Mini Review
PVDF based Nanocomposite Membranes: Application towards Wastewater treatment
1Department of Chemistry, University of Poonch Rawalakot, Azad Kashmir, Pakistan
2NanoScience and Technology Department, National Centre for Physics, Islamabad, Pakistan
*Corresponding author: Nida Salim, Department of Chemistry, University of Poonch Rawalakot, Azad Kashmir, Pakistan, Tel: 009251-2077480 E-mail: a.sam.malik@gmail.com
Received: April 22, 2019 Accepted: April 29, 2019 Published: May 8, 2019
Citation: Salim N, Siddiqa A, Shahida S, Qaisar S. PVDF based Nanocomposite Membranes: Application towards Wastewater treatment. Madridge J Nanotechnol Nanosci. 2019; 4(1): 139-147. doi: 10.18689/mjnn-1000128
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Water is precious natural resource on earth but rapid industrialization and effluent discharge from domestic, agriculture and municipal wastes is polluting water continuously. Membrane technology provide solution to water related problems and used as an attractive tool for removal of pollutants from water. Different types of polymeric membranes are used for wastewater treatment but certain drawbacks are related to polymeric membranes such as hydrophobicity, fouling and low mechanical strength. Incorporation of nanoparticle in polymeric membranes enhances the membrane properties. Recently nanocomposite membranes are developed that increased hydrophilicity, improved mechanical properties and enhanced rejection efficiencies of polymeric membranes. Among different types of polymeric membranes, polyvinylidene fluoride nanocomposites membranes are widely used for removing various contaminants from wastewater. It is reported that polyvinylidene fluoride based nanocomposite membranes possess good separation efficiency for the removal of different pollutants. In this review several polyvinylidene fluoride membranes incorporated with metal oxide such as titanium dioxide, aluminium oxide, silicon oxide, zinc oxide, carbon nanotubes and graphene oxide based nanocomposite membranes have been discussed for wastewater treatment. The current study objective is to summarize the applications of polyvinylidene fluoride based nanocomposite membranes for the removal of different pollutants from wastewater.
Keywords: Polyvinylidene fluoride; Nanocomposites; Hydrophilicity; Modification; Blending; Waste water.
Introduction
Water is basic necessity of life, precious natural source but due to increased population and urbanization its availability becomes limited. Numerous contaminants produced from various industries and domestic discharges pollute the water continuously. Different industries such as agriculture, paper, textile and live stock generate billion gallons of wastewater which continuously degrade the water quality [1]. About 70% of the earth is covered with water. Approximately 97% is covered by sea water which is useless for human consumption due to high salt content, 2% saved in the form of glaciers, ice caps and only 1% available as fresh water. Access to fresh water is one of the major challenges of twenty first century, according to one of the estimation of WHO (World Health Organization) approximately 1.1 billion people lack access to clean water [2]. Water shortage has become more severe due to exploitation of water resources such as industrial, domestic, agriculture and municipal waste effluents discharge in water bodies.
Water pollution cause severe threat to human, plants and animals; pollutants discharged from industries contain salt, surfactant and heavy metals which affect the entire environment. Large number of disease provoke by drinking polluted water such as cholera, diphtheria, hepatitis and malaria etc. To overcome the water related problems there is need to access new water sources or protect existing sources through water treatment techniques. Different conventional methods such as adsorption, advance oxidation process, gravity separation, skimming, coagulation, centrifugation and membrane separation have been applied for water treatment. Membrane technology is considered one of the emerging technologies for wastewater treatments [3] because it is cost effective, simply to operate, possess high separation efficiency and minimal waste production. Other techniques are found to be expensive, difficult to operate and produce toxic waste.
Membrane is a semi permeable barrier which allows wanted materials to pass through it and retain the unwanted material on the surface [4]. Membrane technology is an effective technique for wastewater treatment due to easy operation and high productivity without addition of chemical additives. Processes that are being used in conventional treatment plants such as secondary sedimentation, settling tanks flocculation and granular filtration have been replaced by membrane system [5].
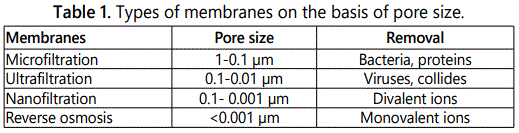
Different types of membranes used for wastewater filtration on the basis of their pore size and rejection mechanismiemicrofiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO) [6]. Microfiltration and ultrafiltration are known as low pressure membranes and nanofiltration/reverse osmosis commonly called high pressure membranes due to small pore size. Different types of membranes, along with pore size are given in table 1.
Membrane has been classified into two major categories i-e inorganic and polymeric membranes. Inorganic membranes are made up of ceramics such as titanium oxide (TiO2), aluminium oxide (Al2O3), zirconium oxide (ZrO2) and silicon oxide (SiO2) [7]. Ceramic membranes are widely used in water treatment applications because they possess high thermal and mechanical strength. Ceramic membranes can be used under extreme pH and high temperature conditions but high cost of ceramic membranes makes them less attractive.
Now a dayʼs polymeric membranes has got much attention for water treatment due to low cost, high flexibility and membrane forming properties. Polymeric membranes consist of organic polymers such as Polysulfones (PSF), Polyether sulfone (PES), Polyacrylonitrile (PAN), Polyvinyl alcohol (PVA), Polypropylene (PP), Poly tetrafluroethylene (PTFE) and Polyvinylidene fluoride (PVDF) [8]. Researchers paid much attention on polyvinylidene fluoride polymer due to its unique properties i-e inert to chemicals and oxidants, membrane forming properties, good mechanical strength and high thermal stability. Owing to these characteristic features polyvinylidene fluoride membranes have been commonly applied for water treatment, recovery of biofuel, gas separation, pollutants removal from water and separator for lithium ion batteries [9]. PVDF is considering as excellent membrane material because easily dissolves in organic solvent and develops porous membrane structure by simple phase inversion method. However applications of PVDF membrane are restricted by major problem such as fouling in wastewater treatment.
Membrane fouling is accumulation of macromolecules, collides, microorganism and salts on the surface of membrane or inside the pores of membranes. Fouling is caused by various factors such as adsorption of organic molecule, particulate deposition and microbial adhesion on the membrane surface. Decline in permeation flux and reduction in membrane life is due to fouling and ultimately leads to failure of membrane performance [10]. Polyvinylidene fluoride membranes are hydrophobic in nature and are easily affected by fouling. Different methods have been adopted to overcome fouling problems related to polyvinylidene fluoride membrane (Figure 1). Pretreatment techniques which reduce fouling are physicaland chemical cleaning of membrane.
