Research Article
Validation of Assay Indicating Method Development of Duloxetine HCl in Bulk and its Tablet Dosage Form by RP-HPLC
1MLR Institute of Pharmacy, Dundigal, Qutbhullapur, RR, Hyderabad, 500043, India
2Yalamarty Pharmacy College, Tarluwada, Anandapuram, Visakhapatnam, Andhra Pradesh, 530052, India
3Osmania University College of Technology, Osmania University, Hyderabad, Telangana, 500007, India
*Corresponding author: Nalini Kanta Sahoo, Professor and H.O.D, Department of PA & QA, MLR Institute of Pharmacy, Dundigal (v), Qutbhullapur (M), RR (Dt), Hyd – 500043, India, Tel: 09550741536, 09396772373, Email: sahoo.nalini@gmail.com
Received: September 1, 2017 Accepted: October 14, 2017 Published: October 20, 2017
Citation: Sahoo NK, Ippili R, Sahu M, Sahoo CK. Validation of Assay Indicating Method Development of Duloxetine HCl in Bulk and its Tablet Dosage Form By RPHPLC. Madridge J Nov Drug Res. 2017; 1(1): 12-17. doi: 10.18689/mjndr-1000103
Copyright: © 2017 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
A novel, simple and economic reverse phase high performance liquid chromatography (RP-HPLC) method has been developed for the quantification of duloxetine HCl (DLX) in bulk and tablet dosage form with greater precision and accuracy. Separation was achieved on inertsil ODS C18 (250mm×4.6×5micron) column in isocratic mode with mobile phase consisting of Acetonitrile: Potassium dihydrogen phosphate buffer (pH 4.9) (43:57v/v) and conditions optimized were: flow rate was 1 ml/min. The detection was carried out at 230 nm. The retention time of Duloxetine HCl was found to be 4.440 min. The method was validated as per ICH guidelines. Linearity was established for Duloxetine HCl in the range10–120μg/ml with R2 value 0.999. The percentage recovery of Duloxetine HCl was found to be in the range 99.82-100.25 %. The high recovery and low relative standard deviation confirm the suitability of the proposed method for the estimation of the drug in bulk and tablet dosage forms. Validation studies demonstrated that the proposed RP-HPLC method is simple, specific, rapid, reliable and reproducible for the determination of Duloxetine HCl in comparison to previous reported methods for Quality Control level.
Keywords: RP-HPLC, Duloxetine HCl, Quality control level.
Introduction
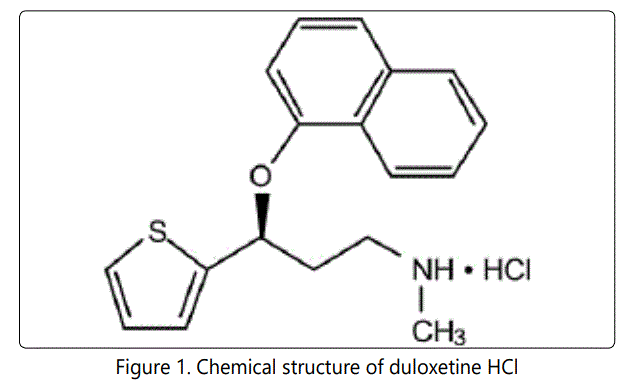
Duloxetine HCl [1] shown in Fig 1, is Methyl [(3S)-3 (naphthalen-1-yloxy)-3- (thiophen-2-yl) propyl] amine. Duloxetine HCl is a selective serotonin and norepinephrine reuptake inhibitor antidepressant (SSNRI). Duloxetine HCl affects chemicals in the brain that may become unbalanced and cause depression [2]. Duloxetine HCl is used to treat major depressive disorder and general anxiety [3] disorder. Duloxetine HCl is also used to treat fibromyalgia (a chronic pain disorder), or chronic muscle or joint pain (such as low back pain and osteoarthritis pain) and pain caused by nerve damage in people with diabetes (diabetic neuropathy). When serotonin and noradrenaline are released from nerve cells in the brain they act to lighten mood. When they are reabsorbed into the nerve cells, they no longer have an effect on mood. It is thought that when depression occurs, there may be a decreased amount of serotonin and noradrenaline released from nerve cells in the brain. Duloxetine HCl works by preventing serotonin and noradrenaline from being reabsorbed back into the nerve cells in the brain. This helps prolong the mood lightening effect of released serotonin and noradrenaline. In this way it helps relieve depression. It may take between two to four weeks to feel the benefits of these medicines. Duloxetine