed in a select subset of this population with favorable anatomy, BAHDs offer rapid and simple rehabilitation with excellent hearing results [2,4]. The absence of the EAC and frequently associated pinna malformations prevents the use of traditional amplification devices. A soft band BAHD can be used during the first years of life. Soft band BAHDs require no surgical intervention, have minimal cutaneous complications with use, and have been shown to provide excellent hearing outcomes in children with congenital aural atresia [5]. However, due to aesthetic and functional concerns, patients and families frequently wish to pursue other methods of hearing rehabilitation, particularly after five years of age.
Percutaneous, surgically implanted BAHD are the longest studied BAHD and have well-documented efficacy and safety, as well as several limitations. Surgery is rapid and simple, and can be performed in one or two stages; the latter divided into initial placement of the titanium implant followed later by fitting of the external abutment. Skin complications, cosmetic concerns, and implant displacements are well-recognized drawbacks of this option. The metal abutment of a traditional surgically implanted BAHD protrudes from the skin and requires meticulous wound care, which can be difficult to achieve in young children. Furthermore, even when not wearing the hearing aid, the metal abutment can be visible. Skin complication rates are frequent, occurring in 22-78% of cases [3,6,7], and 11% develop complete skin overgrowth, covering the abutment, and require revision surgery [3]. Generally, these complications are minor, and managed with topical medications, antibiotics, or simply time in which the device is not used.
Transcutaneous magnetic BAHD (mBAHD) are a newer alternative to bone anchored hearing achieved without an externalized abutment. A titanium implant is secured to the calvarial bone and a base plate, which then exerts a magnetic force on the external processor that can be modulated with intensities ranging from #1 to #6. While this transcutaneous magnet based system still requires surgery, it avoids previously discussed post-operative issues related to abutment cleaning and skin overgrowth. However, the use of mBAHDs in a pediatric population with congenital aural atresia is not well explored. The present study compares children with congenital aural atresia and conductive hearing loss receiving either the transcutaneous magnetic BAHD or the soft band BAHD in terms of hearing thresholds, aided word recognition scores, and complications.
Materials and Methods
We performed a retrospective analysis of all children and adolescents with congenital aural atresia and conductive hearing loss treated at New York Eye and Ear Infirmary of Mount Sinai from January 2014-January 2018. Children implanted with the Cochlear™ Baha? Attract system mBAHD (Cochlear Americas, Centennial, CO) were identified and compared to an age-matched cohort group utilizing a softband BAHD, either the softband Baha® (Cochlear Americas, Centennial, CO) or the softband Ponto® (Oticon Medical, Somerset, NJ). Demographics, hearing thresholds before and after implantation, aided unilateral word recognition scores, and complications were evaluated. Complete audiometric evaluation was completed based on developmental level. Aided testing was performed using the Ling [6]. The Northwestern University Children's Perception of Speech (NUCHIPS), the Northwestern University Auditory Test Number 6 (NU 6), the Phonetically Balanced Kindergarten Test (PBK), the Pediatric AzBio sentence test (PedsAzBio), and/or the Consonant- Vowel Nucleus-Consonant test (CNC) at the discretion of the practicing audiologist.
All surgical procedures for the mBAHD were performed under general anesthesia in one stage. An implant template was used to envision device placement 50-70 mm posterior and superior to the estimated location of the external auditory meatus. Incision was made away from the planned implant site and dissection through the scalp was carried to the pericranium, which was then incised and elevated off the calvarium. A 3 or 4 mm implant was inserted and the bone bed indicator was used to identify any areas of contact between the calvarium and magnet. If identified, a high speed otologic drill was used to contour the surface and remove any bony contacts and reconfirmed with the bone bed indicator circumferentially. The magnet was secured to the implant and tightened to 25 CM. No thinning of the skin flap was performed on any patient. All patients were discharged from the hospital on the day of surgery. Patients waited a minimum of 6 weeks after implantation before loading and magnet fitting to promote Osseo integration prior to use. Length of follow-up, magnet strength, and skin complications were then assessed. Holgers' skin grading system [8] was used to classify skin complications. Two-sided t-tests, F-test of sample variance, and Chi-squared analysis were employed as appropriate with an alpha level of 0.05 to determine statistical significance.
