Research Article
Mechanical Alloying of Nanocrystalline Materials and Nanocomposites
Department of Mechanical and Aerospace Engineering, University of Central Florida, Orlando, USA
*Corresponding author: Challapalli Suryanarayana, Department of Mechanical and Aerospace Engineering, University of Central Florida, Orlando, USA, E-mail: Surya@ucf.edu
Received: January 30, 2019 Accepted: March 5, 2019 Published: March 11, 2019
Citation: Suryanarayana C. Mechanical Alloying of Nanocrystalline Materials and Nanocomposites. Madridge J Nanotechnol Nanosci. 2019; 4(1): 127-134. doi: 10.18689/mjnn-1000126
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Mechanical alloying is an effective method to synthesize nanocrystalline metal powders in the monolithic and composite states. The process involves repeated cold welding, fracturing and rewelding of powder particles in a high energy ball mill. Although originally developed to produce oxide dispersion strengthened superalloys, the technique has been later shown to be capable of producing a variety of metastable phases including nanocrystalline materials. The present article describes the synthesis of nanocrystalline materials and nanocomposites by mechanical alloying. The basic principles of the process, process parameters that affect the constitution and microstructure of the processed powders, and the mechanisms of alloying and grain refinement, including their consolidation to full density while retaining the nanostructures, are described. Methods to achieve the smallest possible grain size are then highlighted. Typical examples of the synthesis of nanocomposites containing a high volume fraction of nanometer-sized reinforcements in aluminum, and process optimization to achieve superplastic behavior in titanium-based nanocomposites are then discussed. The ubiquitous problem of powder contamination during milling and solutions to eliminate or minimize this are also mentioned.
Keywords: Mechanical alloying; Nanocrystalline materials; Nanocomposites; Minimum grain size; Powder consolidation; Al-Al2O3; TiAl-Ti5Si3; Powder contamination and its minimization.
Introduction
Materials scientists and engineers have been developing novel materials to improve the properties and performance of existing materials to meet the demands of the high-technology industries. The second half of the last century has been witness to the development of novel materials such as nanocrystalline materials and nanocomposites, metallic glasses and bulk metallic glasses, high-temperature superconductors, hydrogen storage alloys, superhard carbonitrides, thin film diamond synthesis, quasicrystalline alloys, and high-entropy alloys, to name a few. Several new techniques have also been developed and improved upon to synthesize these novel materials that include rapid solidification processing, mechanical alloying, ion implantation, plasma processing, physical and chemical vapor deposition methods, and others. With the help of these techniques, it has been possible to produce a variety of monolithic and composite materials with vastly improved properties. A common underlying theme in all these processes has been to bring the material into a highly energetic (non-equilibrium) condition by increasing either the temperature or pressure, or by irradiation, or storing of mechanical energy. The material is then “quenched” either to retain the high-temperature/high-pressure phase at lower temperatures and atmospheric pressure or to allow it to slowly transform into more stable phases. Using this approach, it has been possible to modify both the constitution and microstructure of the materials. The advent of new characterization techniques during the same time period has greatly aided in understanding the structure-property correlations in these novel materials. The benefits and deficiencies of these different techniques in developing advanced materials have been discussed in the literature [1].
Nanocrystalline materials are single- or multi-phase polycrystalline solids with a grain size of the order of a few nanometers (1 nm = 10-9 m = 10 Å), typically 1 to 100 nm in at least one dimension. Since the grain sizes are very small, a significant volume of the microstructure in nanocrystalline materials is composed of interfaces, mainly grain boundaries and triple junctions. That is, a large volume fraction of the atoms resides in these interfaces. Consequently, nanocrystalline materials exhibit properties that are significantly different from, and often superior to their conventional coarse-grained polycrystalline counterparts. Compared to the material with a more conventional grain size, i.e., larger than a few micrometers, nanocrystalline materials show increased strength, high hardness, extremely high diffusion rates, and consequently reduced sintering times for powder compaction, and improved deformation characteristics. Several excellent reviews and books are available giving details on different aspects of processing, properties, and applications of these materials [2-6].