HCl is an oral dual reuptake inhibitor that enhances the levels of the NT (5-HT & NE), which are involved in depression. Duloxetine HCl is affective in stress urinary incontinence (SUI) by blocking the reuptake of 5-HT & NE in the spinal cord. This inhibition stimulates increased activity of the pudendal nerve, which may increase contractions of the urethral sphincter at the opening of the bladder. The antidepressant properties are due to blocking the reuptake of serotonin and possibly also noradrenalin within the central nervous system. Major depressive disorder is believed to be due in part to an increase in pro-inflammatory cytokines within the central nervous system. Orally administered duloxetine HCl is well absorbed. C max occurred 6 hrs post dose of duloxetine HCl. Duloxetine is highly bound (>90%) to proteins in human plasma, binding primarily to albumin and α1-acid glycoprotein. The biological half life [4, 5] of duloxetine HCl is 12 hr. Elimination of duloxetine HCl is mainly through hepatic metabolism involving two P450 isozymes, CYP2D6 and CYP1A2. Both CYP1A2 and CYP2D6 are responsible for duloxetine HCl metabolism. Metabolites found in plasma (4-hydroxy duloxetine glucuronide & 5-hydroxy, 6-methoxy duloxetine sulphate). Most of the metabolites are excreted in urine and faeces. Several analytical methods [6-14] for the determination of duloxetine HCl by flourimetry, capillary electrophoresis, spectrophotometry, HPLC, LC/MS and polarography have been reported. The aim of the present work was to develop and validate a sensitive RP-HPLC method that can be implemented for the quantification of duloxetine HCl in bulk as well as in its tablet dosage forms.
Experimental
Materials
Duloxetine HCl working standard was obtained as a gift from Lee Pharma Ltd., Visakhapatnam, Andhra Pradesh, India. Duloxetine hydrochloride pellets sample of batch number DU0212002 was obtained from Lee Pharma Ltd. HPLC grade Potassium dihydrogen ortho phosphate (Merck, India), HPLC grade Triethylamine (Merck, India), HPLC grade Acetonitrile (Merck, India) and Sodium hydroxide were the chemicals used. High-purity water was prepared using TRA water purification system. Solvents were filtered through a 0.45 μm nylon membrane filter and degassed by using ultrasonicator (Enertech, India). All solutions were prepared daily.
Instrumentation
Shimadzu 1800 uv-visible spectrophotometer (Hyderabad), Ultrasonicator, 0.45μm membrane filter, mettler toledo analytical balance, Waters Alliance High Pressure Liquid Chromatography installed with Empower software model: waters e2487 dual absorbance, solvent degasser DCU-20A3 solvent degasser, prominence 10 AT vp binary gradient pumps, SPD 10 A vp uvvis detector with class vp software, column used is Inertsil ODS 3V 5μ, 250 × 4.6 mm, ODS(C18)RP Column (250*4.6mm, i.d; 5μm). A labindia pH meter was used for pH adjustment.
Chromatographic conditions
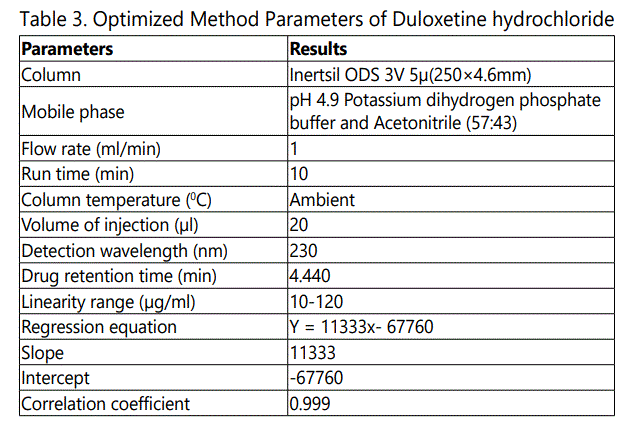
The chromatographic analysis was performed, under ambient conditions and in isocratic mode, with Waters Alliance High Pressure Liquid Chromatography, Model: Waters e2487 dual absorbance, a variable wavelength PDA detector operated at 230 nm was used. The output signal was monitored and integrated using Empower Solutions software. The analysis was performed on a Inertsil ODS 3V 5μ, 250 × 4.6 mm column. The mixture of pH 4.9 Potassium dihydrogen phosphate buffer and acetonitrile was used as mobile phase (57:43, v/v) at a flow rate of 1.0 ml /min. The eluent was filtered through a 0.45 μm nylon membrane filter and degassed by using ultrasonicator. Various trials are shown as in Table 1-3.
