Editorial Article
Mesoporous Materials as Catalyst support for Wastewater Treatment
Department of Chemical and Biological Engineering, The Hong Kong University of Science and Technology, Hong Kong
*Corresponding author: Xijun Hu, Department of Chemical and Biological Engineering, The Hong Kong University of Science and Technology, Hong Kong, Tel: +852 23587134, Fax: +852 23580054, E-Mail: kexhu@ust.hk
Received: May 29, 2019 Accepted: June 6, 2019 Published: June 18, 2019
Citation: Ding Q, Hu X. Mesoporous Materials as Catalyst support for Wastewater Treatment. Madridge J Nanotechnol Nanosci. 2019; 4(2): 160-167. doi: 10.18689/mjnn-1000132
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The scarcity of water resources has stimulated the exploration of sewage treatment technique. On account of flexible separation and recycling, heterogeneous catalysis has aroused strong interest among researchers. In terms of the specific surface area, pore dimension and catalytic sites distribution, porous materials hold infinite potentials in heterogeneous catalysis. Among different porous materials, mesoporous material is most favorable in aqueous system. Moderate diffusion flux and highly efficient mass transfer are achievable due to their appropriate pore dimension, high specific surface area and good organic adsorption capacity. Moreover, most kinds of mesoporous materials are non-toxic, environmental-friendly and highly stable in both acidic and basic conditions. In this review, several kinds of mesoporous materials (such as siliceous mesoporous materials, carbonaceous mesoporous materials and metallic mesoporous materials) are introduced involving their characteristics, advantages, disadvantages as well as their application in wastewater treatment.
Keywords: Mesoporous materials; Catalytic support; Heterogeneous catalysis; Organic pollutants; Wastewater treatment.
Introduction
Water quality is vital to the survival of organisms in nature. With the rapid development of the economy, water consumptions both in household and industry are increasing. Although there exists an abundance of water on the earth, however, less than 1% of the water can be directly exploited by human. The scarcity of water resources has stimulated peopleʼs exploration of sewage treatment technique. Multitudinous methods have been applied to remove contaminants with different characteristics from wastewater. They can be divided into four categories, including physical method (filtration and gravity separation), chemical method (redox method and catalysis), physicochemical method (adsorption and ion exchange) and biological method (aerobic and anaerobic treatment). On account of flexible separation and recycling, heterogeneous catalysis has aroused strong interest among researchers to fulfill the requirements of green and economical treatment of wastewater. There are three primary steps during heterogeneous catalysis: the diffusion and adsorption of the reactants onto the interface of the solid catalyst; redox reactions happened at the catalytically active sites; the desorption and diffusion of the products away from the catalyst.
Since large catalyst surface area and well catalytic sites distribution (e.g. organic functional groups, metals etc.) are beneficial for the interactions between the reactants, porous-structured materials play an significant role in heterogeneous catalysis. Hence, numerous studies have been conducted to apply nanoporous materials as the catalyst or catalyst support for heterogeneous reactions. According to their pore size dimension, porous materials are classified by International Union of Pure and Applied Chemists (IUPAC) into three classes: macroporous material (pore size >50 nm), mesoporous material (pore size=2-50 nm) and microporous material (pore size <2 nm) [1]. Tremendous commercial profits and environmental revolution promote the application of microporous solids, especially microporous zeolite, in wastewater treatment process [2]. However, the poor mass transfer efficiency between microporous materials and large reactive molecules, particularly in liquid-phase systems, greatly restrict their further development in wastewater treatment [3]. Compared with microporous materials, mesoporous materials are more favorable in aqueous system since moderate diffusion flux and highly efficient mass transfer are achievable owing to their high specific surface area, suitable pore size distribution and good organic adsorption capacity [4]. In addition, those mesoporous structure provides large interface area for other guest species to react with. This endows mesoporous material capability to be functionalized. Therefore, the pore dimension, surface polarity, hydrophilicity and textural properties of mesoporous solid can be manipulated through surface modification with different functional groups [5]. This makes mesoporous material an excellent catalytic support for heterogeneous reactions.
The first ordered mesoporous silica was synthesized in 1992 with the reference of a preparation procedure described in a US patent in 1969 [6]. Later, other mesoporous materials (such as mesoporous carbon, mesoporous sulfide and mesopolymer) have been developed as well. The emergence of mesoporous materials attracted considerable attentions from researchers. Consequently, it has been applied in various fields ranging from gas and liquid adsorption, energy storage and conversion, catalysis, chromatography to biological researches [7].
Most of mesoporous materials are non-toxic, environmental-friendly and highly stable both in acidic and basic conditions. Moreover, through altering the structure and properties (like hydrophilic or hydrophobic) of mesoporous materials, it is feasible to change the state of the active centers for a specific reaction. This makes mesoporous material an excellent catalytic support for heterogeneous catalysis and thus endows it with immense potentials in wastewater treatment. To the best of our knowledge, there is no review devoted to mesoporous materials as catalyst support for wastewater treatment. The aim of this review is to present recent literatures concerning the applications of mesoporous materials on wastewater treatment. Different kinds of mesoporous materials were introduced including the characteristics, advantages, disadvantages as well as their applications as catalytic carriers in wastewater treatment.