Mechanical Alloying
Mechanical alloying (MA) is the generic term used to denote processing of metal powders in high-energy ball mills. But, more specifically, MA describes the process when mixtures of powders (of different metals or alloys/compounds) are milled together to produce a homogeneous alloy. Thus, if powders of pure metals A and B are milled together to synthesize a homogeneous alloy phase, the process is referred to as MA. Material transfer is involved in this process to obtain a homogeneous alloy. When powders of uniform (often stoichiometric) composition, such as pure metals, intermetallics, or prealloyed powders, are milled in a high-energy ball mill, and material transfer is not required for homogenization, the process has been termed Mechanical Milling. Only reduction in particle (or grain) size and increase in the surface area are involved in this process and material transfer does not occur.
The process of MA was developed by John Benjamin of INCO International in the late 1960ʼs to produce oxide dispersion strengthened (ODS) nickel-based superalloys [7]. MA involves loading of the blended elemental powders along with the grinding medium in a vial and subjecting the powder to heavy deformation through violent agitation. During this process, the powder particles are repeatedly cold welded, fractured, and rewelded. Sometimes, about 1-2 wt.% of a process control agent (PCA) is added, which is adsorbed on the surfaces of the powder particles and minimizes their excessive cold welding. The PCA, an organic compound, is usually added whenever ductile materials are milled.
Milling can be carried out in high-energy shaker mills (⁓20 g), relatively low-energy planetary ball mills (⁓250 g), or attritors (⁓1 kg). Industrial mills can process several kilograms of powder at a time. While large amounts of powder can be processed only at low speeds, small quantities can be processed at higher speeds. In recent years, cryogenic milling has become a popular method. Facilities to cool the powders to low temperatures or heat them to high temperatures and monitor the pressure and temperature during milling are some of the attachments that are currently available [8,9].
It was realized by mid-1980ʼs that MA can also be utilized to produce metastable phases including supersaturated solid solutions [10-12] intermetallic and quasicrystalline phases [13,14], and amorphous alloys [8,9,15-18]. But, the most notable features observed were that MA can reduce the grain size of milled materials to nanometer dimensions [19] and that nanocomposites containing a high volume fraction of ultrafine (nanometer-sized) reinforcements could be produced [20]. This simple, but effective, processing technique has now been applied to metals, ceramics, polymers, and composite materials. The general attributes of MA have been reviewed earlier [8,9,21]
Mechanism of Alloying
During milling, the powders are heavily deformed leading to the generation of a variety of crystal defects such as dislocations, vacancies, stacking faults, and increased number of grain boundaries. It is also noted that lattice strain in the powder increases with milling time. The presence of defect structure enhances the diffusivity of solute elements in the matrix. Further, the refined microstructural features decrease the diffusion distances. Additionally, the slight rise in temperature during milling further aids the diffusion behavior, and consequently, true alloying takes place amongst the constituent elements. While this alloying generally takes place nominally at room temperature, sometimes it may be necessary to anneal the mechanically alloyed powder at a slightly elevated temperature for alloying to be achieved. This is particularly true when formation of intermetallics is desired.
After milling for a certain length of time, steady-state equilibrium is attained when a balance is achieved between the rate of welding, which tends to increase the average particle size, and the rate of fracturing, which tends to decrease the average composite particle size. Smaller particles are able to withstand deformation without fracturing and tend to be welded into larger pieces, with an overall tendency to drive both very fine and very large particles towards an intermediate size. The particle size distribution at this stage is narrow, because particles larger than average are reduced in size at the same rate that fragments smaller than average grow through agglomeration of smaller particles referred to as steady-state condition.
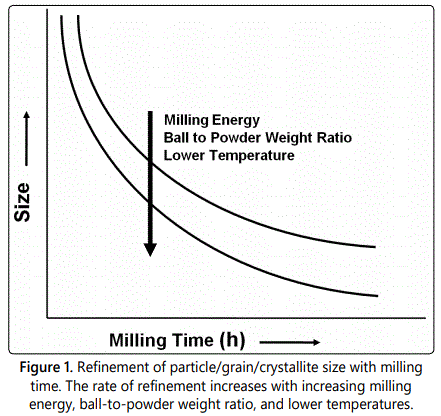
The specific times required to develop a given structure in any system would be a function of the initial powder particle size and the mechanical characteristics of the ingredients as well as the specific equipment used for milling and the operating parameters of the equipment. But, in most cases, the rate of refinement of the internal structure (particle size, crystallite size, lamellar spacing, etc.) is roughly logarithmic with processing time and therefore the size of the starting particles is relatively unimportant. In a few minutes to an hour, the lamellar spacing usually becomes small and the crystallite (or grain) size is refined to nanometer dimensions (Figure 1). Thus, the milled powders will exhibit increased lattice strain and decreased grain size. As a result, the milled powder is in a highly energetic condition. There have been instances, when these ultrafine powders have caught fire on exposure to atmosphere.
