Research Article
Psychometric Properties of a Developed Arabic Instrument to measure Cancer Treatment-Related Fatigue in Saudi Children
1Assistant Professor, College of Nursing, King Saud University, Saudi Arabia
2Associate Professor, College of Nursing, King Saud University, Saudi Arabia
*Corresponding author: Eyad M Alhelih, Assistant Professor, College of Nursing, King Saud University, Saudi Arabia, E-mail: eyad@ksu.edu.sa
Received: April 19, 2018 Accepted: May 7, 2018 Published: May 14, 2018
Citation: Alhelih EM, Baker OG. Psychometric Properties of a Developed Arabic Instrument to measure Cancer Treatment-Related Fatigue in Saudi Children. Madridge J Nurs. 2018; 3(1): 107-112. doi: 10.18689/mjn-1000119
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Fatigue has proven to be a pivotal topic of research and an abstract concept to explore and, as such, has led to debates about whether it will ever be possible to develop a theory concerning its causes, mechanisms, consequences, prevention and treatment. This cross-sectional descriptive study aims to validate a developed Arabic instrument to measure cancer treatment-related fatigue among Saudi children with cancer. The sample consisted of 100 pediatric oncology patients aging 8-12 years recruited from three large ambulatory hospitals in Saudi Arabia. Satisfactory approximations of internal consistency reliability (Cronbach's alpha) were decided upon for the developed instrument subscales: Fatigue (α =0.88), Effect of Fatigue (α =0.86), Compliance to Fatigue (α =0.71), and for Sense of Fatigue (α =0.50). Factorial validity was supported using principal components analysis with varimax rotation that accounted for 40% of the total variance. This validation study warrants using the developed instrument in the assessment of Saudi pediatric cancer patient's related fatigue. Yet, further validation is needed with other types of cancer and treatment.
List of Abbreviations: FAI: Fatigue Assessment Instrument; POMS-SF: Profile of Mood States-Shortened Form; FACT-An: Functional Assessment of Cancer Therapy-Anemia; ACTRFS: Arabic Cancer Treatment – Related Fatigue Scale.
Keywords: Cancer treatment-related fatigue; Children with cancer; Instrument validation; Arabic-language instrument; Saudi Arabia.
Introduction
Fatigue is one of the most widely recognized, prevailing and stressful symptoms during various stages of cancer-related treatment in children with cancer [1-5]. Cancer-related fatigue (CRF) occurs during cancer treatment and after it in approximately 75- 85% of patients according to cancer type and treatment modalities [6,7]. During the late 1990; researches utilized qualitative methods to investigate the concept of cancer–related fatigue among children with cancer [1,8]. There is no single definition is yet widely agreed upon even though the ongoing researchers' efforts in defining cancer-related fatigue due to the ambiguity of determining the underlying mechanisms of fatigue [9]. Though, the widely accepted agreement is that cancer–related fatigue is a multidimensional and subjective phenomenon that needs to be understood from children's' perspective and perception [10,11].
Hinds PS et al [8] defined fatigue for children with cancer aged 7-12 as “a profound sense of being weak or tired or of having difficulty with body movement” Stages of disease and the intensity of treatment influence fatigue and make it dynamic and differ in degrees [11-13].
Cancer-related fatigue can be measured either by self-reports [14-18], reports of parents or health care professional [11,12,19]. As being considered a multidimensional and individual experience [1], fatigue can be clearly defined according to what the child says it is and may thus be more thoroughly assessed when self-reporting is used. Currently, for self-reporting, either a single item from a multiple-symptom assessment scale [14,20] or by multidimensional scales can be used to measure fatigue [15,16,21,22]. Because of the agreement that cancer-related fatigue is a multidimensional phenomenon [1,11], multidimensional assessment of fatigue is assumed to yield a more wide-ranging assessment of cancer – related fatigue in children [9,23].
Currently, researchers and practitioners are using two well-known self-reporting and multidimensional fatigue instruments, in particular the Pediatric Quality of Life Inventory (PedsQL) Multidimensional Fatigue Scale and the Fatigue Scale-Children (FS-C). This 18-item PedsQL Multidimensional Fatigue Scale was derived from reviewing both the adult and pediatric cancer-related fatigue literature [15,24]. On the other hand, FS-C [16] was developed inductively from a conceptual model based on a qualitative study investigating fatigue in children undertaking cancer treatment. It is considered as the only instrument that was related to an overreaching conceptual model and had a conceptual definition for the instrument.
Managing CRF is a crucial concern in clinical practice because occurrence of fatigue negatively affects wide-range perspectives of children's quality of life [12,17,25,26], including psychological, physical and social well-being [11,27]. More above, CRF has also been acknowledged to be interrelated with anxiety [28,29].