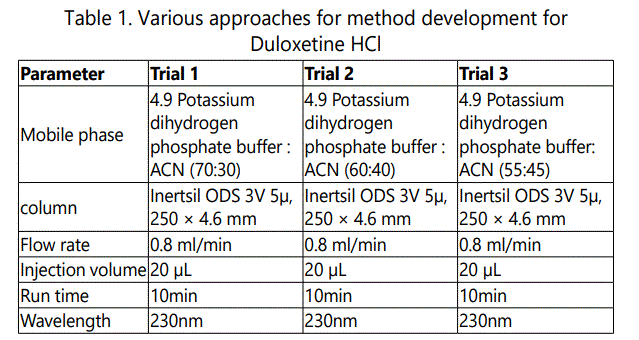
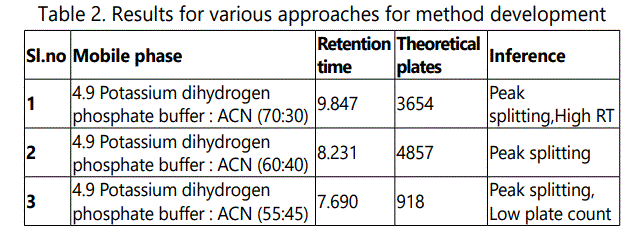
Preparation of mobile phase
Mobile phase was prepared by taking acetonitrile: phosphate buffer (pH 4.9). The pH 4.9 phosphate buffer and acetonitrile were mixed in the ratio of 57:43 to get mobile phase. Mobile phase was filtered through 0.45 μm membrane filter and degassed under ultrasonic bath prior to use. The mobile phase was pumped through the column at a flow rate of 1 ml/min.
Preparation of standard solutions
10g of Duloxetine HCl pellets were weighed and ground into fine powder using a mortar and pestle. Powder equivalent to 40 mg of drug was transferred to a 50 mL volumetric flask and 25mL of mobile phase was added and sonicated for 10 min and diluted to volume with mobile phase. The solution was filtered through a 0.45μm nylon membrane filter. From the filtrate an appropriate aliquot was taken in such a way that the final concentration in 50 mL lies within the linearity range tested.
Assay of duloxetine HCl from marketed tablets
Twenty μL of standard solution was injected three times into the chromatographic system and chromatogram was recorded and peak areas were measured. 20 μL of the sample solution was injected in duplicate into the chromatographic system and chromatogram was recorded and peak areas were measured.
Method validation
The method was validated in accordance with ICH [15] guidelines. The parameters assessed were linearity, accuracy, and precision, reproducibility, robustness and system suitability.
Accuracy
The accuracy of the method was determined by calculating the recoveries of duloxetine HCl by the standard addition method. Known amounts of standard solutions of duloxetine HCl was added at 50%, 100% and 150 % level to pre-quantified sample solution (80μg/ml) of duloxetine HCl and each injected three times into the chromatographic system. The percentage recovery of added drug was estimated by measuring the peak area and by fitting these values to the straight-line equation of calibration curve. Recovery (%), RSD (%) and bias (%) were calculated for each concentration. Accuracy is reported as percentage bias, which is calculated from the expression
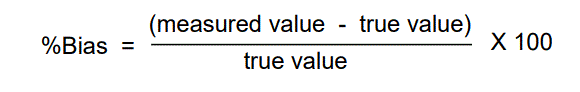
Precision
System Precision
The system precision was established by injecting six replicate injections of standard solution in to the chromatographic system by maintaining the optimized chromatographic conditions.