Powder Consolidation
The product of MA is in powder form. Except in cases of chemical applications, such as catalysis, when the product does not require consolidation, widespread application of such powders requires efficient methods of consolidating them into bulk shapes. Successful consolidation of nanocrystalline powders is a non-trivial problem since fully dense materials should be produced while simultaneously retaining the nanometer-sized grains without coarsening. Conventional consolidation of powders to full density through processes such as hot extrusion and hot isostatic pressing requires use of high pressures and elevated temperatures for extended periods of time to achieve full densification. Unfortunately, however, this results in significant coarsening of the nanometer-sized grains and consequently the benefits of nanostructure processing are lost. On the other hand, retention of nanostructures requires use of low consolidation temperatures and then it is difficult to achieve full interparticle bonding at these low temperatures. Therefore, novel and innovative methods of consolidating nanocrystalline powders are required. The objectives of consolidation of nanocrystalline powders are (i) to achieve full densification (without any porosity), (ii) minimize microstructural coarsening (i.e., retention of nanocrystalline state), and/or (iii) avoid undesirable phase transformations. In fact, the early results of excellent mechanical properties, and most specifically superplasticity at room temperature [22], attributed to nanocrystalline ceramics have not been successfully reproduced, due to incomplete consolidation of the nano powders in the material tested. Thus, consolidation to full density assumes even greater importance.
Because of the small size of the powder particles (typically a few micrometers, even though the grain size is only a few nanometers), nanocrystalline powders pose special problems and some special precautions need to be taken to consolidate them to bulk shapes. For example, they possess very high strength and hardness. They also have a high level of interparticle friction. Further, since the nanocrystalline powders have a large surface area, they also exhibit high chemical reactivity.
Successful consolidation of nanocrystalline powders has been achieved by electro-discharge compaction, plasmaactivated sintering, shock (explosive) consolidation, hotisostatic pressing (HIP), hydrostatic extrusion, strained powder rolling, and sinter forging. By utilizing the combination of high temperature and pressure, HIP can achieve a particular density at a lower pressure when compared to cold isostatic pressing or at a lower temperature when compared to sintering. It should be noted that because of the increased diffusivity in nanocrystalline materials, sintering (densification) takes place at temperatures much lower than in coarse-grained materials. This is likely to reduce the grain growth. Two excellent reviews by Groza [23,1] may be consulted for full details of the methods of consolidation and description of results.
In keeping with the scope of the journal, let us now discuss the synthesis and applications of nanocrystalline materials and nanocomposites by mechanical alloying – an advanced powder processing technique.
Nanocrystalline Materials
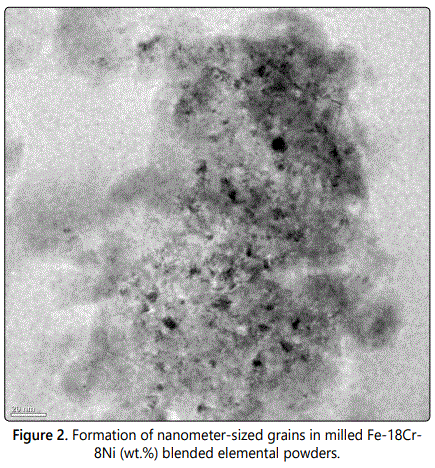
A number of different techniques have been employed to produce nanocrystalline materials including inert gas condensation, mechanical alloying, plasma processing, and others [2,3,6]. However, mechanical alloying (in spite of some limitations) has been one of the most popular techniques [24]. Grain sizes with nanometer dimensions have been observed in almost all mechanically processed pure metals, intermetallics, and alloys (if they continue to be crystalline) (Figure 2). The minimum grain size achieved has been reported to be a few nanometers, ranging typically from about 5 to 50 nm, but depending on the material and processing conditions. Thus, it appears that synthesis of nanostructures by MA is a ubiquitous phenomenon and that nanostructures could be produced in every material. In fact, the ease with which nanostructured materials can be synthesized is one reason why mechanical alloying has been extensively employed to produce nanocrystalline materials. In spite of this, there have not been many detailed investigations to explain why and how nanometer-sized grains are obtained in these materials.