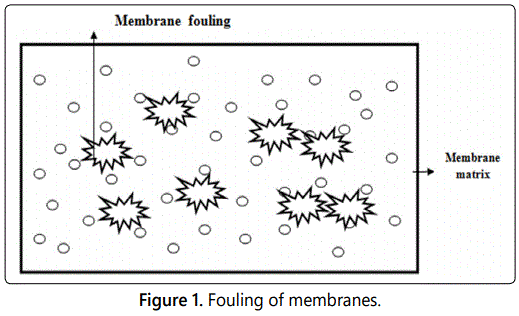
Physical cleaning performed by backwashing of membranes while chemical cleaning achieved by using various chemicals (acids, caustic soda etc). Chemical cleaning of membrane damage the membrane material so it is not an effective method to improve fouling therefore modification of PVDF is necessary [11].
This review provides an overview of the different modification methods for polyvinylidene fluoride based membranes for improving hydrophilicity. This review also provides an insight on various polyvinylidene fluoride metal oxide based nanocomposite membranes for wastewater treatment. Different carbon based polyvinylidene fluoride nanocomposite membranes such as carbon nanotubes based composite membranes and graphene based nanocomposite membrane for wastewater treatment have been also discussed in detail.
Modification of PVDF Membranes
As discussed previously, hydrophobic nature of PVDF reduces the applications of polyvinylidene fluoride membranes in separation and purification of wastewater. Various methods have been applied for modification of PVDF membrane to overcome fouling (i) bulk modification blending of polymer with hydrophilic additives, (ii) surface modification (coating of PVDF with hydrophilic polymer). Hydrophilic modification of PVDF can be attained during preparation process.
Bulk modification of PVDF membrane
Bulk modifications of PVDF can be attained during preparation process. Bulk modifications can be done in two ways (i) polymer blending (polymer addition) and (ii) incorporation of nanomaterial.
Polymer blending: One approach to reduce fouling and increase the hydrophilicity of polyvinylidene fluoride membrane is blending of PVDF with hydrophilic polymer. Different polymers are used to increase the hydrophilicity of PVDF membrane such as polyacrylonitrile (PAN) [12], polyvinyl alcohol (PVA) [13], polyethylene glycol (PEG) [14] and polyvinyl pyrrolidene (PVP) etc., [15]. Among them polyvinyl alcohol is well known polymer for fabricating membranes with good hydrophilic properties [16]. PVA blend with polyvinylidene fluoride stronger intermolecular interaction develops among two polymer chains of PVDF and PVA which improve the hydrophilicity [17].
Poly (vinyl pyrrolidone) PVP is widely used hydrophilic polymer for preparing PVDF membranes to regulate pore size and prevent fouling due to its hydrophilic nature [18]. PVP promotes the formation of large finger like macrovoids during immersion in the coagulation bath [19]. By increasing the concentration, significant increase in surface porosity and pore size of the membrane thus increases the permeation flux [20].
Another well known pore forming agent is polyethylene glycol which favors microvoid formation during the fabrication and impart hydrophilic character to the polyvinylidene fluoride membrane. Blending of PEG with PVDF adjust the thermodynamics and kinetics of casting solution and control the morphology of membrane and reduce fouling.
Poor compatibility of polymer with hydrophobic PVDF matrix is one of the predominantly issue in polymer blending process. PVDF/PVA blends, revealed incompatibility [13], during the phase separation technique. So, some researchers have looked into the use of amphiphilic copolymer as modifier to solve this issue.
Amphiphilic modifier possesses both hydrophilic and hydrophobic properties [21]. During phase inversion process the hydrophobic chains ensure the compatibility with host PVDF polymer, while the hydrophilic chains reinforce onto the membrane pore surface [22]. For the fabrication of hydrophilic PVDF membranes commonly used amphiphilic copolymers are PS-b-PEGMA (polystyrene–polyethylene glycol methacrylate [10], P(MMA- r-POEM) polymethyl methacrylate [23], and PVC-g-P(PEGMA) [24].
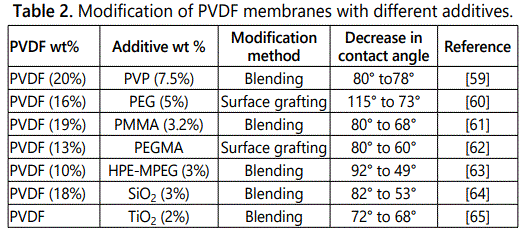
Liu et al. fabricated copolymer consist of polyvinylidene fluoride backbone and polyacrylomorpholine (PACMO) side chain membrane by radical polymerization technique, the resultant membranes showed better resistance to fouling and excellent hydrophilicity [25]. Table 2 represents the modification of PVDF membranes with different additives [26-32,60-66].
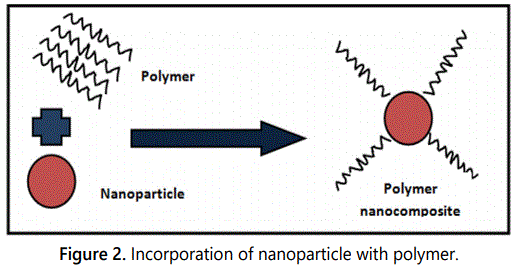
Incorporation of nanomaterials: The inorganic nanomaterials are promising modifier apart from hydrophilic polymer to minimize the fouling. The incorporation of nano material into polymer matrix has become an interesting approach for reducing fouling of PVDF membranes [33,34]. Blending of PVDF with nanomaterial, alter the intrinsic features of the composites. Various nanomaterials such as carbon nanotubes, graphene, alumina, titanium oxide and other nano scale materials are used for enhancement of hydrophilicity, antifouling and separation properties of PVDF membrane (Figure 2). The introduction of inorganic nano material influences the membrane performance in following ways:
- Nanomaterials improve the fouling and the hydrophilicity of membranes [35].
- Amplify the mass transfer during the prevaporation technique [36].
- Enhance selectivity and solute rejection efficiency of membrane [37].