Siliceous Mesoporous Materials
Naturally sourced mesoporous silica
The clays, formed by tetrahedral silicate [SiO4] and octahedral aluminate [AlO6] sheets, are a class of natural soil with a parallel layered structure. They are often detected from the soil sediments as well as bentonite (an aluminum phyllosilicate clay primarily comprised with montmorillonite and beidellite) [8,9]. Since the structure of the clays results from the crevices and staggered layer edges of the soil particles, the clays generally has a wide pore dimension distribution ranging from micropore to mesopore [10]. Cation ions (such as Na+, Ca2+, K+) are commonly found between the oxygen layers of the clay structure to maintain the charge balance. This provides the clay the capability of accepting guest species to insert into the interlayer space via ion-exchange [11]. The surface area and pore volume of the clays is designable through introducing different kinds of cation ions into the silicate layers. For example, larger cations generally create larger surface area and pore volume. The intercalation of guest species leads to the opening of the interlayer region, which is beneficial for the diffusion of the reactive molecules [12]. Besides, another outstanding advantage of the clay is its abundance in nature. Therefore, mesoporous clay as catalytic support for wastewater treatment has attracted lots of attention in the past decades.
Bentonite-clay-based Fe nanocomposite (Fe-B) was successfully synthesized via exchanging iron cations into the bentonite layers [13]. The catalytic activity of the prepared Fe-B was evaluated in photo-Fenton system to degrade the organic pollutant (Orange II, a sort of dye) with the reinforcement of an 8W UVC light source. Compared with amorphous FeOOH, calcined FeOOH and hematite (α-Fe2O3), Fe-B exhibited the best photocatalytic activity in both discoloration and mineralization of Orange II. Besides, loading copper onto acid-activated bentonite was also conducted and the as-prepared copper-bentonite composite (Cu-B) showed effective catalytic performance on the elimination of the textile organic pollutants [14]. Compared with mono-metallic bentonite composite, higher TOC removal percentage and less pH-sensitivity was reached by a bimetallic bentonite (Cu-Fe-B), which is attributed to the synergistic effect between copper and iron cations [15].
However, the natural clays often possess many impurities since their sources and formation environment are complicated. This problem can be solved by synthesized clay, which is commonly obtained from modification of natural inorganic minerals, oxides and glasses [16]. Through varying the preparation conditions, the physical and chemical properties of synthetic clays are adjustable. Laponite is one of the most commonly used synthetic clay. Laponite clay-based iron nanocomposite, primarily composed of Fe2O3 and Fe2Si4O10(OH)2, was successfully fabricated with pillaring technique and it displayed good catalytic activity in the degradation of several kinds of dyes, such as Reactive Red HE-3B, Acid Black 1 and Orange II [17-19].
Although the synthetic clay remedies the defects of natural clays, it suffers from severe swelling once contacted with water or other polar solvents. Furthermore, during the dehydration process, it is difficult to restore the original structure of the clays, and irreversible collapse of the mesoporous structure happened when the dehydration temperature is higher than 120°C [12]. Therefore, further investigation is required to obtain ideal synthetic clays for sewage treatment.
Sythesized mesoporous silica
Since first reported in 1992, ordered mesoporous silicas have become the focus of research [20]. The utilization of nano-templating method endows the synthesized silica an extremely ordered pore structure. In the past decades, various mesoporous silicas have been fabricated with different morphologies (such as sphere, rods, discs) and pore geometries. Commonly used mesoporous silicas include the Mobil Composition of Matter (MCM) family, the Santa Barbara Amorphous (SBA) family, the Michigan State University (MSU) family, the Fudan University (FDU) family and the Korea Adv. Inst. of Science and Technology (KIT) family [7].
The surface area, pore geometry and pore volume of the prepared mesoporous silicas is affected by the synthesis conditions, involving pH (acidic or basic), water content, temperature, the type of surfactant or template (anionic or cationic or zwitterionic) and the silicate source [21]. In general, mesoporous silica is formed in liquid phase through introducing a silicate source onto the surface of surfactant micelle. Therefore, the mesostructure of the prepared material strongly depends on the self-assembly behavior of the amphiphilic surfactant, which can be evaluated by the surfactant packing parameter. Essentially, ideal lamellar, cylindrical and spherical morphologies have surfactant packing parameters equal to 1, 1/2 and 1/3, respectively, which means a smaller surfactant packing parameter tend to form a mesophase with a higher surface mean curvature [22,23]. The packing mode of surfactant molecules in merely water system is primarily dominated by their concentration and geometry. However, the synthetic environment of the mesoporous silica is much more complicated, because the present of silicates could interfere the packing process of surfactant molecules via intermolecular force [24]. Through changing the pH, surfactant species and the concentration of silica, three kinds of MCM mesoporous material were formed [25]. According to the pore structure, they were classified into MCM-41 (hexagonal), MCM-48 (cubic) and MCM-50 (lamellar). In order to serve as an excellent catalytic support, mesoporous silica should possess large surface area (>500 m2/g), appropriate range of pore size (2–10 nm), and satisfied pore volume. Basically, pure mesoporous silica has almost no catalytic activity for chemical reactions due to their inert tetravalent Si atoms [5]. The existence of many accessible hydroxyl groups at the edge of ordered mesoporous silicas are not only favorable for them to be functionalized, but also beneficial for the mass transfer of the reactants [26].
Generally, incorporating active heteroatom or metal complex into the framework of mesoporous silica seems an intelligent approach to obtain mesoporous materials with good catalytic activity. With chemical vapor deposition (CVD) method, iron and copper were successfully loaded onto the surface of MCM-41. After photo-Fenton-like oxidation of Orange II, 80% of Total Organic Carbon (TOC) removal was achieved with the assistance of Cu/MCM-41 catalyst, while 93% of TOC removal was reached in the presence of bimetallic FeCu/MCM-41 catalyst [27,28]. In addition, Cu/MCM-41 was also applied for oxidation of phenol. In the presence of UV light and hydrogen peroxide, oxidation efficiency of phenol reached 70% within 90 min [29]. However, metal-leaching is obviously during the reactions because of the weak bonding between metal particles and MCM-41 supports. An in-situ oxidation method was carried out to mitigate this issue via the formation of metal-O-Si bonds to strengthen the bonding between metal particles and MCM-41 carriers [30,31]. In addition to MCM-41, SBA-15, another commonly used mesoporous molecular sieve, was also studied as a catalytic support. Applying copper (II) acetylacetonate as the precursor, hydrogen and ammonia as the reactant gas in the process of chemical vapor deposition, copper nanorod and copper(I) nitride nanorod were successfully synthesized onto the surface of SBA-15 [32]. Other metal oxides or nitrides were also be loaded onto the mesoporous silica support through varying the precursor and carrier gas.