Results
Nine children (11 ears) with congenital aural Atresia and conductive hearing loss underwent surgical implantation with the mBAHD and were compared to nine children (11 ears) who utilized soft band BAHD. Patient demographics were similar between groups with a trend toward an older age (p=0.077) and more frequent auricular reconstruction (p=0.0006) in the mBAHD group (Table 1). Associated syndromes trended to be more common in the soft band BAHD group (p=0.055), with two patients having Treacher Collins Syndrome and one patient with Goldenhar compared to one patient with Treacher Collins in the mBAHD group.
The median age of implantation was 8.7 years old. After surgery, patients waited an average 3.1 months (range 1.5 - 5 months) prior to loading the external device. Prior to implantation of the mBAHD, seven ears had used a soft band BAHD but patients and their families were unhappy due to aesthetic concerns of the band. Furthermore, one patient had a percutaneous BAHD that had to be removed secondary to recurrent infections of the abutment and pursued a transcutaneous mBAHD.
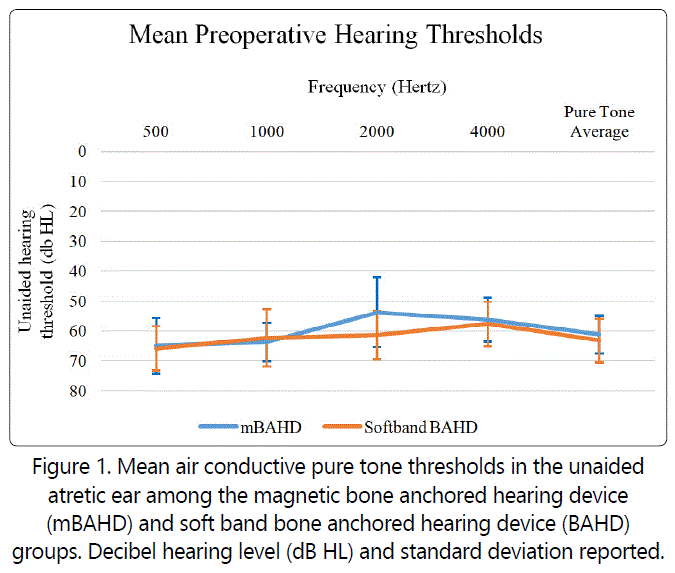
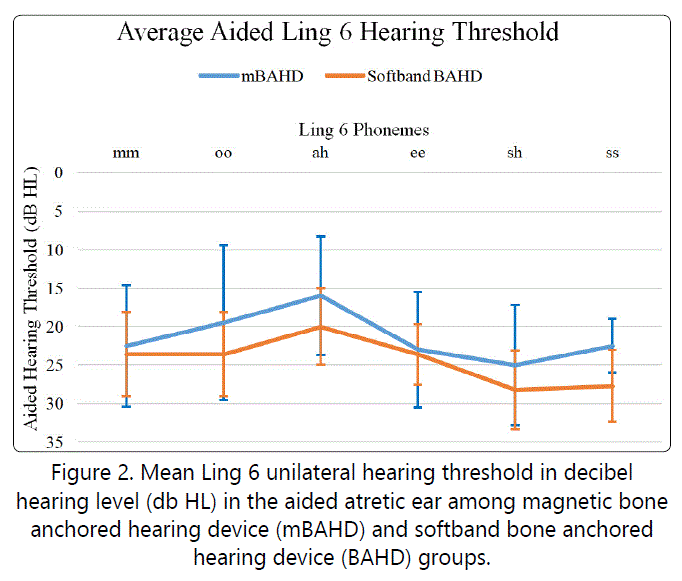
During follow-up, patients in both groups had comprehensive audio logic assessment as detailed in the Methods section. Follow-up ranged from 13-43 months, with a mean of 26.5 months in the mBAHD group and 29.9 months in the soft band BAHD group (Table 1). Preoperative hearing thresholds in the atretic ears were similar between groups with an average pure tone loss of 61.2 decibel (dB) hearing level (HL) in the mBAHD group and 63.2 dB HL in the soft band BAHD group (Figure 1). After implantation, aided speech reception thresholds and average gain was comparable between the mBAHD and softband BAHD groups (Table 2). Mean aided unilateral Ling 6 thresholds in the atretic ear were comparable between groups (Figure 2) with a mild improvement in the hearing threshold among the mBAHD group with the 'ss' phoneme: 22.5 ± 3.5dB HL versus 27.7 ± 4.7dB HL in the soft band BAHD group (p=0.009).