- Improve the mechanical and thermal properties [38,39].
Surface modification of PVDF membrane
Surface modification is effective strategy to increase hydrophilicity of PVDF membranes. Purpose of surface modification is formation of hydrophilic layer on membrane surface which prevent the contact between membrane surface and pollutants thus diminishing fouling. Surface modification categories as physical modification and chemical modification.
In physical modification hydrophilic modifier bound to PVDF membrane by physical interaction not by covalent bonding, the chemical composition of the membranes remains unchanged. Physical modification of polyvinylidene fluoride membrane can be attained by the membrane surface directly coated with hydrophilic polymer or membrane is coated by solution of chemically active monomers. Xi et al. demonstrated study on physical modification of hydrophobic polyvinylidene fluoride membrane by coating with dopamine. The dopamine firmly attachs to membrane surface and contact angle of dopamine coated membrane reduce remarkably as compared to pristine membrane. Decrease in contact angle suggested improved hydrophilicity due to addition of hydroxyl, carboxyl and amino groups at membrane surface [40].
Chanachai and his cowokers researched on coating of hydrophobic PVDF membrane with chitosan by dip coating method. Coating of hydrophilic chitosan polymer increases the hydrophilicity of membrane by decreasing the repulsive forces between hydrophobic PVDF and water molecules. Diffusion of water through hydrophilic chitosan layer enhanced water flux resulted in increased hydrophilicity [41].
In chemical modification polyvinylidene fluoride membrane modified through covalent bonding interaction PVDF chains firstly activated by chemical reaction or high energy radiation such as plasma graft, UV photo irradiation and electron beam radiations.
Yang et al. fabricated hydrophilic polyvinylidene fluoride membrane by electron beam induced graft polymerization. Fourier transform infrared spectroscopy verified the successful attachment of hydrophilic monomers on membrane surface by polymerization. Contact angle decrease from 93° to 35° indicated the improved hydrophilicity of modified polyvinylidene fluoride membranes [42].
Han et al. modified hydrophobic PVDF membranes through defluorination and sulfonation. SEM analysis confirmed that chemical modification did not adversely affect the membrane structure and pore size. Incorporation of hydrophilic sulfonic acid moieties increased the surface charge and wettability results in increased membrane hydrophilicity and water flux [43]. Chemical modification usually requires costly chemicals and special instruments which limited their practical application.
Mixed Matrix Membranes
Mixed matrix membranes are modified polymeric membranes with nano material dispersed in their matrix. During the synthesis of membrane, nano filler is incorporated into polymer so called mixed matrix or nanocomposite membranes. Nanocomposite membranes have received worldwide attention in gas separation, sensor application, direct methanol fuel cell and water treatment industry. Nanocomposite membranes able to face all challenges of polymeric membranes like fouling and its applications in waste water treatment.
Incorporation of nanoparticle into water filtration membranes change the membrane properties such as wastewater rejection capacity, increase water permeability, enhance flux and antifouling behavior [44]. Nanoparticles provide better hydrophilicity, pore channels and large surface area to polyvinylidene fluoride matrix [45]. Different metal oxide nanoparticles such as titanium oxide, aluminum oxide, silicon oxide and zinc oxide are incorporated into PVDF membranes for wastewater treatment.
PVDF Metal Oxide based Nanocomposite
Titanium dioxide based nanocomposite membrane
TiO2 is considered as a promising material for development of nanocomposite ultrafiltration membrane due to its hydrophilic character. It is used as filler with different polymers like polyvinylidene fluoride, cellulose acetate, polyether sulfone and polypropylene. Different phases of TiO2 play very important role in the solute transport of polymeric membranes [46]. PVDF titanium dioxide based nanocomposite not only improves the hydrophilicity, but also increase water flux and reduce the fouling issue of polyvinylidene fluoride [35].
Yuliwati et al. developed PVDF titanium nanocomposite membranes by phase inversion method and used for the filtration of oily wastewater. Addition of 1.95% TiO2 resulted in higher hydrophilicity, small pore size and porosity. This is due to the reason that TiO2 attract the water molecules inside the composite membrane, enhancing flux and rejection capacity [47].
Teow et al. developed polyvinylidene fluoride titania mixed matrix membrane by phase inversion and colloidal precipitation method. The performance of the fabricated ultrafiltration membranes was evaluated by measuring the membrane permeate flux and humic acid rejection. Results demonstrated that improvement of membrane flux and rejection of humic acid reached to 98.44% due to pore enlargement and enhanced hydrophilicity resulted from close polymer chain packing by titania nanoparticles [48].
Ong et al. synthesized series of PVDF-PVP-TiO2 composite membrane by dry jet wet spinning method and used for the treatment of oily wastewater. Membranes properties were characterized in terms of pure water flux and oil rejection. When 2 wt% of TiO2 was added in PVDF maximum flux of 70.48% and 99.7% of oil rejection was attained by using 250 ppm oily solution. This was due to increased hydrophilicity and pore size upon the addition of highly hydrophilic inorganic additive [49].
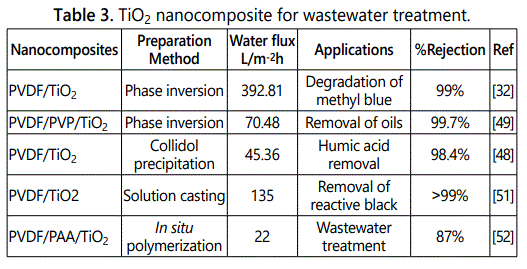
Babak et al. fabricated thin film PVDF-PVA nanocomposite membranes by immersion precipitation method incorporated with carboxylated titania nanoparticles to improve the separation performance of membranes. Separation of various solutes divalent salt, organic solute and bovine serum albumin was determined. Solute rejection and antifouling properties of the membranes were improved due to carboxylated titinia nanoparticles which provide good dispersion and adhesion with the polymer [50]. Different types of titinia nanocomposites [48,49,32,51,52] for wastewater treatment are given in table 3.
Aluminium oxide based nanocomposite membranes
Aluminum oxide nanoparticles are highly reactive, non toxic resistant to chemicals and possess large surface area. Aluminium oxide is inorganic metal oxide material form nanocomposite membrane with organic polyvinylidene fluoride membrane increased the hydrophilicity and suppress the fouling of polyvinylidene fluoride membrane.