Regardless of those benefits mentioned above, there are still some defects in ordered mesoporous silicas. In most cases, their preparation processes require extreme pH, high temperature and toxic chemicals, which are very expensive and harmful for the environment. The low yield of ordered mesoporous silicas, due to the complicated synthesis procedures, has limited their application in practical industry [33].
Carbonaceous Mesoporous Material
Ordered mesoporous carbons (OMCs)
OMCs, synthesized by nanocasting method, often hold the characteristics of large surface area, periodically arranged pore structure and relatively uniform pore size distribution. The purpose of nanocasting is using nanoparticles or nanoporous materials as the template to obtain the replica with reverse structure of the template. Nanocasting method, to some degree, could make up for the shortcomings of surfactant-assisted method. And it has been widely used in the preparation of mesoporous carbon materials, mesoporous metal oxide and other non-siliceous materials [34,35]. The synthesis process of OMCs mainly includes the selection of template and carbon precursor (acetylene gas, acrylonitrile, pyrrole, furfuryl alcohol, sucrose, etc.), carbonization under non-oxidizing atmosphere (N2 or inert gas) and removal of the template with strong acidic or alkaline solution. According to the characteristic of the template, it was divided into hard templating (using MgO nanoparticles, mesoporous silicas, colloidal silicas, alumina microfibers, or porous clay as the template), soft templating (using block copolymer surfactants, or metal-organic frameworks, as the template) and dual templating [36].
The development of OMCs is greatly attributed to the successful synthesis of various mesoporous silicas. The first reported OMCs, named as CMK-1 was synthesized in 1999 by infiltrating sucrose into the void of mesoporous silica sieves (MCM-48), followed by carbonization and silica removal [37]. Subsequently, CMK-3, a typical carbon replica of SBA-15, was also synthesized and has the structure of hexagonally arrayed carbon rods cross linked with each other. The synthesis process of CMK-3 and cubic CMK-1 is shown in figure 1 [38]. CMK-3 is considered as one of the most useful OMC support due to its uniform network, which makes the mass transfer of active substances easier inside the channels.
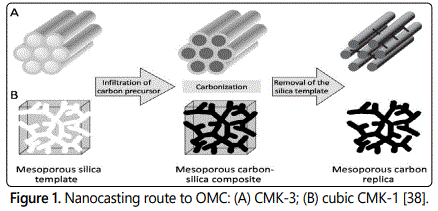
Generally, catalyst supported on mesoporous carbon would exhibit higher catalytic activities toward both degradation and TOC removal of organic pollutants, in comparison with the sole catalyst or catalyst loaded on conventional carbon black [36]. The enhanced performance is attributed to two reasons. One is that the dispersion of the small-sized metal particles onto the surface of mesoporous carbon can increase the interaction frequency between the reactants and catalytic metal particles. The other is that the good affinity between the mesoporous carbon support and the organic pollutant is favorable for the improvement of the catalytic performance. Large amount of studies has indicated that there exist high affinities between ordered mesoporous carbon (CMK-3) and many kinds of organic pollutants (such as orange II, reactive red 2 and acid black 1) [39,40]. This accelerates the diffusion of pollutants from the bulk area to the surface of the catalyst and thus promotes the interaction of reactants with catalytic sites. Besides, by means of incipient wetness impregnation method, Fe/OMC was successfully fabricated via loading iron particles onto the surface of OMC. 4-chlorophenol (4-CP) aqueous solution was used to evaluate the catalytic performance of the prepared Fe/OMC. Results indicated that 96.1% of degradation efficiency and 47.4% of TOC removal were achieved after 4.5 h under the best condition. Also, 88% of degradation efficiency was retained after three runs. The degradation of 4-CP was mainly attributed to the hydroxy radicals formed on the surface of the catalyst and the iron cations leached out [41].
Amorphous mesoporous carbons
Different from OMCs, the pore size and structure in amorphous carbons are irregular. Many approaches have been used to prepare amorphous mesoporous carbon, such as high temperature thermal activation, catalytic activation with metallic materials, siliceous template-based method, and carbonization of mesoporous polymers or organic aerogels [42]. In contrast to OMCs, the synthesis process of amorphous mesoporous carbons is relatively simple and less expensive. However, in most cases, the mesoporous voids in amorphous mesoporous carbon are isolated or interconnected randomly. Due to the principle of similar compatibility, carbonaceous mesoporous material has high adsorption capacity for organics, which is generally beneficial for the removal of organic contaminants. However, in some cases, the carbonaceous mesoporous support can be destroyed by the strong oxidizing species (e.g. hydroxyl radicals) during the catalytic reactions. Therefore, carbonaceous mesoporous materials are mostly applied in the adsorption of organic pollutants instead of working as the catalytic support.
Metallic Mesoporous Material
Mesoporous metal oxides
Similar to mesoporous carbon material, the invention of metallic mesoporous material was also initiated by the discovery of ordered mesoporous silica. Great efforts have been put on the synthesis of different kind of metallic mesoporous materials for wastewater treatment [43]. The techniques for the fabrication of mesoporous metal oxide can be summarized into five categories including soft-templating method, hard-templating method, reinforced crystallization method, post synthesis from solid–solid conversion, and self-assembly of nanoparticles. Owing to its special physicochemical properties, such as internal surfaces with redox activity, crosslinked pore network and unique d-shell electrons, mesoporous metal oxide can directly catalyze many kinds of reaction, apart from being the support for heterogeneous catalysis [44].