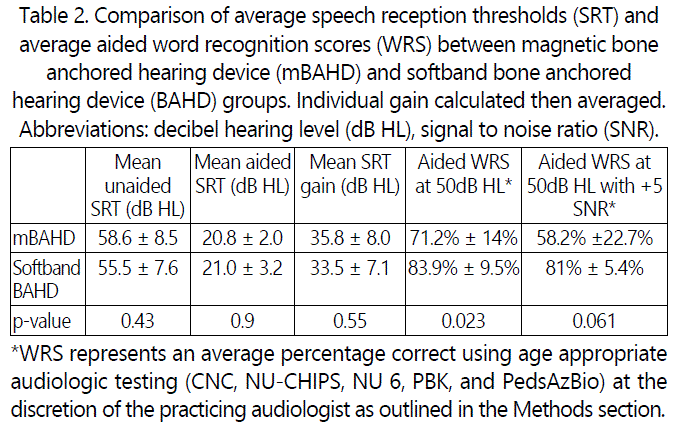
However, using a broad range of pediatric word recognition scores based on child's developmental age and audiologists' discretion, the aided unilateral word recognition scores (WRS) differ between the two groups. Among those who underwent mBAHD implantation, the average unilateral aided (WRS) at 50dB HL was 71.2% ± 14% versus 83.9% ± 9.5% (p=0.023). Similarly, when tested in noise, with an added +5dB signal to noise ratio (SNR), the mean unilateral aided WRS at 50dB HL was 58.2% ± 22.7% among the mBAHD group versus 81% ± 5.4% in the softband BAHD group (p = 0.061).
With respect to skin complications, 6 of 11 implanted ears (54.5%) had skin complications over the magnet site, not the incision, limiting device use (Table 3.). All six instances had pain, four of which also had accompanying erythema. Using the Holger skin grading system [8], five of the skin complications were scored as grade 1, and one was grade 2. All skin complications occurred with magnet strength #3 or greater, and all patients with magnet strength #5 or greater developed skin complications limiting use (Table 3). In both instances where loading occurred prior to 3 months post-operatively, skin complications developed. In 5 of 6 instances, successful treatment included decreasing the magnet strength and adding additional soft pads. One instance with moderate to severe erythema and pain required topical antibiotic ointment, oral antibiotic use, further reduction in magnet strength, and limited device use time. There were no skin complications, including pain, erythema, or treatments such as antibiotics or device rest in the softband BAHD cohort. In both groups, external processor malfunctions occurred requiring replacement or repair. During the follow-up period, two mBAHDs external processors required replacement. Two softband BAHD required replacement, and one required repair.
Discussion
Amplification options for children with congenital aural atresia include atresiaplasty and/or use of BAHD, including soft band, external abutment and magnetic systems. This study compared 2 groups of children with aural atresia and conductive hearing loss treated with either soft band BAHD or a magnetic bone anchored hearing device (mBAHD). Adequate access to sound is crucial for appropriate speech and language development and intervention at an early age with non-surgical options such as a soft band BAHD is recommended and encouraged.
Consideration of surgical intervention incorporates a multitude of patient- and provider specific factors, including temporal bone anatomy, audiologic parameters and patient and parent preference. Atresiaplasty requires surgical expertise, favorable anatomy, and post-operatively is often complicated by restenosis and recurrent otitis externa [2]. To optimize outcomes, the Jarhsdoerfer computed tomography (CT) grading system [9] uses a temporal bone CT scan to establish the presence of middle and inner ear structures and assign a grade. A score of 6 or greater is associated with good candidacy for atresiaplasty [2]. Overall, however, hearing outcomes with atresia surgery are generally inferior to that achieved with BAHDs, requiring some patients to use conventional hearing aids or BAHDs after atresiaplasty. Additionally, 65-84% of children with congenital aural atresia also have microtia [3]. And atresiaplasty is typically delayed until after microtia reconstruction to avoid compromising temporal blood supply to cutaneous flaps. Postoperatively, otitis externa remains a significant concern occurring in 42% of cases and up to 30% of patients may require revision surgery for canal restenosis [3]. Therefore, while atresiaplasty remains part of the otologists' armamentarium, there is an ongoing paradigm shift to increased use of implantable bone anchored hearing aids to manage the conductive hearing loss in children with congenital aural atresia.