Zheng et al. blended aluminium silicate clay particles with polyvinylidene fluoride for the removal of direct dyes from wastewater. Novel nanocomposite membranes results showed that dye rejection ratio exceed to eighty six percent for direct red, eighty five percent for direct yellow and ninety three percent for direct blue which was attributed to electrostatic repulsion between membrane surface and dyes. Antifouling results for direct dyes demonstrated that nanocomposite membranes showed outstanding antifouling behavior.
Li et al. synthesized PVDF aluminium oxide based nanocomposite membranes and used for the separation of bovine serum albumin. Nanoparticles could directly bond to PVDF chains due to the formation of conjugated double bond through acid catalyzed grafting reactions. Hydrophilic aluminium oxide improves the surface hydrophilicity, bovine serum albumin rejection efficiency and antifouling performance of the nanocomposite membranes [53]. Yan et al. studied PVDF ultrafiltration membrane blended with aluminium oxide nanoparticles in the presence of hexadisodium phosphate as the dispersant and polyvinyl pyrrolidene as pore former [54] for the treatment of oily wastewater. Addition of aluminium oxide nanoparticles into PVDF membrane enhances permeation flux and separation efficiency due to the hydrophilic inorganic nanoparticle.
Silicon oxide based nanocomposite membranes
Silica nanoparticles are recognized as inorganic additive in the fabrication of organic membranes due to its mild reactivity, mechanical strength, nontoxic nature, chemicaly and thermaly stable applied in various fields including catalysis, ceramics and chromatography. Now a dayʼs applications extended to separation process, nanoporous silica provide inner channels for water molecules and enhance water flux also offer attractive possibilities for the preparation of ultrafiltration nanocomposite membrane.
Xiao et al. fabricated modified PVDF membrane by blending nanosilica, PVA and styrene maleic anhydride. Membranes properties were studied for the filtration of dyes i-e congo red and reactive black. Results showed that the modified silicon dioxide polyvinylidene fluoride based nanocomposite possess high rejection capacity for both dyes as compared to neat polyvinylidene fluoride membrane due to addition of additives [55].
Sun et al. developed PVDF/PVA/SiO2 nanocomposite membrane ultrafiltration membrane by non solvent induces phase separation and studied membrane properties i-e pure water flux, percentage rejection and antifouling. The results demonstrated that hydrophilicity, water flux and rejection of bovine serum albumin increased due to hydroxyl groups of polyvinyl alcohol and silica particles [56].
Simmeng et al. synthesized polyvinylidene fluoride silica phosphorylated nanocomposite membrane by phase inversion process and used for the separation of oily wastewater. Results showed that the permeate flux of the composite membrane was better as compared to the neat membrane. Highest oil rejection was attained as compared to the neat membrane which indicated that the pore diameter of PVDF membrane increased due to phosphorylated silica particles [57].
Similar studies were carried out by Wang and found that oil rejection efficiency increased from 86.0% to 91.2% after blending polyvinylidene fluoride with silica nanoparticle.
Zinc oxide based nanocomposite membranes
Zinc oxide is multifunctional inorganic nanoparticle and attracted attention due to high surface area as compared to other inorganic nanoparticles. Zinc oxide used as nanofiller with various polymers (cellulose acetate, polyether sulfone, polyvinylidene fluoride) in the fabrication of different nanocomposite membranes. Incorporation of zinc oxide in polymeric membranes enhances properties of polymer such as hydrophilicity, fouling resistance and chemical and mechanical properties.
Liang et al. synthesized novel ZnO blended PVDF membranes for the treatment of synthetic wastewater (humic acid, bovine serum albumin, sodium azide and sodium alginate. Filtration experiments revealed zinc oxide incorporated nanocomposite membranes showed better antifouling and high separation efficiency for wastewater due to increased hydrophilicity [58].
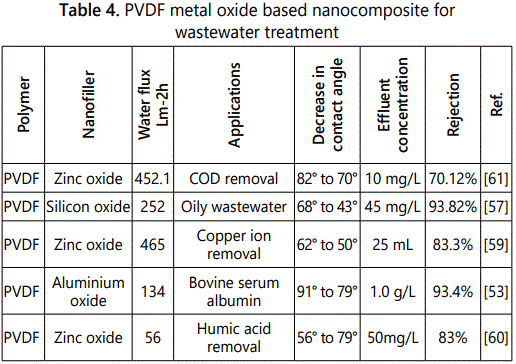
Xia et al. developed hybrid PVDF/ZnO membranes by physical blending method. Performance of hybrid membranes was determined through measurement of water flux, contact angle measurement and removal of copper ions. Results demonstrated higher membrane performance for heavy metal separation due to deposition of zinc oxide nanoparticle on the surface of membrane [59]. Polyvinylidene fluoride metal oxide nanocomposites [53,57,59,60,61] are given in table 4.
Carbon based Nanocomposite Membranes
Carbon based nanomaterials have characteristic property of sp2 hybridized carbon bonds with outstanding physical and chemical properties and surface adsorption properties at the nanoscale. Fullerenes, graphene, carbon Nanotubes and nanodiamonds are different allotropic form of carbon. Carbon based nanomaterials attracted worldwide attention in different fields like contaminants removal (organic compounds, dyes, pesticides) environmental remediation and drug delivery system. In water and wastewater treatment carbon nanomaterials used as adsorbent, photocatalyst and disinfection.
Carbon nanotubes based composite membranes
Carbon nanotubes are also called bucky tubes one dimensional nanomaterials classified as single-walled (SWCNTs) or multi-walled (MWCNTs) on the basis of carbon layer. Carbon nanotubes exhibit large surface area, rich hollow and layered structure which helps in removal of organic contaminants and heavy metals. Carbon nanotubes could be incorporated into polymers to develop multifunctional membranes with improves selectivity, permeability and fouling resistance.
Musthafa et al. fabricated dual layer polyvinylidene fluoride carbon nanotubes blended membranes. Carbon nanotubes was efficiently immobilized on the surface of membrane and used for the removal of methylene blue. The composite membrane exhibited high methylene blue removal efficiency due to more open pore structure and presence of different functional groups on the surface of blend membrane [60].