Hellstern et al. [25] have studied the evolution of nanostructure formation in mechanically milled AlRu compound through detailed transmission electron microscopy (TEM) techniques. From high-resolution TEM observations, it was observed that deformation was localized within shear bands in the early stages of milling, due to the high deformation rates experienced by the powder particles. These shear bands, which contain a high density of dislocations, have a typical width of approximately 0.5 to 1.0 µm. Small grains, with a diameter of 8–12 nm, were seen within the shear bands and electron diffraction patterns suggested significant preferred orientation. With continued milling, the average atomic level strain increased due to increasing dislocation density, and at a certain dislocation density within these heavily strained regions, the crystal disintegrated into subgrains that are separated by low-angle grain boundaries. This resulted in a decrease of the lattice strain. The subgrains formed this way were of nanometer dimensions and are often between 20 and 30 nm.
On further processing, deformation occurred in shear bands located in previously unstrained parts of the material. The grain size decreased steadily and the shear bands coalesced. The small-angle boundaries were replaced by higher angle grain boundaries, implying grain rotation, as reflected by the absence of texture in the electron diffraction patterns and random orientation of the grains observed from the lattice fringes in the high-resolution electron micrographs. Consequently, dislocation-free nanocrystalline grains were formed. This is the currently accepted mechanism of nanocrystal formation in MA processed powders.
Minimum Grain Size
As noted earlier, the grain size of the milled materials decreases with milling time and reaches a saturation level when a balance is established between the fracturing and cold-welding events. This minimum grain size, dmin is different depending on the material and milling conditions. Some efforts have been made in recent years to rationalize the obtainable dmin in different materials in terms of the material properties. The value of dmin achievable by milling is determined by the competition between the plastic deformation via dislocation motion that tends to decrease the grain size, and the recovery and recrystallization behavior of the material that tends to increase the grain size. This balance gives a lower bound for the grain size of pure metals and alloys.
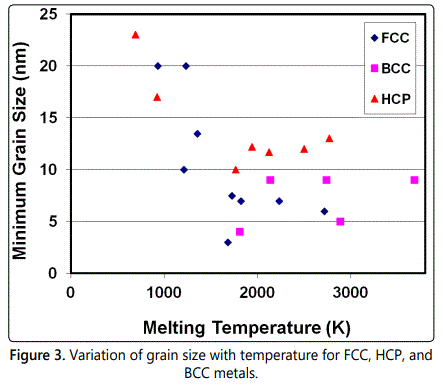
The dmin obtained is different for different metals and also is found to vary with their crystal structures (Figure 3). In most of the metals, the minimum grain size attained is in the nanometer dimensions. But, metals with the BCC crystal structure reach much smaller values in comparison to metals with the other crystal structures. This is probably related to the difficulty of extensive plastic deformation and consequent enhanced fracturing tendency during milling. Ceramics and compounds are much harder and usually more brittle than the metals on which they are based. Therefore, intuitively, one expects that dmin of these compounds is smaller than those of the pure metals; but it does not appear to be always the case.
Trying to relate the dmin obtained during milling, it was noted that the grain size decreases with an increase in the melting temperature of the metal [26]. This trend is amply clear in the case of metals with close-packed structures (Figure 3). Another point of interest is that the difference in grain size is much less amongst metals that have high melting temperatures; the minimum grain size is virtually constant. Thus, for the HCP metals Co, Ti, Zr, Hf, and Ru, the minimum grain size is almost the same even though the melting temperatures vary between 1495°C for Co and 2310°C for Ru. An inverse relation as above is less obvious in the case of BCC metals.
The existing data on dmin of mechanically processed pure metals was analyzed and correlated with different properties of the metals [27,28]. It was shown that the normalized minimum grain size, dmin/b, where b is the Burgers vector of the dislocations in that crystal structure, deceases with increasing melting temperature, activation energy for self diffusion, hardness, stacking fault energy, bulk modulus, and the equilibrium distance between two edge dislocations.