Bone anchored hearing devices rely on transmission of sound input through bone directly to the inner ear. There are three distinct bone conduction hearing rehabilitation options available for children in the United States: 1) softband transcutaneous BAHD, 2) traditional percutaneous BAHD connected to osseointegrated implant, and 3) transcutaneous mBAHD. The best studied is the traditional percutaneous BAHD, with well documented safety and efficacy.
Percutaneous BAHDs are FDA approved for implantation in children over 5 years old and have excellent hearing outcomes [4,10]. The average aided hearing gain achieved with percutaneous BAHDs in conductive hearing loss ranges from 32 to 39.8 dB1 [1-13]. Although heavily influenced by how skin complications are defined such as "irritation", "pain", "erythema", and/or "cellulitis", skin complications with percutaneous, BAHD use range from 22-78% [3,6,7,14,15]. Furthermore, in implant displacement or fixture loss has been reported to occur between 5-26% of cases [3,6,15-18]. Finally, revision surgery due to fixture displacement, complete skin overgrowth, or recurrent infections, is reported to occur in 7.5%-25.9% of cases [14]. Therefore, the hearing outcomes associated with percutaneous BAHDs are excellent and must be balanced against the known drawbacks of cosmesis, skin complications, wound care, and implant displacement or loss.
A soft band BAHD relies on transcutaneous transmission of signal to the calvarium via an external processor held in place by a headband. There is no surgical implantation required and improvement in gain is reported to be comparable to percutaneous BAHDs. In one study, the gain in pure tone averages was 33dB ± 6dB in children with congenital aural atresia [5]. Which is similar to this study, which found an improvement of 33.5dB ± 7.1dB in the soft band BAHD group? Soft band BAHDs are ideal in children under 5 years old and can be utilized for early hearing rehabilitation. However, the headband has two key drawbacks. First, it is readily removable and may not be tolerated by young children reducing the hearing rehabilitation benefit. Second, the device and band are distinctly visible and the poor cosmesis becomes an influential factor as children enter school. In this study's mBAHD cohort, 6 of the 9 patients (7 of the 11 ears) had previously used a soft band BAHD and were unhappy due to aesthetic concerns of the band.
Transcutaneous mBAHDs are newer and less well studied overall, particularly in the pediatric population. Two prior studies in the pediatric conductive hearing loss population investigate a different transcutaneous magnetic Sophono™ Alpha® system (Sophono Inc., Boulder, CO). Denoyelle et al. examined 15 children with congenital aural atresia and found an improvement in the SRT from 71.73 ± 9.2dB to 38.27 ± 4.54dB and demonstrated non-inferiority in its hearing rehabilitation compared to soft band BAHD [19]. Furthermore, they report good cutaneous tolerance overall, but note several episodes of skin edema, inflammation, and pain associated with use, typically treated with decreasing the magnet strength and stopping use for 1-8 days. Additionally, this led to a shift in the institution's protocol surrounding loading of mBAHD instead starting with the weakest magnet intensity (#0 or #1) and gradually increasing the duration of use from 2 hours to 6 hours to permanent use over the course of three weeks. If an increase in magnet strength was required, this graduated exposure was again employed [19]. While O'Niel et al. evaluated 14 cases of children implanted with this separate transcutaneous mBAHD system and found the overall skin complication rate was 35.7%, with two cases of skin breakdown and one case requiring revision surgery and a cellular dermal matrix placement [13].
In this investigation, we examined children with congenital aural atresia and conductive hearing loss, treated with surgical implantation of mBAHD and compared hearing thresholds, aided word recognition scores, and complications to a matched group with congenital aural atresia using softband BAHD. Preoperative characteristics were similar between groups (Table 1, Figure 1) with comparable improvement in speech reception thresholds with aiding and similar Ling 6 hearing thresholds (Table 2, Figure 2). The word recognition scores showed a modest benefit of softband BAHD over mBAHD at 50 dB HL (83.9% vs 71.2%, respectively, p=0.023) and this WRS improvement in the soft band group was reduced to a trend toward improved performance when noise was introduced (81.0% vs 58.2%, p=0.061). Improved speech understanding in the soft band group should be interpreted cautiously as speech perception testing utilized a variety of age-appropriate standardized tests, but was reported as a global percentage correct across ages and tests. In addition, this difference between groups may reflect other factors not controlled for in this study but known to affect rates of speech perception, including length of device use, age at amplification, cognitive ability and dual-language exposure. All forms of BAHD examined in the study demonstrated improved rates of speech understanding in the aided compared with unaided condition and support the efficacy of BAHD for hearing rehabilitation in this population.