PVDF/MWCNTs nanocomposite membrane was synthesized by Ma et al. In order to reduce the fouling through poly (amine-ester) functionalized multiwalled carbon nanotubes, poly amine ester groups facilitated the dispersion of multiwall carbon nanotubes in the casting solvent. The water permeability/recovery and fouling properties of nanocomposite membranes depend on the weight percent and dispersed state of MWCNTs in the polymer matrix.
Shan and Murthy et al. carried the preparation of amino-functionalized-multi-walled CNTs-polysulfone composite membranes and used for heavy metal removal Cr(VI) and Cd(II). The membranes displayed maximum removals about 94.2% and 78.2%, respectively which was just 9% and 10% respectively with pristine membranes. The percentage rejection of heavy metal for these composite was found to increase with increasing the loading of multi walled carbon nanotubes [62].
Ma et al. synthesized polyvinylidene ultrafiltration membranes with pristine and oxidized multi-walled carbon nanotubes. Results demonstrated that contact angle decrease water flux increased and bovine serum albumin rejection increased for oxidized carbon nanotubes. With addition of 2 wt% of oxidized carbon Nanotubes increased the viscosity of solution this also prevents the exchange between dimethyl acetamide and water leads to slow down the precipitation of membrane. As a result porous membrane was formed and rejection of bovine serum albumin increased due to presence of hydrophilic oxygen containing groups on the surface of membrane.
Graphene oxide based nanocomposite membranes
Graphene oxide is sp2 oxidized derivative of graphene exhibits hydrophilic nature [63]. The presence of oxygen functional groups hydroxyl, carbonyl, epoxy and carboxyl groups at basal plane and edges impart hydrophilicity to graphene oxide. Graphene oxide used with different polymers such as polyamide, polysulfone, cellulose ester, and polyvinylidene fluoride improve thermal and mechanical properties of polymeric membranes [64]. Graphene oxide nanocomposite membranes attracted great attention for water treatment application including removal of toxic ions, water desalination and organic molecules in polluted water [65].
Zhao et al. incorporated 2 wt% graphene oxide in polyvinylidene fluoride ultrafiltration rejection. Increased in pure water flux was attributed to high hydrophilicity due to the presence of abundant oxygen containing functional groups on the GO surface. These functional groups attracted water molecules inside the membrane matrix and facilitated passage of water molecules through the membranes. Rejection of BSA increases due to the formation of hydrated layer on the membrane surface and the slow change of flux ratio indicated better antifouling properties due to the introduction of hydrophilic grapheme oxide in the composite [66].
Zhang et al. [67] cross-linked graphene oxide with isophoronediisocyanate (IPDI), and then coated on polyvinylidene fluoride ultrafiltration membrane by surface modification. The tendency of dye removal exceeded to 96% and heavy metal ions rejection increased to 40-70% as compared to neat PVDF membranes without the addition of graphene oxide.
Synergistic effects of GO and PVP on ultrafiltration polyvinylidene fluoride membrane performance investigated by Chang et al. for the treatment of bovine serum albumin. The results showed that the membrane hydrophilicity, rejection efficiency and the antifouling performance was improved by the addition of graphene and polyvinyl pyrrolidone. It is reported that this improvement is due to the formation of hydrogen bonds between PVP and GO [68].
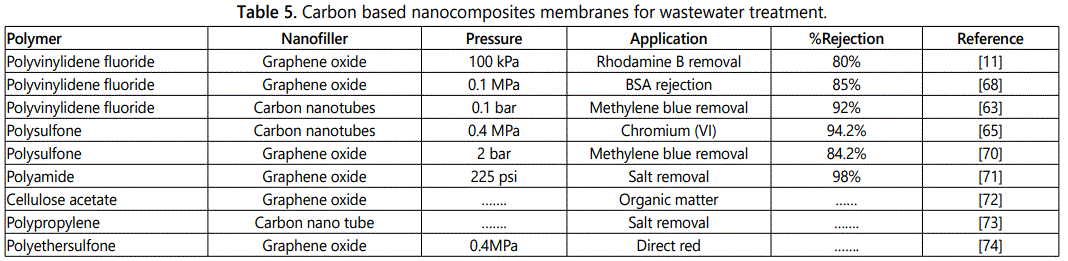
Zhenya et al. carried out study for hazardous dye rejection rhodamine B by introducing graphene oxide and lithium chloride into PVDF membrane. Because of graphene and lithium chloride the decolorization rate and flux recovery ratio of nanohybrid membrane exceed to 80% and 78% respectively. This is due to presence of many carboxyl and hydroxyl groups on the surface of nanohybrid facilitating hydrogen bonding with dye molecule [11]. Table 5 represents the carbon based nanocomposite [11,63,65,68-74] for water treatment.
Conclusion
Polyvinylidene fluoride is most commonly used membrane material for wastewater treatment and has gained significant consideration in recent years due to its outstanding anti-oxidation activity, high thermal stability, remarkably organic selectivity, excellent chemical resistance and membrane forming ability. Polyvinylidene fluoride membranes have been abundantly used in water treatment for purification purposes for example textile wastewater treatment, reuse of municipal wastewater, and heavy metals removal etc.
Major problem in water treatment applications is fouling due to its hydrophobicity, which can be improved by various modification methods. Incorporation of nanomaterial in PVDF change the membrane properties like hydrophilicity, porosity, charge density, thermal and mechanical stability that provide unique properties to membrane. Literature study showed that polyvinylidene fluoride based nanocomposite membranes are highly efficient in removing various pollutants both organic as well as heavy metals from wastewater. This review mainly focuses on use of polyvinylidene fluoride-metal oxide based nanocomposite i-e PVDF-TiO2, PVDF-Al2O3, PVDF-SiO2, PVDF-ZnO and carbon based nanocomposite PVDF-CNT and PVDF-GO for wastewater treatment. It gives new direction to design the next generation of polymeric membranes with high separation capability and anti fouling properties.
References
- Otitoju TA, Ahmad AL, Ooi BS. Polyvinylidene fluoride (PVDF) membrane for oil rejection from oily wastewater: A performance review. Journal of Water Process Engineering. 2016; 14: 41-59. doi: 10.1016/j.jwpe.2016.10.011
- Zahid M, Rashid A, Akram S, Rehan ZA, Razzaq W. A Comprehensive Review on Polymeric Nano-Composite Membranes for Water Treatment. Journal of Membrane Science & Technology. 2018; 8(1): 1-20. doi: 10.4172/2155-9589.1000179
- Siddiqa A, Razzaq H, Qaisar S, Liaqat S, Arshad M, Gill R. PVDFNanodiamonds Composite Membranes: Fabrication, Characterization and Water Treatment Applications. Der Pharma Chemica. 2017; 9(15): 32-40.