Method Precision
Six replicate samples of drug product (n=6) at 100% of concentration (80 μg/ml) were prepared and injected into the chromatographic system. The results were reported in terms of relative standard deviation (% RSD).
Ruggedness/Inter day precision
Six preparations individually using single batch of duloxetine HCl drug substance as per test method and injected each solution induplicate on the same day in to HPLC using different column, system and analysts on different days. And %RSD value was calculated to determine inter-day precision.
Robustness
The concept of robustness of an analytical procedure has been defined by the ICH as "a measure of its capacity to remain unaffected by small but deliberate variations in method parameters". To determine the robustness of the method experimental conditions are purposely altered and chromatographic characters are evaluated. Influence of small changes in chromatographic conditions such as change in flow rate (± 0.1ml/min), wavelength of detection (±2nm) and acetonitrile content in mobile phase (±2%) were studied to determine the robustness of the method
Limit of detection (LOD)
The limit of detection (LOD) of an analytical method may be defined as the concentration, which gives rise to an instrument signal that is significantly different from the blank. For spectroscopic techniques or other methods that rely upon a calibration curve for quantitative measurements, the IUPAC approach employs the standard deviation of the intercept (Sa), which may be related to LOD and the slope of the calibration curve, b, by
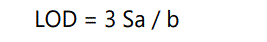
Limit of quantitation (LOQ)
The LOQ is the concentration that can be quantitated reliably with a specified level of accuracy and precision. The LOQ represent the concentration of analyte that would yield a signal-to-noise ratio of 10.
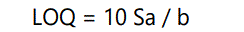
Where, Sa is the standard deviation of the peak area ratio of analyte to IS (6 injections) of the drugs and b is slope of the corresponding calibration curve.
Linearity and Range
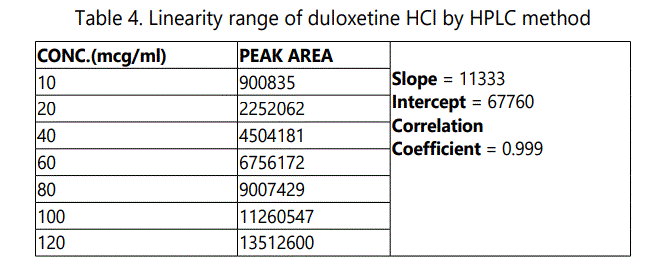
Linearity indicates the ability of analytical procedures to produce results that are directly proportional to the concentration of analyte in the given sample. A series of solutions of drug substance standard were prepared in the specified concentration range. These solutions were injected into the HPLC and linearity was determined by observing peak areas. A graph of peak area versus concentration (on X-axis concentration and on Y-axis Peak area) was plotted and the correlation coefficient was calculated.
Results and Discussion
Linearity
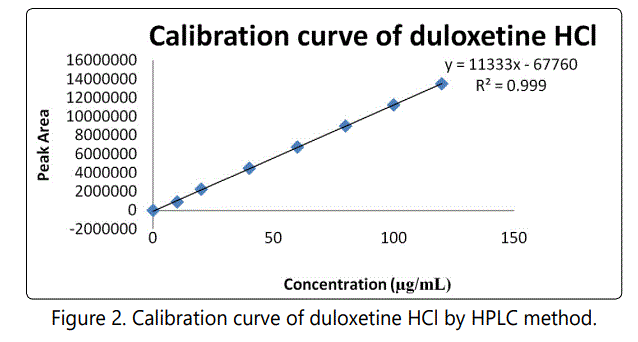
The evaluation of the linearity was performed with a seven-point calibration curve over the concentration range of 20–120 μg mL-1 with a % RSD of less than 2. The slope and intercept of the calibration graph was calculated by using linear regression analysis. The regression equation of the calibration curve was: y = 11333x- 67760; R2 = 0.999 and shown in Fig.2. A correlation coefficient of 0.999 suggests that the developed HPLC method had an excellent linearity over the investigated range and the results obtained in Table 4.