Even with the CRF is recognized as a foremost issue for children with cancer; still it lacks proper assessment and management [30]. This could be attributed to three primary problems that limited our knowledge about CRF despite of tremendous efforts in this field of science. First, practitioners and researchers often studied CRF without explicit or implicit conceptual definition. Second, hypotheses engendered for testing have been theoretical or lacked logical consistency with the conceptual model or theory cited for the study. Finally, the soundness for the results of published studies has been challenged by the use of instruments that either lacked (a) reliability and validity estimates among children diagnosed with cancer, or (b) logical consistency with conceptual definitions of CRF.
A reliable and valid Arabic measure to assess cancer -related treatment fatigue could afford valuable evidence for managing this disturbing symptom. Unfortunately, for Arab children, no reliable and valid Arabic tool has been yet available. Such lack of a tool has been an obstacle to properly manage fatigue, clinically, in pediatric oncology patients in the Arab nation. Therefore, this study sought to evaluate the psychometric properties of a developed Arabic Cancer Treatment-Related Fatigue Scale (ACTRFS) in a sample of Saudi children.
Methods
Study Sample and setting
This cross-sectional study was conducted between April, 2016 and June, 2017 in three large ambulatory hospitals that treat children with cancer in Saudi Arabia. Eligibility criteria included Saudi children aging 8-12 years old, diagnosed with cancer, being actively treated and speak Arabic fluently. Children having other chronic diseases were not included because their fatigue might vary from that experienced by cancer patients.
Ethical protocols and hospital's IRB application for the protection of patients' rights, privacy and confidentiality were firmly followed. The authors provided children and their guardians with oral and written clarifications about the purpose of the study and its procedures and asked them if they were willing to participate in the study. After obtaining children's assent and guardians' consent, Arabic versions of Fatigue Assessment Instrument (FAI), Profile of Mood States-Shortened Form (POMS-SF) and Functional Assessment of Cancer Therapy-Anemia (FACT-An) were made available to each participant during a face-to-face interview. The English version of the used tools was forward and backward translated into Arabic by independent expert and underwent pilot testing with representative homogenous sample [31,32]. Patient identifiers on the survey package were removed instantly after completing data collection and all packages were kept in a protected office to guarantee confidentiality and anonymity.
Tools
Fatigue Assessment Instrument (FAI)
This 29-item; validated self-reporting instrument was designed to capture fatigue including qualitative and quantitative components. It consists of four subscales: Global Severity, Consequences of Fatigue, Situation-specific Fatigue, and Responses to rest or sleep. Based on their experiences within the past two weeks; participants were encouraged to respond to each item by indicating their degree of agreement on a 7-point, Likert-type scale (1= completely disagree to 7= completely agree). The sum of item scores on each subscale creates a subscale score. Higher subscale scores signify increased severity, more severe consequences, more situational triggers to fatigue, and improvement in fatigue with rest and sleep, respectively.
Profile of Mood States - Shortened Form (POMS-SF)
This validated tool is a 37-item; self-reporting adjective checklist intended to measure mood states. The POMS-SF has six subscales: Tension-anxiety (6 items), Depression-dejection (8 items), Anger-hostility (7 items), Vigor-activity (6 items), Fatigue-inertia (5 items), and Confusion-bewilderment (5 items). Participants rate each item on a 5-point Likert scale (0=Not at all, 1=A little, 2=moderately, 3=Quite a bit, and 4=extremely). Responses represent how individual has felt over the past seven days.
Functional Assessment of Cancer Therapy- Anemia (FACT-An)
The Functional Assessment of Cancer Therapy-Anemia (FACT-An) is a 50-item, valid self-reporting scale intended to assess general quality of life and the effect of fatigue and other anemiarelated symptoms experienced by children with cancer. The original questionnaire, Functional Assessment of Cancer Therapy-General, includes 30 items. The scale consists of four subscale scores: physical, functional, emotional, and social well-being. Two subscales, the Impact of Fatigue -13 items (FACT-F) and Impact of anemia related symptoms-7 items (FACT-Anemia) were added to the FACT-G to create the FACT-An. Participants respond to each statement on a 5-point, Likert-type scale (0=Not at all, 1 =A little bit, 2=somewhat, 3=Quite a bit, and 4=Very much).
The developed Arabic Cancer Treatment – Related Fatigue Scale (ACTRFS)
ACTRFS is a 26-item, self-reporting instrument developed by the corresponding author to measure the appraisal of cancer treatment -related fatigue among children with cancer. The ACTRFS covers four content areas and has four subscales: Fatigue (5 items), Sense of Fatigue (6 items), Effect of Fatigue (7 items), and Compliance to Fatigue (8 items). Readability level was calculated as second grade using the SMOG Readability formula. Less than 35 minutes were needed to complete the instrument.