- Krishnaveni S, Meera V. A Review on the Use of Nanocomposite Membranes as an Emerging Technology for Water Wastewater Treatment. International Journal of Innovative Research in Science Engineering and Technology. 2016; 5(15): 230-236.
- Lai CY, Groth A, Gray S, Duke M. Nanocomposites for Improved Physical Durability of Porous PVDF Membranes. Membranes (Basel). 2014; 4(1): 55-78. doi: 10.3390/membranes4010055
- Warsingera DM, Chakraborty S, Tow EW, et al. A review of polymeric membranes and processes for potable water reuse. Prog Polym Sci. 2016; 81: 209-237. doi: 10.1016/j.progpolymsci.2018.01.004
- Ulbricht M. Advanced functional polymer membrane. Polymer (Guildf). 2006; 47(7): 2217-2262. doi: 10.1016/j.polymer.2006.01.084
- Ursino C, Munzo RC, Drioli E, Gzara L, Albeirutty MH, Figoli A. Progress of Nanocomposite Membranes for Water Treatment. Membranes (Basel). 2018; 8(2): 1-40. doi: 10.3390/membranes8020018
- Kang GD, Cao YI. Application and modification of poly(vinylidene fluoride) (PVDF) membranes-A review. J Memb Sci. 2014; 463: 145-165. doi: 10.1016/j.memsci.2014.03.055
- Rezakazemi M, Dashti A, Hossein RH, Hajilari N, Inamuddin I. Foulingresistant membranes for water reuse. Environ Chem Lett. 2018; 16(3): 715-763. doi: 10.1007/s10311-018-0717-8
- Zhu Z, Wang L, Xu Y, Li Q, Jiang J, Wang X. Preparation and characteristics of graphene oxide-blending PVDF nanohybrid membranes and their applications for hazardous dye adsorption and rejection. J Colloid Interface Sci. 2017; 504: 429-439. doi: 10.1016/j.jcis.2017.05.068
- Liu TY, Lin WH, Huang LY, Chen SY, Yang MC. Surface characteristic and hemocompatibility of PAN/PVDF blend membranes. Polym Adv Technol. 2005; 16(5): 413-419. doi: 10.1002/pat.592
- Li N, Xiao C, An S, Hu X. Preparation and properties of PVDF/PVA hollow fiber membranes. Desalination. 2010; 250(2): 530-537. doi: 10.1016/j.desal.2008.10.027
- Pezeshk N, Rana D, Narbaitz RM, Matsuura T. Novel modified PVDF ultrafiltration flat-sheet membrane. J Memb Sci. 2012; 389(1): 280-286. doi: 10.1016/j.memsci.2011.10.039
- Bi QY, Li Q, Tian Y, Lin YK, Wang XL. Hydrophilic Modification of poly (vinylidene fluoride) membrane with poly(vinylpyrrolidone) via cross liking reaction. J Appl Polym Sci. 2013; 127(1): 394-401. doi: 10.1002/app.37629
- Turner JE, Shen M, Lin CC. Hydrophilic behavior of HSPAN/PVA membrane. J Appl Polym Sci. 1980; 25(7): 1287-1296. doi: 10.1002/app.1980.070250703
- Linares A, Nogales A, Rueda DR, Ezquerra TA. Molecular dynamics in PVDF/PVA blends as revealed by dielectric loss spectroscopy. J Appl Polym Sci. 2007; 45(13): 1653-1661. doi: 10.1002/polb.21210
- Zhang J, Xu Z, Mai W, et al. Improved hydrophilicity,permeability, antifouling and mechanical performance of PVDF composite ultrafiltration membrane tailored by oxidized low dimensional carbon nanomaterials. J Mater Chem A. 2013; 1(9): 3101-3111. doi: 10.1039/C2TA01415G
- Wang D, Li K, Teo WK. Preparation and characterization of polyvinylidene fluoride(PVDF) hollow fiber membrane. J Memb Sci. 1999; 163(2): 211-220. doi: 10.1016/S0376-7388(99)00181-7
- Kong J, Li K. Preparation of PVDF hollow fiber membranes via immersion precipitation. J Appl Polym Sci. 2001; 81(7): 1643-1653. doi: 10.1002/app.1595
- Venault A, Liu YH, Wu JR, et al. Low-biofouling, of the membranes prepared by liquid-induced phase separation of the PVDF/polystyrene-bpoly(ethyleneglycol)methacrylate blend. J Memb Sci. 2014; 450: 340-350. doi: 10.1016/j.memsci.2013.09.004
- Kasemset S, Lee A, Miller DJ, Freeman BD, Sharma MM. Effect of polydopamine deposition condition on fouling resistance,physical properties, and permeation properties ofvreverse osmosis membranes in oil/water separation. J Memb Sci. 2013; 425-426(1): 208-216. doi: 10.1016/j.memsci.2012.08.049
- Hester JF, Banerjee P, Mayes AM. Preparation of protein-resistant surfaces on poly(viylidene fluoride) membrane via surface segregation. Macromolecules. 1999; 32(5): 1643-1650. doi: 10.1021/ma980707u
- Shao XS, Li JH, Zhou Q, Miao J, Zhang QQ. Amphiphilic poly (vinyl chloride)-g-poly(ethyleneglycol)methyletheracrylate) copolymer for the surface hydrophilic modification of poly(vinylidene fluoride). J Appl Polym Sci. 2013; 129(5): 2472-2478. doi: 10.1002/app.38891
- Liu J, Shen X, Zhao YP, Chen L. Acryloylmorpholine-grafted PVDF membrane with improved protein fouling resistance. Ind Eng Chem Res. 2013; 52(51): 18392-18400. doi: 10.1021/ie403456n
- Wang P, Tan KL, Kang ET, Neoh KG. Plasma-induced immobilization of poly(ethylene glycol) onto poly(vinylidene fluoride) microporous membrane. J Memb Sci. 2002; 195(1): 103-114. doi: 10.1016/S0376-7388(01)00548-8
- Nunes SP, Peinemann KV. Ultrafiltration membranes from PVDF/PMMA blends. J Memb Sci. 1992; 73(1): 25-35. doi: 10.1016/0376-7388(92)80183-K