It was suggested that dmin is determined by the minimum grain size that can sustain a dislocation pile-up within a grain and by the rate of recovery. Based on the dislocation pile-up model, the critical equilibrium distance between two edge dislocations in a pile-up, Lc (which could be assumed to be the crystallite or grain size in milled powders), was calculated [29] using the equation:
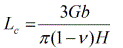
where G = shear modulus, b = Burgers vector, ν = Poissonʼs ratio, and H = hardness of the material. According to the above equation, increased hardness results in smaller values of Lc (grain size) and an approximate linear relationship was observed between Lc and the minimum grain size obtained by milling of a number of metals. Other attempts have also been made to theoretically predict dmin on the basis of thermodynamic properties of materials [30].
An interesting observation made is that the grain size in these mechanically processed materials is reasonably stable and that nanograin sizes have been retained up to fairly high temperatures. For example, nanograin sizes are stable up to about 900°C in titanium-based materials [31].
Effect of Process Variables
MA is a complex process and involves a number of process variables, including the type of mill, milling container, milling energy/speed, milling time, type, size, and size distribution of the grinding medium, ball-to-powder weight ratio, extent of filling the vial, milling atmosphere, nature and amount of the process control agent, and temperature of milling. Some information is available in the literature on the minimum grain size achieved under different processing conditions. Amongst these, the effect of milling energy, milling temperature, and alloying effects have been reported. The grain size obtained is smaller the higher the milling energy, the lower the milling temperature, and the higher the alloying content.
Nanocomposites
Metal matrix composites (MMCs) combine the good ductility and toughness of the metal matrix and the high strength and stiffness of the ceramic reinforcement. MMCs find niche applications in the automobile and aerospace industries [32]. In comparison to continuous fiber-reinforced MMCs, particulate-reinforced MMCs are attractive since they are easier to manufacture, exhibit isotropic properties, and are often cheaper. Since the conventional reinforcements are large (tens to hundreds of micrometers in size), MMCs exhibit low ductility and toughness, mainly due to the easy initiation and propagation of cracks in the ceramic particles at the interfaces.
Achievement of a uniform distribution of the reinforcement in a matrix is essential to achieve good mechanical properties of the composites. Further, the mechanical properties of the composite tend to improve with increasing volume fraction and decreasing particle size of the reinforcement [33]. Traditionally, a reasonably large volume fraction of the reinforcement could be added, if the size of the reinforcement is large (on a micrometer scale). But, if the reinforcement size is very fine (of submicrometer or nanometer dimensions), then the volume fraction added is limited to about 2–4%. This is because the fine powders tend to float to the top of the melt during processing of the composites through solidification processing.
Reinforcing of metals with small particles (in the submicron range) leads to increased strength and improved performance. However, inhomogeneous dispersion, settling down of reinforcements, and poor wettability are serious issues. Even subsequent thermomechanical processing may not be effective in eliminating these drawbacks. The ductility and toughness of MMCs, along with the strength, can be significantly improved by reducing the reinforcement size to nanometer levels, in the so-called nanocomposites [18,34,35]. Thus, if we are able to introduce a large volume fraction of nanometer-sized reinforcement, the mechanical properties and performance of the composite are likely to be vastly improved. Such a situation is only possible through solidstate processing methods such as MA.
In our investigations, during the past few years, we have successfully achieved a very uniform distribution of the reinforcement phases in different types of matrices through the solid-state powder processing technique of MA [36-44]. These include homogeneous dispersion of lead in Al and Al– Cu alloys [36], graphite in an Al-6061 alloy matrix [37], effect of clustering of the reinforcement on the mechanical properties of the composites [38], dispersion of a high volume fraction of Al2O3 in Al [20] and Mg [39], SiC in Al [40], AlN in Mg [41], Ti5Si3 in γ-TiAl [42,43], and synthesis of MoSi2+ Si3N4composites for high temperature applications [44]. However, in this article, we will describe our new results obtained in two systems.
Some of the specific goals sought in processing of nanocomposite materials through MA include (i) incorporation of a high volume percentage of ultrafine reinforcements and (ii) achievement of high ductility, and even superplasticity in some composites. It is also possible that due to the increased ductility, the fracture toughness of these composites could be higher than in their coarse-grained counterparts.