Each subscale of the ACTRFS is scored separately. A total scale score is not calculated. Fatigue subscale, is reversely scored. The scores on each of the five items were summed to produce a fatigue score. Scores range from 0 to 15 with a higher score representing a higher, more persistent level of fatigue.
The scores on each of the five items for sense of fatigue subscale are summed to produce a sense of fatigue score with a higher score indicating a more positive outlook on fatigue. For the Effect of Fatigue subscale, the importance score and energy score for each item are summed to produce an item score. Then, the item scores are totaled to produce the total subscale score, with a higher positive score indicating a higher effect. The energy score and the effectiveness score are summed to produce an item score. Item scores are summed to produce the Compliance to Fatigue score, with a higher score representing higher compliance to fatigue.
Data Analysis
Data analyses were conducted using the IBM SPSS 20.0. Descriptive statistics were used to describe the demographics of the sample with Mean and standard deviations were used to describe the distributions of subscales and the total scale. Reliability of the total scale and subscales was calculated by Cronbach's alpha coefficients, corrected item-total correlations and item-subscale correlations. Construct validity was estimated by using factorial validity.
Results
Subject Characteristics
A total of 106 children diagnosed with cancer who were receiving treatment at large ambulatory hospitals were approached to participate in the study. Five children refused to participate; three due to time constraints and two of them because of illness severity. One subject completed the assent form and demographic data but did not complete the instruments. The final study sample who completed the package of questionnaires without difficulty was 100 subjects.
Most of the participants were treated in an inpatient care setting (78%). Males comprised the majority (62%) of the sample. The subjects were Saudi with preparatory level of education (100%).
Reliability assessment
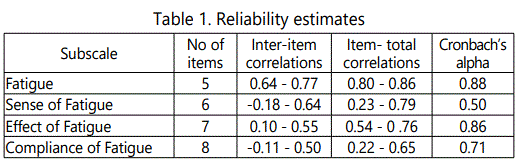
Table 1 represents the internal consistency reliability, inter-item and item-total correlations for each ACTRFS subscales.
The maximum of inter-item and item-total correlations was higher for the fatigue subscale and lower for the sense of fatigue subscale. Reliability estimates as measured by Cronbach's alpha for the four subscales ranged from 0.50 to 0.88.
Validity assessment
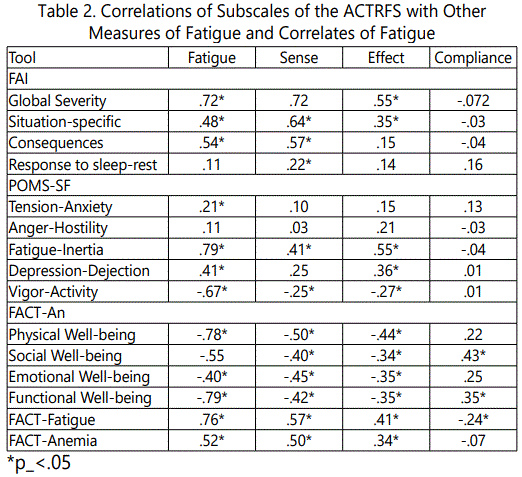
Regarding the criterion validity of the ACTRFS, all correlations were found to be substantial. Nevertheless, the most significant correlation found was between ACTRFS and FAI, which directly illustrates the criterion validity of ACTRFS, where the two fatigue scales were positively and significantly correlated.
Convergent validity was appraised by testing a series of hypotheses about the direction and strength of correlation between the subscales of the ACTRFS and selected subscales of the Fatigue Assessment Instrument, Profile of Mood StatesShortened Form and the Functional Assessment of Cancer Therapy-An. Table 2 reveals these findings.
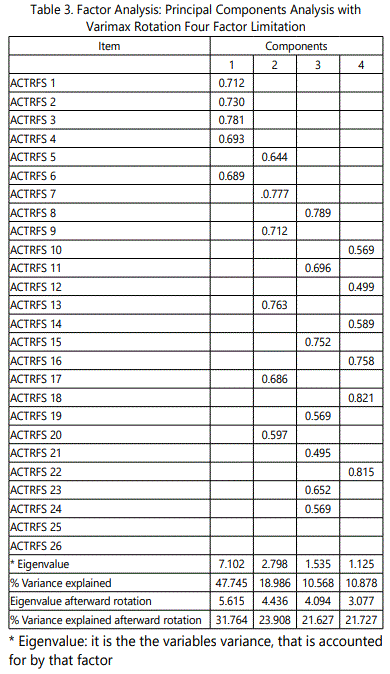
The construct validity of the ACTRFS was appraised using a factor analysis with a Varimax rotation. Support for a four-factor solution was found for the 26-item ACTRFS. Item loadings on the factors are exposed in Table 3. Twenty-four items with factor loadings greater than (0 .40) were retained for further analysis. Two items did not load on any of the four factors. Five items loaded on Factor 1; 6 items loaded on Factor 2; 7 items loaded on Factor 3; and 6 items loaded on Factor 4.