Accuracy: Recovery study
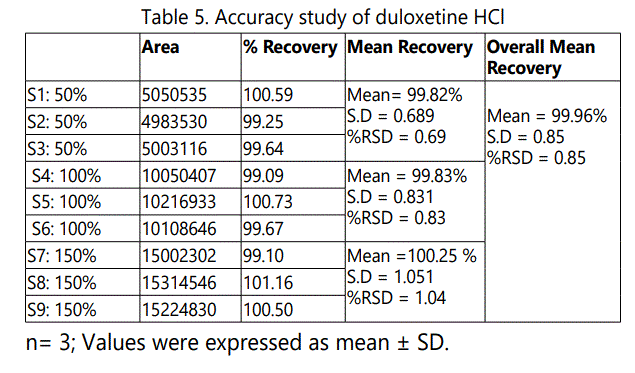
The accuracy of the method was determined. The mean recovery was found to be 99.96% and the %RSD was found to be 0.85 (%RSD ≤2%). The accuracy study indicates that the developed HPLC method was accurate and there was no interference from the exipients present in the dosage form and shown in Table 5 and Fig.3-5.
Precision
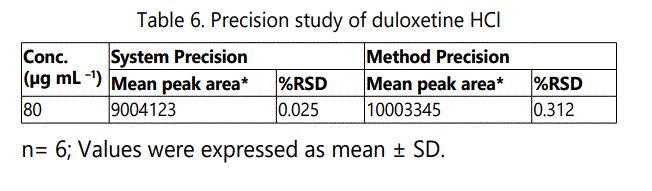
The precision of the method was evaluated with standard samples at 100% concentration using six replicates. The System and Method precisions were satisfactory with %RSD less than 2 and shown in Table 6 &7 and Fig. 6-7. This indicates that the developed HPLC method was reproducible.
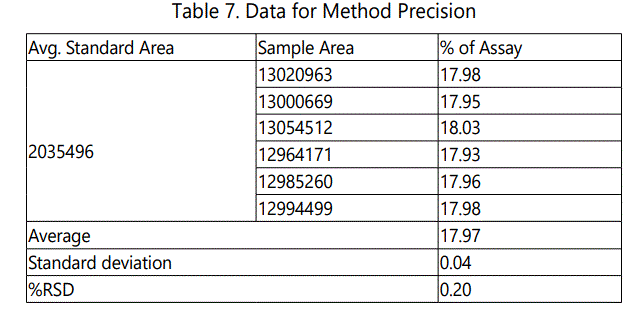
Acceptance Criteria: The % RSD should be less than 2.0%
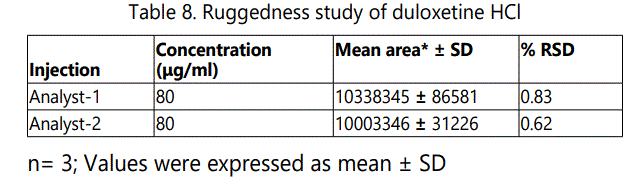
Ruggedness
The ruggedness of the method was determined by preparing standard solution by two analysts (n=6). The relative standard deviation was < 2%. The results of the study prove that the method is rugged and shown in Table 8.
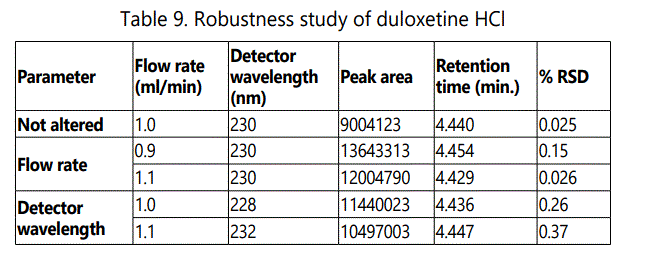
Robustness
When the optimized chromatographic conditions were deliberately altered, the chromatographic parameters such as peak area, retention time and theoretical plates (N) were remained within the acceptance limits. The results prove that the method is robust and is shown in Table 9.
Limit of detection
The limit of detection of duloxetine HCl was found to be 1.24 μg/ml.
Limit of quantification
The limit of quantification of duloxetine HCl was found to be 4.092 μg/ml.
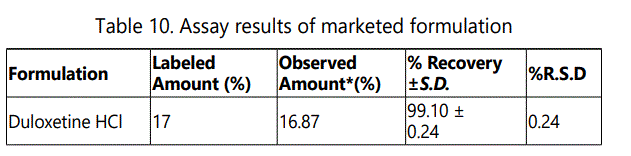
Assay of marketed formulation
Assay results of the marketed formulation is shown in Table no.10 and Fg.no.8.