Among the mesoporous metal oxide family, mesoporous titanium oxide has attracted lots of attention due to its semiconductor property. Apart from its distinctive photosensitivity, those mesoporous channels existed in mesoporous titanium dioxide provide larger surface area and increased approachability for organic pollutants. Carbon-titanium oxide nanocomposites with a highly ordered 2D hexagonal mesostructure were synthesized by means of in-situ crystallization [45]. Carbon nanowires in the prepared nanocomposite served as adhesive to crosslink TiO2 nanocrystals. The photocatalytic activity of this nanocomposite was evaluated in Rhodamine B degradation process, and 89% of Rhodamine B was effectively degraded within 3 h. This was attributed to the synergistic effect of carbon and the titanium dioxide nanocrystals, where the former hold good adsorption capacity for Rhodamine B which increased mass transfer between contaminant molecules and titanium dioxide nanocrystals (active sites). In the light of the successful synthesis of mesoporous metal oxide from active carbon [46,47], combination of two nanocasting procedures was suggested to synthesize monodispersed mesoporous spheres, such as mesoporous TiO2, ZrO2 and γ-Al2O3 spheres [48]. The first nanocasting step was to form mesoporous carbon sphere with the template of mesoporous silica sphere. Then, using the as-prepared mesoporous carbon sphere as the template, divergent mesoporous metal oxide spheres can be created in second nanocasting step via varying the applied precursor.
In most cases, embedding other components, such as noble metal or semiconductor nanoparticles, onto the surface of mesoporous metal oxide is regarded as an effective way to enhance the photocatalytic activity of the material. An impregnation method was developed to incorporate metal nanoparticles into the framework of mesoporous materials, through immersing the mesoporous oxides in metallic solution, followed by an in-situ reduction process [49]. Nevertheless, this method cannot effectively manage the size of those synthetic metal particles, and they tend to be formed within the pore channel instead of on the surface of the mesoporous oxides. Another study was conducted to embed gold nanoparticles homogeneously into the framework of mesoporous TiO2 by a multicomponent assembly method [50]. Compared with bare mesoporous TiO2, enhanced photocatalytic activity was observed in this mesoporous Au/ TiO2 composite. The spectral absorption range, quantum effect and photoactivity of the prepared composite can be manipulated by varying the loading amount and particle dimension of gold. In addition, modification of mesoporous TiO2 with cadmium sulfide quantum dots (CdS QDs) was also investigated [51]. Cadmium oxide crystals were introduced into the porous structure of TiO2 and then transformed into CdS QDs via ion exchange method. Under the irradiation of visible light, the as-prepared composite exhibited much better catalytic performances than bare mesoporous TiO2 in both NOx oxidation and photodegradation of organic contaminants. The presence of CdS QDs not only enlarged the absorption spectra range of mesoporous TiO2 but also reduced the recombination rate of photoinduced charges through accelerating their transportation speed from inorganic sensitizer to TiO2.
Mesoporous metal sulfides/phosphate
In-depth studies on mesoporous metal oxides shed light on other kinds of metallic mesoporous materials, such as metal sulfide and metal phosphate. Contrast to mesoporous metal oxide, conventional nano-casting method is not applicable to the synthesis of mesoporous metal sulfide/ phosphate. The large volume difference between metal sulfide and its precursor (i.e., metallic salts) often result in the formation of individual metal sulfide particles instead of an interconnected pore framework of metal sulfide [52].
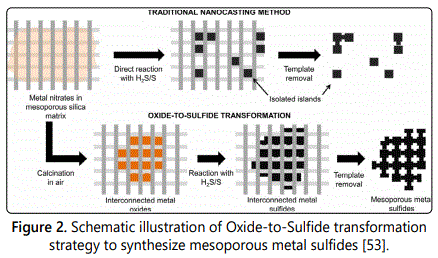
In order to avoid this problem, an “oxide-to sulfide” strategy (Figure 2) was proposed [53]. Making use of the mature preparation system of mesoporous metal oxide, mesoporous metal sulfide can be fabricated by directly transforming the metal oxide precursor into metal sulfide under an H2S/sulfur vapor atmosphere. Consequently, several mesoporous metal sulfides including FeS2, CoS2 and NiS2 were synthesized, and their performance was evaluated by removing methylene blue from aqueous solution. Compared with their corresponding nonporous catalyst, all of these three mesoporous metal disulfides displayed an enhanced adsorption capacity and photodegradation efficiency for methylene blue. The catalytic activity of mesoporous NiS2 was much lower than that of mesoporous FeS2 and CoS2. This is so because the states of Fe and Co element can easily change from 2+ to 3+, which is favorable for the formation of oxidizing radicals and thus promote the catalytic reactions [54,55].
Also, mesoporous metal phosphate (ZrP and AlP) spheres were prepared using the two-step nanocasting method mentioned above, of which the precursors contain phosphorus elements (e.g., triethylphosphate) [48]. The preparation process of mesoporous metal phosphate is relatively complicate [56]. Due to the fast reaction rate between metal cations and phosphate anions, the mesoporous structure is easy to collapse during the process of surfactants assembly or template removal [57]. In order to overcome these drawbacks, a polymeric micelle assembly method was designed and employed for the fabrication of mesoporous iron phosphate [58]. Asymmetric triblock co-polymer (PS-PVP-PEO) replaced conventional surfactants to form polymeric micelle, where polystyrene (PS), poly-2-vinylpyridine (PVP) and poly-ethylene oxide (PEO) separately served as porogens, reaction sites for inorganic precursors, and crosslinker to interlock the composite micelles during evaporation.