Discussion
This study is the first study conducted to validate Arabic Instrument to measure Cancer Treatment-Related Fatigue among children with cancer. The characteristics of the sample recruited for this study were significant for the purpose of generalizability of the scale for use in the population. The mean age of the sample was 10.8 years, with a minimum of 8 years and a maximum of 12 years. The sample was representative of an older population than expected. This finding might be related to the study settings.
Hematological cancers were over-represented in the sample which presents a prospect to address a gap in the literature. Patients with lymphomas, leukemia, and myeloma were more probable to experience treatments that are more toxic, dose-intensive, and extended than those received by patients with solid tumors [14-18]. Only a few studies were recognized in the literature that explicitly addressed the experience of fatigue in this population [15,24].
The high rate of participation in the study can be attributed to two factors. Co-researchers for the study were nurses who worked in the outpatient or inpatient care units. Participants also indicated that they were committed to completing the study in order to “help others in the future.” No data were missing in the final study. High completion rates for the research instruments could be contributed to three factors. First, instructions for completing the instruments were reviewed orally with each subject. Second, subjects were told to answer each question and to review their responses prior to submitting them to the researcher.
Oversights (96%), instead of various responses to a single item, accounted for most of the half-finished or unclear items. Time allotted for completion of the instrument was less than 35 minutes. Nevertheless, the number of instrument items and time to complete remains long to be used as fatigue assessment instrument in busy outpatient care settings or with severely ill hospitalized patients.
For all dimensions of the ACTRFS, the internal consistency was found to be acceptable. The Fatigue and Effect of Fatigue subscales demonstrated high internal consistency. Moderate internal consistency was demonstrated for the Compliance to Fatigue subscale. Estimates of internal consistency for the fourth subscale, Sense of Fatigue, resulted in low internal consistency reliability (r=0.50). The ACTRFS and other three used fatigue assessment tools were correlated significantly in almost all of their subscales.
Authors generated hypotheses about the relationship of the ACTRFS subscales and selected subscales of the Profile of Fatigue Assessment Instrument, Mood States-Shortened Form, and Functional Assessment of Cancer Therapy-Anemia to assess construct validity. Hypotheses were verified and gave rise to weak to moderate correlations among the subscales in the directions hypothesized with the exception of the correlations of the compensation to Fatigue subscale and the Effect of Fatigue subscale and the POMS Tension-anxiety subscale and the FACT-An Emotional and Functional Well-being subscales.
ACTRFS construct validity was established through a principal components factor analysis with varimax rotation. A four-factor solution with item loadings of 0.40 or greater on each factor was determined. The factors were described and named: Fatigue, Effect of Fatigue, Compliance to Fatigue, and Sense of Fatigue. Two items of the ACTRFS did not load on any of the four factors. Items from the original Sense of Fatigue subscale either loaded on the Fatigue, Effect of Fatigue subscale or on Compliance to Fatigue. Results supported the initial factor structure for the developed instrument. Removal of subscale items based on the factor analysis procedures yielded a 24-item ACTRFS.
Practitioners have failed to report adequately fatigue in children experiencing cancer [33-35]. Barriers to care have included the subjective nature of fatigue; lack of ease of use, reliable, and valid instruments to measure changes in fatigue over time; and the lack of empirical data on which to base the selection of interventions to modify the fatigue experience [36-38]. Development and validation of a new instrument to measure fatigue is a continuous process. Data made from supplementary studies using the instrument creates the pieces of the puzzle required to support or disprove consistent patterns of reliability and validity for the developed instrument.
Conclusion
ACTRFS is a valid and reliable instrument to measure the appraisal of fatigue among children with cancer in Saudi Arabia. This conclusion was established based on an acceptable internal consistency estimates and multiple validity examinations, including moderate to high content validity, acceptable goodness of fit of the instrument by executing confirmatory factor analysis and a significant association.
Due to the important advances in the areas of cancer treatment and patients' quality of life, relieving distressing symptoms has become a pivotal issue. Thus, having a reliable and valid instrument for measuring fatigue related to cancer treatment is crucial in clinical practice to guide clinical efforts toward releasing fatigue-related distress. Authors believe that the ACTRFS fills and satisfies the need in Arab world for such a tool. Testing should be done by applying it on a new or different patient population.
Acknowledgements
The authors would like to thank all the patients participated in the current study. The original study yielded the first version of ACTRFS is funded by SANAD Society for Cancer Children Support in Riyadh- Saudi Arabia.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication.
References