Although the mBAHD offered a comparable hearing outcome to the transcutaneous softband BAHD, the mBAHD was associated with significantly more skin complications. Six of eleven mBAHD cases developed erythema and/or pain at the site limiting use (54.5%). Additionally, skin complications occurred only in patients with magnets at #3 strength or greater, and all patients using #5 or #6 strength magnets developed an adverse outcome (Table 3). It is unclear the optimal waiting time after implantation prior to loading, but both instances of loading prior to 3 months developed skin complications. While skin complications were relatively mild and successfully treated, usually by a reduction in magnet strength (Table 3), one instance was Holger's grade II [8], and did required topical and oral antibiotics, a second reduction of magnet strength, and halting device use. Additionally, it is important to highlight that skin complications from transcutaneous mBAHD may not only occur during the acute postoperative period after loading but even a long time after surgery, occurring 29 months after surgery in this study (Table 3).
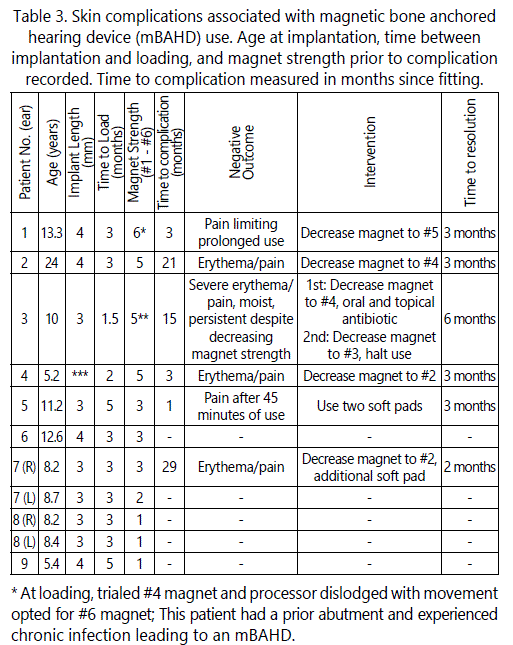
**At loading, trialed #3 magnet and processor dislodged with movement. Opted for #4 magnet, but felt it wasn't secure, and increased to #5 magnet prior to complication.
***mBAHD Cochlear BAHA Attract implantation surgery performed at an outside hospital, patient followed at our institution throughout the postoperative period for insurance reasons.
This study is limited by the size of the cohort populations. Given that congenital aural atresia is rare, the population available to study is small, which limits the analysis and conclusions that can be drawn. Furthermore, this is a retrospective review with patients and their families opting to undergo mBAHD implantation and thus susceptible to the introduction of confounding and bias. Finally, initial magnet strength was decided at the discretion of the audiologist at the time of loading, without the implementation of a standardized graded protocol. As a result, this study is meant to further define the risk of skin complications and highlight the hearing rehabilitation associated with mBAHD use and to form a framework for future study.
Conclusions
Transcutaneous magnetic BAHDs represent a realistic third option for rehabilitating conductive hearing loss in children with congenital aural atresia. It is safe, effective and results in similar hearing outcomes compared with softband BAHDs, with improved cosmesis. Although it does not have the same complication profile as the percutaneous BAHD with skin overgrowth, wound care, and skin flap infections; skin erythema and pain are also common with mBAHD use. Complications may be mitigated by prompt evaluation following reported pain and are frequently resolved by utilization of the lowest feasible magnet strength at initial loading and consideration of a gradual increase in magnet strength over time if needed. Further study to define an optimal graduated use loading protocol could be beneficial.
Support: We have no funding or sources of support to report.
Disclosures & Conflicts of Interest: We have no disclosures or conflicts of interest to report.