- Chang Y, Ko CY, Shih YJ, et al. Surface grafting control of PEGylated poly(vinylidene fluoride) antifouling membrane via surface-initiated radical graft copolymerization. J Memb Sci. 2009; 345(1-2): 160-169. doi: 10.1016/j.memsci.2009.08.039
- Zhao YH, Zhu BK, Kong L, Xu YY. Improving hydrophilicity and protein resistance of PVDF membrane by blending with amphilic hyperbranched star polymer. Langmuir. 2007; 23(10): 5779-5786. doi: 10.1021/1a070139o
- Yu LY, Xu ZL, Shen HM, Yang H. Preparation and characterization of PVDF/SiO2 composite hollow fiber UF membrane by sol-gel method. J Memb Sci. 2009; 337(1-2): 257-265. doi: 10.1016/j.memsci.2009.03.054
- Oh SJ, Kim N, Lee YT. Preparation and characterization of PVDF/TiO2 organic-inorganic composite membranes for fouling resistance improvement. J Memb Sci. 2009; 345(1-2): 13-20. doi: 10.1016/j.memsci.2009.08.003
- Ngang HP, Ooi BS, Ahmad AL, Lai SO. Preparation of PVDF-TiO2 mixedmatrix membrane and its evaluation on dye adsorption and UV cleaning properties. Chem Eng J. 2012; 197: 359-367. doi: 10.1016/j.cej.2012.05.050
- Dzunuzovi E, Jeremic K, Nedeljkovi JM. In situ radical polymerization of methyl methacrylate in a solution of surface modified TiO2 nanoparticles. Eur Polym J. 2007; 43(9): 3719-3726. doi: 10.1016/j.eurpolymj.2007.06.026
- Lalia BS, Burreiza EG, Arafat HA, Hashaikeh R. Fabrication and characterization of polyvinylidene fluoride-co-hexafluoropropylene(PVDFHFP) electrospun membranes for direct contact membrane distillation. J Memb Sci. 2013; 428: 104-115. doi: 10.1016/j.memsci.2012.10.061
- Cao X, Ma J, Shi X, Ren Z. Effect of TiO2 nanoparticles size on the performance of PVDF membrane. Appl Surf Sci. 2006; 253(4): 2003-2010. doi: 10.1016/j.apsusc.2006.03.090
- Ji W, Sikdar SK. Pervaporation using adsorbent-filled membranes, Prevaporization using adsorbent- filled membranes. Ind Eng Chem Res. 1996; 35(4): 1124-1132. doi: 10.1021/ie9503609
- Pandey P, Chauhan RS. Membranes for gas separation. Prog Polym Sci. 2001; 26(6): 853-893. doi: 10.1016/S0079-6700(01)00009-0
- Li LH, Deng JC, Deng HR, Liu ZL, Xin L. Synthesis and characterization of chitosan/ZnO nanocomposite membranes. Carbohydr Res. 2010; 345(8): 994-998. doi: 10.1016/j.carres.2010.03.019
- Smith RC, Liang C, Landry M, Nelson JK, Schadler LS. The mechanism leading to the useful electrical properties of polymer nanodielectrics. IEEE Trans Dielectr Electr Insul. 2008; 15(1):187-196. doi: 10.1109/T-DEI.2008.4446750
- Xi ZY, Xu YY, Zhu LP, Wang Y, KZhu B. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly(DOPA) andpoly(dopamine). J Memb Sci. 2009; 327(1-2): 244-253. doi: 10.1016/j.memsci.2008.11.037
- Chanachai A, Meksup K, Jiraratananon R. Coating of hydrophobic hollow fiber PVDF membrane with chitosan for protection against wetting and flavor loss in osmotic distillation process. Sep Purif Technol. 2010; 72(2): 217-224. doi: 10.1016/j.seppur.2010.02.014
- Xu B, Li Hy. Preparation of a hydrophilic PVDF membranes by electron beam induced grafting polymerization of acrylic acid. Advanced Materials Research. 2013; 625: 273-276. doi: 10.4028/www.scientific.net/AMR.625.273
- Han MJ, Barona GNB, Jung B. Effect of surface charge on hydrophilically modified poly(vinylidene fluoride) membrane for microfiltration. Desalination. 2011; 270(1-3): 76-83. doi: 10.1016/j.desal.2010.11.024
- Jordan J, Jacob KI, Tannenbaum R, Sharaf MA, Jasiuk I. Experimental trends in polymer nanocomposites- A review. Mater Sci Eng A Struct Mater. 2005; 393(1-2): 1-11. doi: 10.1016/j.msea.2004.09.044
- Lofrano G, Carotenuto M, Libralato G, et al. Polymer functionalized nanocomposites membranes for metals removal from water and wastewater. An overview. Water Res. 2016; 92: 22-37. doi: 10.1016/j.watres.2016.01.033
- Bae TH, Tak TM. Effect of TiO2 nanoparticles on fouling mitigation of ultrafiltration membrane for activated sludge filtration. J Memb Sci. 2005; 249(1-2): 1-8. doi: 10.1016/j.memsci.2004.09.008
- Yuliwati E, Ismail AF, Matsuura T, Kassim MA, Abdullah MS. Effects of modified PVDF hollow fiber submerged ultrafiltration membrane for refinery wastewater treatment. Desalination. 2011; 283: 214-220. doi: 10.1016/j.desal.2011.03.049
- Teow YH, Ooi BS, Ahmad AL, Lim JK. Mixed-Matrix Membrane for Humic Acid Removal: Influence of Different Types of TiO2 on Membrane Morphology and the Performance. Int J Chem Eng Appl. 2012; 3(6): 374- 379. doi: 10.7763/IJCEA.2012.V3.222
- Ong CS, Lau WJ, Goh PS, Ng BC, Ismail AF. Preparation and characterizationof PVDF–PVP–TiO2 composite hollow fiber membranes for oily wastewater treatment using submerged membrane system. Desalination Water Treat. 2015; 53(5): 1213-1223. doi: 10.1080/19443994.2013.855679
- Rajaeian B, Heitz A, Tade MO, Liu S. Improved separation and antifouling performance of PVA thin film nanocomposite membranes incorporated with carboxylated TiO2 nanoparticles. J Memb Sci. 2015; 485: 48-59. doi: 10.1016/j.memsci.2015.03.009