Overall, it can be concluded that the preparation of ordered mesoporous metal compound is a complex, expensive and scale-limited process. Despite of those drawbacks, the application potential of metallic mesoporous materials is still promising, since their unsaturated d-orbitals endow them the capability of accepting foreign electrons and donating electrons from d-orbitals. Therefore, metallic mesoporous material is considered to be an extraordinary catalytic support for heterogeneous reactions and could make great contributions for wastewater treatment in future.
Other Mesoporous Materials
Except those commonly used mesoporous materials, some atypical mesoporous materials were also investigated. For example, natural wood has numerous long, partially aligned mesoporous structure and nanochannels along with its growth direction. As illustrated in figure 3, palladium nanoparticles (Pd NPs) were in-situ formed in a three dimensional (3D) mesoporous basswood, in the purpose of dealing with wastewater containing methylene blue (MB) and NaBH4 [59]. The results showed that 99.8% of MB was efficiently degraded when the water flow rate was up to 1 × 105 L·m−2·h−1. In the porous structure of the hardwood, hydroxyl-rich cellulose and uniformly distributed lignin (worked as reducing agent) provide favorable conditions for the immobilization and in-situ synthesis of Pd NPs. Besides, the irregular channel structure (different channel diameters, vessel pits, and spiral thickenings) benefits the mass transfer of the contaminants in water as well as their interactions with the catalytic sites (Pd NPs).
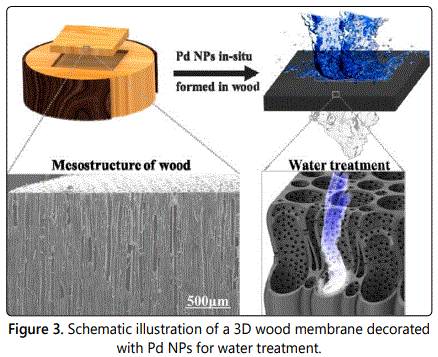
Note: the magnified images show the mesostructure of the wood perpendicular to the growth direction and in-situ formed Pd NPs within the wood where lignin acts as the reducing agent [59].
Owing to its mesoporous structure, organic framework, and high physicochemical stability in acidic or basic system, mesopolymer is also an ideal support for heterogeneous catalysis. It can be functionalized easily through post-graft or co-condensation approaches. Metallophthalocyanine (MPcs), a type of very significant photosensitizers, has been widely investigated in the field of organics photodegradation due to its extraordinary capability of absorbing energy from light source to generate high-active oxygen species [60,61]. However, when placed in aqueous medium, severe aggregation of MPcs would occur and thus result in self-quenching and deactivation of the excited species. Anchoring MPcs onto nanoporous materials is viable to solve these intrinsic obstacles. A sulfonated palladium phthalocyanine (PdPc) modified FDU-15 mesoporous polymer (FDU-PdPc) has been synthesized through chemical modification to disperse PdPc molecules evenly into the confined pore structure of mesoporous FDU-15 polymer [62]. π-π interactions between PdPc molecules and the organic framework dominated the process of preventing PdPc molecules from aggregation. With external addition of hydrogen peroxide, the prepared FDU-PdPc effectively catalyzed the degradation of 4-chlorophenol (4-CP) under visible light illumination. On the one hand, the affinity between 4-CP molecules and the aromatic species in the framework shortened their distance with the active sites. On the other hand, monodispersed PdPc molecules could avail themselves of high singlet oxygen yields and photostability [63,64].
In addition, using zeolite as the support for catalyst is considered as a profitable strategy. However, conventional zeolite is microporous substance with pore diameter in the range of 0.4-1.2 nm, which strongly affect the mass transfer in liquid phase and limit their application in water purification [65]. Using sphere-like carbon nanoparticles, nanotubes or nanofibers as the template, mesoporous zeolite with different pore geometry (cave-like mesopores or mesoporous channels) have been successfully prepared [66]. Compared with conventional zeolite, it is much easier to disperse catalytic active particles into the pore structure of mesoporous zeolitic support. For instance, previous studies successfully loaded metal (Pt), alloy (PtSn), and metal carbide in mesoporous zeolite for catalytic reactions [67]. Same as mesoporous silica, mesoporous zeolites possess promising potentials in water purification.
Conclusion
Summarily, this review introduced many kinds of mesoporous materials involving siliceous mesoporous material, carbonaceous mesoporous materials, metallic mesoporous materials and other mesoporous materials. Meanwhile, their characteristic, preparation method, advantage and disadvantage as well as their application in wastewater treatment were also discussed.
Due to its low cost and abundant resources, the clay, especially bentonite and laponite, has attracted lots of attention in wastewater treatment. However, there are lots of impurities in natural clays due to their variable resources and formation environment. Although the synthetic clay has a higher purity than natural clay, its swelling problems when contacted with water or other polar solvents lead to poor recyclability in dehydration process. Ascribed to the utilization of nano-templating method, ordered mesoporous silicas possess highly ordered pore structure, adjustable morphology and narrow pore diameter distribution. The inert framework of ordered mesoporous silicas and the accessible hydroxyl groups at their wall provide them the capability to be functionalized as well as to facilitate the mass transfer of the reactants. Besides, the morphology and porous structure of the prepared mesoporous silica can be manipulated through varying the synthesis conditions. However, in most cases, the preparation process of mesoporous silica requires extreme pH condition, high temperature and toxic chemicals, which is very expensive and harmful. Also, the low yield of ordered mesoporous silicas, due to the complicated synthesis procedures, limited their application in practical industry. Due to similar compatibility, carbonaceous mesoporous material generally has good affinity with organic molecules. This can accelerate the mass transfer between the contaminants and active sites and thus benefit the catalytic performance. However, carbonaceous mesoporous support is relatively unstable under extreme conditions. Moreover, the synthesis cost of OMCs is more expensive than that of ordered mesoporous silica since most of OMCs is obtained from ordered mesoporous silica. Due to its special properties of cross linked pore network and unique d-shell electron, the most distinctive feature of metallic mesoporous material is that the metallic framework itself has catalytic activity on some reactions. Until now, many kinds of metallic mesoporous materials have been prepared, such as mesoporous metal oxide, mesoporous metal sulfide and mesoporous metal phosphate. In most cases, their synthesis process is not a direct one-step method but complicated, expensive and hard to scale up. Investigation of an alternative synthesis method is necessary. Except those commonly used mesoporous materials, some atypical mesoporous materials were also elaborated including natural mesoporous wood, polymer-based mesoporous material and mesoporous zeolite.