References
- Kelley PE, Scholes MA. Microtia and congenital aural atresia. Otolaryngol Clin North Am. 2007; 40: 61-80. doi: 10.1016/j.otc.2006.10.003
- Yellon RF. Atresiaplasty versus BAHA for congenital aural atresia. Laryngoscope. 2011; 121(1): 2-3. doi: 10.1002/lary.21408
- Farnoosh S, Mitsinikos FT, Maceri D, Don DM. Bone-Anchored Hearing Aid vs. Reconstruction of the External Auditory Canal in Children and Adolescents with Congenital Aural Atresia: A Comparison Study of Outcomes. Front Pediatr. 2014; 2(5): 1-7. doi: 10.3389/fped.2014.00005
- Pfiffner F, Kompis M, Stieger C. Bone Anchored Hearing Aids: correlation between Pure-Tone Thresholds and outcomes in three user groups. Otol Neurotol. 2009; 30: 884-890. doi: 10.1097/MAO.0b013e3181b4e8eb
- Verhagen CV, Hol MK, Coppens-Schellekens W, Snik AF, Cremers CW. The BAHA Softband. A new treatment for young children with bilateral congenitalaural atresia. Int J Pediatr Otorhinolaryngol. 2008; 72(10): 1455- 1459. doi: 10.1016/j.ijporl.2005.02.010
- Lloyd S, Almeyda J, Sirimanna KS, Albert DM, Bailey CM. Updated surgical experience with bone-anchored hearing aids in children. J Laryngol Otol. 2007; 121: 826-31. doi: 10.1017/S0022215107003714
- Kraai T, Brown C, Neeff M, Fisher K. Complications of bone-anchored hearing aids in pediatric patients. Int J Pediatr Otorhinolaryngol. 2011; 75: 749-53. doi: 10.1016/j.ijporl.2011.01.018
- Holgers KM. Characteristics of the Inflammatory Process around Skin- Penetrating Titanium Implants for Aural Rehabilitation. Audiology. 2000; 39(5): 253-9.
- Yeakley J, Jahrsdoerfer RA. CT evaluation of congenital aural Atresia: what the radiologist and surgeon need to know. J Comput Assist Tomogr. 1996; 20: 724-31.
- Doshi J, Sheehan P, McDermott AL. Bone anchored hearing aids in children: an update. Int J PediatrOtorhinolaryngol. 2012; 76(5): 618-22.
- Bouhabel S, Arcand P, Saliba I. Congenital aural atresia: bone-anchored hearing aid vs external auditory canal reconstruction. Int J Pediatr Otorhinolaryngol. 2012; 76(2): 272-77. doi: 10.1016/j.ijporl.2011.11.020
- Lustig LR, Arts HA, Brackmann DE, Francis HF, Molony T, Megerian CA, et al. Hearing rehabilitation using the BAHA bone-anchored hearing aid: results in 40 patients. Otol Neurotol. 2001; 22(3): 328-34.
- Hol MKS, Nelissen RC, Agterberg MJH, Cremers CW, Snik AF. Comparison between a new implant able transcutaneous bone conductor and percutaneous bone-conduction hearing implant. Otol Neurotol. 2013; 34(6): 1071-75. doi: 10.1097/MAO.0b013e3182868608
- O'Neil MB, Runge CL, Friedland DR, Kerschner JE. Patient Outcomes in Magnet-Based Implantable Auditory Assist Devices. JAMA Otolaryngol Head Neck Surg. 2014; 140(6): 513-20. doi: 10.1001/jamaoto.2014.484
- Mc Dermott AL, Sheehan P. Bone anchored hearing aids in children. Curr Opin Otolaryngol Head Neck Surg. 2009; 17(6): 488-93. doi: 10.1097/ MOO.0b013e32833237d7
- deWolf MJ, Hol MK, Huygen PL, Mylanus EA, Cremers CW. Nijmegen results with application of a bone-anchored hearing aid in children: simplified surgicaltechnique. Ann Otol Rhinol Laryngol. 2008; 117(11): 805-14. doi: 10.1177/000348940811701103
- Mc Dermott AL, Sheehan P. Pediatric BAHA®. Adv Otorhinolaryngol. 2011; 71: 56-62. doi: 10.1159/000323580
- Stewart CM, Clark JH, Niparko JK. Bone-anchored devices in single-sided deafness. Adv Otorhinolaryngol. 2011; 71: 92-102. doi: 10.1159/000323589
- Denoyelle F, Coudert C, Thierry B, et al. Hearing rehabilitation with the closed skin bone-anchored implant Sophono Alpha1: results of a prospective study in 15 children with ear atresia. Int J Pediatr Otorhinolaryngol. 2015; 79(3): 382- 7. doi: 10.1016/j.ijporl.2014.12.032