Al-Al2O3 Nanocomposites
Aluminum-based metal matrix composites possess light weight and high strength-to-weight ratio and are therefore ideal materials for structural applications in the aircraft and automotive industries. Reinforcement of the ductile aluminum matrix with stronger and stiffer second-phase reinforcements like oxides, carbides, borides, and nitrides provides a combination of properties of both the metallic matrix and the ceramic reinforcement. Uniform dispersion of the fine reinforcements, especially at large volume fractions, and a fine grain size of the matrix contribute to improving the mechanical properties of the composite.
Our investigations were focused on reinforcing the aluminum metal matrix with different volume fractions (20, 30, and 50%) of fine (50 nm, 150 nm) and coarse (5 µm) Al2O3 particles through MA and determine whether there is a maximum volume fraction beyond which it will be difficult to achieve uniform dispersion and consolidate to full density, and to see if there is a minimum particle size below which again it is difficult to obtain a uniform dispersion.
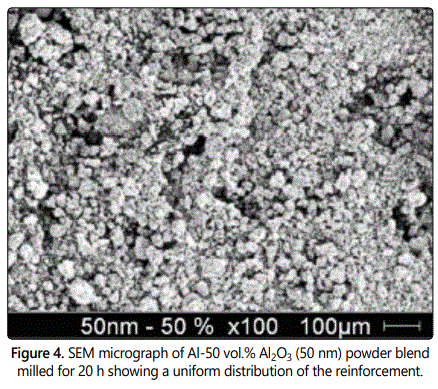
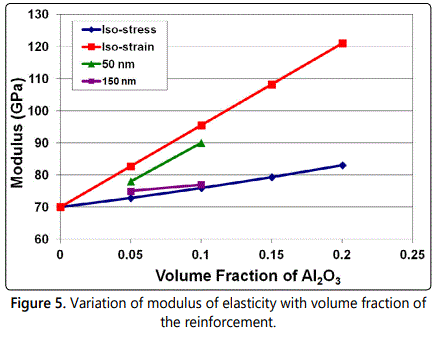
Powder mixtures of the above combinations were mechanically milled in a SPEX shaker mill and it was noted that a fairly uniform distribution of Al2O3 was obtained in all the alloy combinations on milling the powder blend for 20 h. Scanning electron microscopy (SEM) studies indicated a uniform dispersion of Al2O3 in Al even when the volume fraction was 0.5 and the reinforcement size was 50 nm (Figure 4). These composites were very hard and strong, especially when the reinforcement size was small and its volume fraction was large. Accordingly, these powders could be consolidated to near full density only with a combination of vacuum hot pressing and hot isostatic pressing. The measured mechanical properties of these composites were very similar to those obtained by the rule of mixtures. The strength and modulus increased with increasing volume fraction and decreasing reinforcement size (Figure 5).
TiAl-Ti5Si3 Nanocomposites
Light weight intermetallic alloys based on γ-TiAl are promising materials for high-temperature structural applications, e.g., in aircraft engines or stationary turbines. Even though they have many desirable properties such as high specific strength and modulus both at room and elevated temperatures, and good corrosion and oxidation resistance, they suffer from inadequate room temperature ductility and insufficient creep resistance at elevated temperatures, especially between 800 and 850°C, an important requirement for elevated temperature applications of these materials. Therefore, current research programs have been addressing the development of high-temperature materials with adequate room temperature ductility for easy formability and ability to increase the high-temperature strength by a suitable heat treatment or alloying additions to obtain sufficient creep resistance.
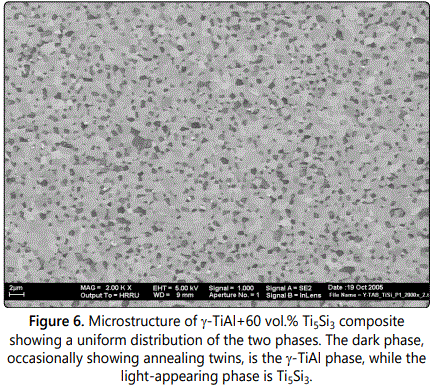
Different amounts (0 to 60 vol.%) of the Ti5Si3 intermetallic have been incorporated into the γ-TiAl matrix by MA methods and the resultant powders were consolidated to full density by hot isostatic pressing methods. The microstructure and mechanical properties of the fully dense Ti5Si3– γ-TiAl composites were investigated. Composites containing a low volume fraction of Ti5Si3 showed that the Ti5Si3 phase was distributed along the grain boundaries of the γ-TiAl matrix. On the other hand when the volume fraction was larger, both the Ti5Si3 and γ-TiAl phases were of sub-micrometer grain size and uniformly distributed as alternate grains (Figure 6). Materials exhibiting such a microstructure (roughly equal amounts of the two phases, small grain sizes of the two phases, and a uniform distribution of the two phases) are expected to be deformed superplastically.
