3rd International Nanotechnology Conference & Expo
May 7-9, 2018, Rome, Italy
Hydrogen Evolution Reactions of Conducting Polymer-Metal Organic Framework Nanocomposites
1Department of Chemistry, University of Limpopo, South Africa
2Sensor Lab, Department of Chemistry, University of the Western Cape, South Africa
The development of highly efficient electrocatalysts for hydrogen evolution reaction is a fundamental undertaking of the hydrogen economy. Herein, we investigated the electrocatalytic performance of conducting polymer (polyaniline, poly (3-aminobenzoic acid)/metal organic framework (HKUST-1) nanocomposites for hydrogen evolution reactions. The results show that the synthesized nanocomposites exhibit the best electrocatalytic efficiency at lower overpotential and the Tafel analysis ( transfer coefficient (α) and Tafel slope (b)) suggests that the rate-determining step is the Volmer (electrochemical discharge) coupled with either Tafel (chemical desorption) or Heyrovsky (electrochemical desorption) reactions.
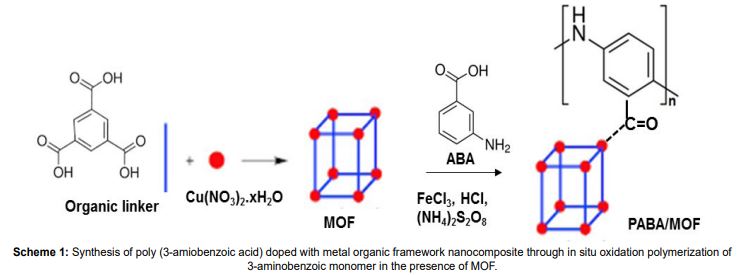
Keywords: Electrocatalyst, Hydrogen Evolution Reaction, Conducting Polymer, Metal Organic Framework, Tafel Analysis