Conclusion
A New RP-HPLC method indicating assay of DLX in bulk and in pharmaceutical dosage forms is established. This method is simple, reliable, linear, accurate, sensitive and reproducible as well as cost effective for the effective quantitative analysis of duloxetine HCl in bulk and tablet formulations. The method was completely validated showing satisfactory data for all the method validation parameters tested and method is free from interference of the other active ingredients and additives used in the formulations. Therefore the method is suitable for use of the routine quality control analysis of duloxetine HCl in API or in pharmaceutical dosage forms.
Acknowledgements
The authors would like to acknowledge the contributions of MNR College of Pharmacy, Fasalwadi, Sangareddy, Medak, Telangana, India for providing necessary facilities to carry out the research work.
Competing Interests
The authors declare that they have no competing interests.
References
- Johnson JT, Oldham SW, Lantz RJ and DeLong AF. High performance liquid chromatographic method for determination of duloxetine and desmethyl duloxetine in human plasma. J Liq Chromatogr Rel Tech. 1996; 19(10): 1631-41. doi: 10.1080/10826079608005497
- Lantz RJ, Gillespie TA, Rash TJ, Kuo F, Skinner M, Kuan HY and Knadler MP. Metabolism, Excretion and pharmacokinetics of duloxetine in healthy human subjects. The American Society for Pharmacology and Experimental Therapeutics. 2003; 31(9): 1142-1150. doi: 10.1124/dmd.31.9.1142
- Ma N, Zhang BK, Li HD, Chen BM, Xu P, Wang F, Zhu RH, Feng S, Xiang DX and Zhu YG. Determination of duloxetine in human plasma via LC/MS and subsequent application to a pharmacokinetic study in healthy Chinese volunteers. Clinica Chimica Acta. 2007; 380(1-2): 100-105. doi: 10.1016/j. cca.2007.01.018
- Mercolini L, Mandrioli R, Cazzolla R, Amore M and Raggi MA. HPLC analysis of the novel antidepressant duloxetine in human plasma after an original solid phase extraction procedure. Journal of Chromatography B. 2007; 856: 81-87. doi: 10.1016/j.jchromb.2007.05.031
- Elisabetta B, Samuele F, Giovanni F, Claudio F and Luciana M. Isolation and characterization of a phenolic impurity in a commercial sample of duloxetine. J Pharm Biomed Anal. 2007; 43: 1573-5. doi: 10.1016/j.jpba.2006.11.026
- Dhaneshwar SS, Despande P, Patil M, Vandnerkar G and Dhaneshwar SR. Development and validation of HPTLC method for estimation of duloxetine hydrochloride in bulk drug tablet dosage form. Indian Journal of Pharmaceutical Sciences. 2008; 70(2): 233-236. doi: 10.4103/0250- 474X.41463
- Senthamil SP, Gowda KV, Mandal U, Sam SWD and Pal TK. Determination of duloxetine in human plasma by liquid chromatography with atmospheric pressure ionization tandem mass spectrometry and its application to pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 858(1-2): 269-275. doi: 10.1016/j.jchromb.2007.09.002
- Waldschmitt C, Vogel F, Maurer C and Hiemke C. Measurement of duloxetine in blood using High Performance Liquid Chromatography with spectrometric detection and column switching. Ther Drug Monit. 2007; 29(6): 767-772. doi: 10.1097/FTD.0b013e31815d0dfa
- Yunoos M, Sankar DG, Kumar BP, Hameed S and Hussain A. Simple UV Spectrophotometric determination of duloxetine hydrochloride in bulk and in pharmaceutical formulations. E Journal of Chemistry. 2010; 7(3): 785-788. doi: 10.1155/2010/709176
- Puranik M, Wadherb S and Sharma K. A simple novel validated stability indicating RP-HPLC method for estimation of duloxetine hydrochloride in capsule pharmaceutical formulation. Indian J of Pharmaceutical Education and Research. 2014; 48(3): 91-98. doi: 10.5530/ijper.48.3.11