Using mesoporous material as support endows the catalyst benefits of moderated pore structure, high surface area and well-dispersed active centers. This could not only improve the selectivity of the catalyst for a certain reaction, but also accelerate the mass transfer of the intermediates inside the mesopores, and thus enhance catalytic performance of the catalyst. It is undoubtable that mesoporous materials have infinite potentials in wastewater treatment. Further studies are required to overcome those drawbacks presented in current mesoporous materials.
Acknowledgement
This work was financially supported by the Innovation and Technology Commission of Hong Kong and Guangdong Guanghua Sci-Tech Company Ltd through a grant number of GHX/017/18.
References
- Sing KSW, Everett DH, Haul R, et al. Reporting physisorption data for gas/solid systems with Special Reference to the Determination of Surface Area and Porositys. Pure Appl Chem. 1985; 57(4): 603-619. doi: 10.1515/iupac.57.0007
- Li Z, Zhou Y, Tao D, Huang W, Chen X, Yang Z. MOR zeolite supported Brønsted acidic ionic liquid: an efficient and recyclable heterogeneous catalyst for ketalization. RSC Adv. 2014; 4(24): 12160-12167. doi: 10.1039/C4RA00092G
- Venuto PB. Organic catalysis over zeolites: a perspective on reaction paths within micropores. Microporous Materials. 1994; 2(5): 297-411. doi: 10.1016/0927-6513(94)00002-6
- Pal N, Bhaumik A. Mesoporous materials: versatile supports in heterogeneous catalysis for liquid phase catalytic transformations. RSC Adv. 2015; 5(31): 24363-24391. doi: 10.1039/C4RA13077D
- Taguchi A, Schüth F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005; 77(1): 1-45. doi: 10.1016/j.micromeso.2004.06.030
- Di Renzo F, Cambon H, Dutartre R. A 28-year-old synthesis of micelletemplated mesoporous silica. Microporous Materials. 1997; 10(4-6): 283-286. doi: 10.1016/S0927-6513(97)00028-X
- Suib SL. A review of recent developments of mesoporous materials. Chem Rec. 2017; 17(12): 1169-1183. doi: 10.1002/tcr.201700025
- Ng E, Mintova S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008; 114(1-3): 1-26. doi: 10.1016/j.micromeso.2007.12.022
- Chiou CT, Rutherford DW. Effects of exchanged cation and layer charge on the sorption of water and EGME vapors on montmorillonite clays. Clays Clay Miner. 1997; 45: 867-880. doi: 10.1346/CCMN.1997.0450611
- McKissock I, Gilkes RJ, Walker EL. The reduction of water repellency by added clay is influenced by clay and soil properties. Appl Clay Sci. 2002; 20(4-5): 225-241. doi: 10.1016/S0169-1317(01)00074-6
- Rutherford DW, Chiou CT, Eberl DD. Effects of exchanged cation on the microporosity of montmorillonite. Clays Clay Miner. 1997; 45(4): 534-543. doi: 10.1346/CCMN.1997.0450405
- Smart LE, Moore EA. Solid state chemistry: an introduction. 3rd edition. Boca Raton: Taylor & Francis; 2005.
- Feng J, Hu X, Po LY. Discoloration and mineralization of Orange II using different heterogeneous catalysts containing Fe: A comparative study. Environ Sci Technol. 2004; 38(21): 5773-5778. doi: 10.1021/es049811j
- Yip AC, Lam FL, Hu X. A novel heterogeneous acid-activated clay supported copper catalyst for the photobleaching and degradation of textile organic pollutant using photo-Fenton-like reaction. Chem Commun. 2005; (25): 3218-3220. doi: 10.1039/B501531F
- Yip AC, Lam FL, Hu X. Novel bimetallic catalyst for the photo-assisted degradation of Acid Black 1 over a broad range of pH. Chem Eng sci. 2007; 62(18): 5150-5153. doi: 10.1016/j.ces.2007.01.014
- Kloprogge JT. Synthesis of Smectites and Porous Pillared Clay Catalysts: A Review. Journal of Porous Materials. 1998; 5(1): 5-41. doi: 10.1023/A:1009625913781
- Feng J, Hu X, Yue PL, Zhu HY, Lu GQ. Discoloration and mineralization of Reactive Red HE-3B by heterogeneous photo-Fenton reaction. Water Res. 2003; 37(15): 3776-3784. doi: 10.1016/S0043-1354(03)00268-9
- Sum OSN, Feng J, Hu X, Yue PL. Pillared laponite clay-based Fe nanocomposites as heterogeneous catalysts for photo-Fenton degradation of acid black 1. Chem Eng sci. 2004; 59(22-23): 5269-5275. doi: 10.1016/j.ces.2004.09.032
- Yue PL, Feng JY, Hu X. Photo-Fenton reaction using a nanocomposite. Water Sci Technol. 2004; 49(4): 85-90. doi: 10.2166/wst.2004.0228
- Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992; 359(6397): 710-712. doi: 10.1038/359710a0
- Sayari A, Hamoudi S. Periodic mesoporous silica-based organic-inorganic nanocomposite materials. Chem Mater. 2001; 13(10): 3151-3168. doi: 10.1021/cm011039l
- Israelachvili JN, Mitchell DJ, Ninham BW. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J Chem Soc Faraday Trans 2. 1976; 72: 1525-1568. doi: 10.1039/F29767201525
- Israelachvili JN. Intermolecular and Surface Forces. 3rd edition. 2011. doi: 10.1016/C2011-0-05119-0
- Huo Q, Margolese DI, Stucky GD. Surfactant control of phases in the synthesis of mesoporous silica-based materials. Chem Mater. 1996; 8(5): 1147-1160. doi: 10.1021/cm960137h
- Hoffmann F, Cornelius M, Morell J, Fröba M. Cover Picture: Silica-Based Mesoporous Organic–Inorganic Hybrid Materials (Angew. Chem. Int. Ed. 20/2006). Angew Chem Int Ed Engl. 2006; 45(20): 3187-3187. doi: 10.1002/anie.200690070
- Schwarz JA, Contescu CI, Putyera K. Dekker encyclopedia of nanoscience and nanotechnology. New York: CRC Press; 2004.