- Damodar RA, You SJ, Chou HH. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J Hazard Mater. 2009; 172(2-3): 1321-1328. doi: 10.1016/j.jhazmat.2009.07.139
- Madaeni SS, Zinadini S, Vatanpour V. A new approach to improve antifouling property of PVDF membrane using in situ polymerization of PAA functionalized TiO2 nanoparticles. J Memb Sci. 2011; 380(1-2): 155-162. doi: 10.1016/j.memsci.2011.07.006
- Liu F, Moghareh Abed MR, Li K. Preparation and characterization of poly(vinylidene fluoride) based ultrafiltration membranes using nano γ-Al2O3. J Memb Sci. 2011; 366(1-2): 97-103 doi: 10.1016/j.memsci.2010.09.044
- Yan L, Li YS, Xiang CB. Preparation of poly(vinylidene fluoride) PVDF ultrafiltration membrane modified by nano-sized alumina (Al2O3) and its antifouling research. Polymer (Guildf). 2005; 46(18): 7701-7706. doi: 10.1016/j.polymer.2005.05.155
- Meng XR, Zhang N, Wang XB, Wang L, Huang DX, Miao R. Dye effluent treatment using PVDF UF membranes with different properties. Desalination Water Treat. 2013; 52(25-27): 5068-5075. doi: 10.1080/19443994.2013.862033
- Sun D, Yue D, Li B, Zheng Z, Meng X. Preparation and the performance of the novel PVDF ultrafiltration membranes blending with PVA modified SiO2 hydrophilic nanoparticles. Polymer Engineering and Science. 2018; 59: E412-E421. doi: 10.1002/pen.25002
- Zhang S, Wang R, Zhang S, Li G, Zhang Y. Treatment of wastewater containing oil using phosphorylated silica nanotubes (PSNTs)/polyvinylidene fluoride (PVDF) composite membrane. Desalination. 2014; 332(1): 109-116. doi: 10.1016/j.desal.2013.11.008
- Liang S, Xiao K, Mo Y, Huang X. A novel ZnO nanoparticle blended polyvinylidene fluoride membrane for anti-irreversible fouling. J Memb Sci. 2012; 394-395: 184-192. doi: 10.1016/j.memsci.2011.12.040
- Zhang X, Wang Y, Liu Y, Xu J, Han Y, Xu X. Preparation and performance of PVDF/ZnO hybrid membranes and their application in removal of copper ions. Appl Surf Sci. 2014; 316: 333-340. doi: 10.1016/j.apsusc.2014.08.004
- Mavukkandy MO, Zaib Q, Arafat HA. CNT/PVP blend PVDF membrane for the removal of organic pollutants from simulated treated wastewater effluent. J Environ Chem Eng. 2018; 6(5): 6733-6740. doi: 10.1016/j.jece.2018.10.029
- Hong J, He Y. Effects of nano sized zinc oxide on the performance of PVDF micro filtration membranes. Desalination. 2012; 302: 71-79. doi: 10.1016/j.desal.2012.07.001
- Shah P, Murthy CN. Studies on the porosity control of MWCNT/polysulfone composite membrane and its effect on metal removal. J Memb Sci. 2013; 437: 90-98. doi: 10.1016/j.memsci.2013.02.042
- Jhaveri JH, Murthy ZVP. Nanocomposite membranes. Desalination Water Treat. 2016; 57(55): 1-17. doi: 10.1080/19443994.2015.1120687
- Ionita MP, A.M, Crica L, Pilan L. Improving the thermal and mechanical properties of polysulfone by incorporation of graphene oxide. Compos B Eng. 2014; 59: 133-139. doi: 10.1016/j.compositesb.2013.11.018
- An D, Yang L, Wang TJ, Liu B. Separation Performance of Graphene Oxide membrane in aqueous solution. Ind Eng Chem Res. 2016; 55(17): 4803-4810. doi: 10.1021/acs.iecr.6b00620
- Zhao C, Xu X, Chen J, Yang F. Effect of graphene oxide concentration on the morphologies and antifouling properties of PVDF ultrafiltration membranes. J Environ Chem Eng. 2013; 1(3): 349-354. doi: 10.1016/j.jece.2013.05.014
- Zhang P, Gong JL, Zeng GM, et al. Cross-linking to prepare composite graphene oxide-framework membranes with high-flux for dyes and heavy metal ions removal. Chem Eng J. 2017; 322: 657-666. doi: 10.1016/j.cej.2017.04.068
- Chang X, Wang Z, Quan S, Xu Y, Jiang Z, Shao L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl Surf Sci. 2014; 316: 537-548. doi: 10.1016/j.apsusc.2014.07.202
- Fontananova E, Jansen JC, Cristiano A, Curcio E, Drioli E. Effect of additives in the casting solution on the formation of PVDF membranes. Desalination. 2006; 192(1-3): 190-197. doi: 10.1016/j.desal.2005.09.021
- Badrinezhad L, Ghasemi S. Preparation and characterization of polysulfone/graphene oxide nanocomposite membrane for the separation of methylene blue from water. Polym Bull. 2017; 75(2): 469-484: doi: 10.1007/s00289-017-2046-7
- Chae HR, Lee J, Lee CH, Kim IC, Park P. Graphene oxide-embedded thinfilm composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J Memb Sci. 2015; 483: 128-135. doi: 10.1016/j.memsci.2015.02.045
- Pastrana-Martinez LM, Morales-Torres S, Figueiredo JL, Faria JL, Silva AMT. Graphene oxide based ultrafiltration membranes for photocatalytic degradation of organic pollutant in salty water. Water Res. 2015; 7: 179-190. doi: 10.1016/j.watres.2015.03.014
- Ghethard K, Sae-Khow, Mirta H. Water desalination using carbon nano enhance membrane distillation. ACS Appl Mater Interfaces. 2011; 3(2): 110-114. doi: 10.1021/am100981s
- Zinadini S, Zinatizadeh AA, Rahimi M, Vatanpour V, Zanghene H. Preparation of novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J Memb Sci. 453: 292-301. doi: 10.1016/j.memsci.2013.10.070