- Lam FLY, Yip ACK, Hu X. Copper/MCM-41 as a highly stable and pHinsensitive heterogeneous photo-Fenton-like catalytic material for the abatement of organic wastewater. Ind Eng Chem Res. 2007; 46(10): 3328-3333. doi: 10.1021/ie061436b
- Lam FL, Hu X. A high performance bimetallic catalyst for photo-Fenton oxidation of Orange II over a wide pH range. Catal Commun. 2007; 8(12): 2125-2129. doi: 10.1016/j.catcom.2007.04.025
- Hu X, Lam FL, Cheung LM, Chan KF, Zhao XS, Lu GQ. Copper/MCM-41 as catalyst for photochemically enhanced oxidation of phenol by hydrogen peroxide. Catal Today. 2001; 68(1-3): 129-133. doi: 10.1016/S0920-5861(01)00273-5
- Lam FL, Hu X. In situ oxidation for stabilization of Fe/MCM-41 catalyst prepared by metal organic chemical vapor deposition. Catal Commun. 2007; 8(11): 1719-1723. doi: 10.1016/j.catcom.2007.02.009
- Lam FL, Hu X. pH-Insensitive bimetallic catalyst for the abatement of dye pollutants by photo-fenton oxidation. Ind Eng Chem Res. 2013; 52(20): 6639-6646. doi: 10.1021/ie302864e
- Zhang Y, Lam FL, Hu X, Yan Z. Fabrication of copper nanorods by lowtemperature metal organic chemical vapor deposition. Chin Sci Bull. 2006; 51(21): 2662-2668. doi: 10.1007/s11434-006-2128-7
- Diagboya PNE, Dikio ED. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Microporous Mesoporous Mater. 2018; 266: 252-267. doi: 10.1016/j.micromeso.2018.03.008
- Chang H, Joo SH, Pak C. Synthesis and characterization of mesoporous carbon for fuel cell applications. J Mater Chem. 2007; 17(30): 3078-3088. doi: 10.1039/b700389g
- Deng X, Chen K, Tuüysuüz H. Protocol for the nanocasting method: Preparation of ordered mesoporous metal oxides. Chem Mater. 2017; 29(1): 40-52. doi: 10.1021/acs.chemmater.6b02645
- Inagaki M, Toyoda M, Soneda Y, Tsujimura S, Morishita T. Templated mesoporous carbons: Synthesis and applications. Carbon N Y. 2016; 107: 448-473. doi: 10.1016/j.carbon.2016.06.003
- Ryoo R, Joo SH, Jun S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J Phys Chem B. 1999; 103(37): 7743-7746. doi: 10.1021/jp991673a
- Walcarius A. Recent trends on electrochemical sensors based on ordered mesoporous carbon. Sensors (Basel). 2017; 17(8): 1863. doi: 10.3390/s17081863
- Peng X, Hu X, Fu D, Lam FLY. Adsorption removal of acid black 1 from aqueous solution using ordered mesoporous carbon. Appl Surf Sci. 2014; 294: 71-80. doi: 10.1016/j.apsusc.2013.11.157
- He C, Hu X. Anionic Dye Adsorption on Chemically Modified Ordered Mesoporous Carbons. Ind Eng Chem Res. 2011; 50(24): 14070-14083. doi: 10.1021/ie201469p
- Duan F, Yang Y, Li Y, Cao H, Wang Y, Zhang Y. Heterogeneous Fenton-like degradation of 4-chlorophenol using iron/ordered mesoporous carbon catalyst. J Environ Sci. 2014; 26(5): 1171-1179. doi: 10.1016/S1001-0742(13)60532-X
- Kyotani T. Control of pore structure in carbon. Carbon N Y. 2000; 38(2): 269-286. doi: 10.1016/S0008-6223(99)00142-6
- Ren Y, Ma Z, Bruce PG. Ordered mesoporous metal oxides: synthesis and applications. Chem Soc Rev. 2012; 41(14): 4909-4927. doi: 10.1039/c2cs35086f
- Rao Y, Antonelli DM. Mesoporous transition metal oxides: characterization and applications in heterogeneous catalysis. J Mater Chem. 2009; 19(14): 1937-1944. doi: 10.1039/b813533a
- Liu R, Ren Y, Shi Y, et al. Controlled synthesis of ordered mesoporous C-TiO2 nanocomposites with crystalline titania frameworks from organicinorganic-amphiphilic coassembly. Chem Mater. 2008; 20(3): 1140-1146. doi: 10.1021/cm071470w
- Schwickardi M, Johann T, Schmidt W, Busch O, Schüth F. High surface area metal oxides from matrix assisted preparation in activated carbons. In: Gaigneaux E, De Vos DE, Jacobs PA, et al. (eds). Scientific Bases for the Preparation of Heterogeneous Catalysts. Boston: Elsevier; 2002. 93-100.
- Wakayama H, Itahara H, Inagaki N, Fukushima S, Tatsuda Y. Nanoporous metal oxides synthesized by the nanoscale casting process using supercritical fluids. Chem Mater. 2001; 13(7): 2392-2396. doi: 10.1021/cm001408y
- Dong A, Ren N, Tang Y, et al. General synthesis of mesoporous spheres of metal oxides and phosphates. J Am Chem Soc. 2003; 125(17): 4976-4977. doi: 10.1021/ja029964b
- Wang X, Yu JC, Ho C, Mak AC. A robust three-dimensional mesoporous Ag/TiO2 nanohybrid film. Chem Commun. 2005; 17: 2262-2264. doi: 10.1039/b500605h
- Li H, Bian Z, Zhu J, Huo Y, Li H, Lu Y. Mesoporous Au/TiO2 nanocomposites with enhanced photocatalytic activity. J Am Chem Soc. 2007; 129(15): 4538-4539. doi: 10.1021/ja069113u
- Li G, Zhang D, Yu J. A New Visible-Light Photocatalyst: CdS Quantum Dots Embedded Mesoporous TiO2. Environ Sci Technol. 2009; 43(18): 7079. doi: 10.1021/es9011993
- Shi Y, Wan Y, Liu R, Tu B, Zhao D. Synthesis of highly ordered mesoporous crystalline WS2 and MoS2 via a high-temperature reductive sulfuration route. J Am Chem Soc. 2007; 129(30): 9522-9531. doi: 10.1021/ja072910n
- Yonemoto B, Hutchings G, Jiao F. A General Synthetic Approach for Ordered Mesoporous Metal Sulfides. J Am Chem Soc. 2014; 136(25): 8895-8898. doi: 10.1021/ja504407e
- Houas A, Lachheb H, Ksibi M, Elaloui E, Guillard C, Herrmann J. Photocatalytic degradation pathway of methylene blue in water. Appl Catal B. 2001; 31(2): 145-157. doi: 10.1016/S0926-3373(00)00276-9
- Wu C, Chern J. Kinetics of photocatalytic decomposition of methylene blue. Ind Eng Chem Res. 2006; 45(19): 6450-6457. doi: 10.1021/ie0602759
- Wan Y, Yang H, Zhao D. “Host-guest” chemistry in the synthesis of ordered nonsiliceous mesoporous materials. Acc Chem Res. 2006; 39(7): 423-432. doi: 10.1021/ar050091a
- Lin RH, Ding Y. A Review on the Synthesis and Applications of Mesostructured Transition Metal Phosphates. Materials (Basel). 2013; 6(1): 217-243. doi: 10.3390/ma6010217
- Bastakoti BP, Li Y, Guragain S, et al. Synthesis of Mesoporous TransitionMetal Phosphates by Polymeric Micelle Assembly. Chemistry. 2016; 22(22): 7463-7467. doi: 10.1002/chem.201600435
- Chen F, Gong AS, Zhu M, et al. Mesoporous, Three-Dimensional Wood Membrane Decorated with Nanoparticles for Highly Efficient Water Treatment. ACS Nano. 2017; 11(4): 4275-4282. doi: 10.1021/acsnano.7b01350
- Meunier B, Sorokin A. Oxidation of pollutants catalyzed by metallophthalocyanines. Acc Chem Res. 1997; 30: 470-476. doi: 10.1021/ar960275c
- Rawling T, Mcdonagh A. Ruthenium phthalocyanine and naphthalocyanine complexes: Synthesis, properties and applications. Coord Chem Rev. 2007; 251(9): 1128-1157. doi: 10.1016/j.ccr.2006.09.011
- Xing R, Wu L, Fei Z, Wu P. Palladium phthalocyaninesulfonate functionalized mesoporous polymer: A highly efficient photocatalyst for degradation of 4-chlorophenol under visible light irradiation. J Mol Catal A Chem. 2013; 371: 15-20. doi: 10.1016/j.molcata.2013.01.022
- Xiong Z, Xu Y. Immobilization of palladium phthalocyaninesulfonate onto anionic clay for sorption and oxidation of 2,4,6-trichlorophenol under visible light irradiation. Chem Mater. 2007; 19(6): 1452-1458. doi: 10.1021/cm062437x
- Sun A, Xiong Z, Xu Y. Removal of malodorous organic sulfides with molecular oxygen and visible light over metal phthalocyanine. J Hazard Mater. 2008; 152(1): 191-195. doi: 10.1016/j.jhazmat.2007.06.105
- Corma A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem Rev. 1997; 97(6): 2373-2419. doi: 10.1021/cr960406n
- Meng X, Nawaz F, Xiao F. Templating route for synthesizing mesoporous zeolites with improved catalytic properties. Nano Today. 2009; 4(4): 292-301. doi: 10.1016/j.nantod.2009.06.002
- Christensen CH, Schmidt I, Carlsson A, Johannsen K, Herbst K. Crystals in Crystals Nanocrystals within Mesoporous Zeolite Single Crystals. J Am Chem Soc. 2005; 127(22): 8098-8102. doi: 10.1021/ja050380u