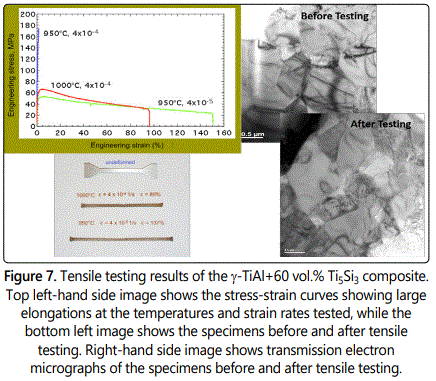
The hardness and strength of these composites were very high. To determine whether it is possible to achieve superplastic deformation in these composites, both compression and tensile testing were conducted at different temperatures and strain rates. It was noted that their strength decreased with increasing temperature and decreasing strain rate. The γ-TiAl+60 vol.% Ti5Si3 composite exhibited large ductility of 150% when tested at 950°C and a strain rate of 4 x 10-5 s-1 and about 100% when tested at 1000°C and a strain rate of 4 x 10-4 s-1 (Figure 7). Transmission electron micrographs suggested that there was only grain rotation and no grain elongation during testing confirming that superplastic deformation was observed. Considering that this composite is based in a ceramic and that monolithic Ti5Si3 does not exhibit superplastic deformation and that the γ-TiAl phase exhibits superplasticity only around 1300°C, the present result is very significant.
Powder Contamination
A major concern in MA processed metal powders is the nature and amount of impurities that get incorporated into the powder and contaminate it. The small size of the powder particles and consequent availability of large surface area, formation of fresh surfaces during milling, and wear and tear of the milling tools, all contribute to contamination of the powder. Thus, it appears as though powder contamination is an inherent drawback of the method unless special precautions are taken to avoid/minimize them. But, it is important to keep in mind that all applications do not require ultraclean powders and that the purity desired will depend on the specific application. The magnitude of powder contamination appears to depend on the time of milling, intensity of milling, atmosphere in which the powder is milled, nature and size of the milling medium, and differences in the strength/hardness of the powder and the milling medium and the container [8,9].
Contamination of milled metal powders can be traced to (i) chemical purity of the starting powders, (ii) milling atmosphere, (iii) milling equipment (milling container and grinding medium), and (iv) process control agents (PCA) added to the powders during milling. The amount of impurities increases with milling time and with increasing ball-to-powder weight ratio and reaches a saturation value.
One way of minimizing contamination from the grinding medium and the milling container is to use the same material for the container and the grinding medium as the powder being milled [45]. If a container of the same material to be milled is not available, then a thin adherent coating on the internal surface of the container (and also on the surface of the grinding medium) with the material to be milled will minimize contamination. In general, a simple rule that should be followed to minimize contamination from the milling container and the grinding medium is that the container and grinding medium should be harder/stronger than the powder being milled.
Milling atmosphere is another important parameter that needs to be controlled. Even though nominally pure gases are used to purge the glove box before loading and unloading the powders, even small amounts of impurities appear to contaminate the powders, especially if they are reactive. In fact, the best solution one could think of will be to place the mill inside a chamber that is evacuated and filled with highpurity argon gas. Since the whole chamber is maintained under argon gas atmosphere (continuously purified to keep oxygen and water vapor below 1 ppm each), and the container is inside the mill, which is inside the chamber, contamination from the atmosphere is minimum [46,47].
Contamination from PCAʼs is perhaps the most ubiquitous. Since most of the PCAʼs used are organic compounds, which have low melting and boiling points, they decompose during milling due to the heat generated. The decomposition products consisting of carbon, oxygen, nitrogen, and hydrogen react with the metal atoms and form undesirable carbides, oxides, nitrides, etc.
Conclusion
Nanocrystalline materials have been synthesized by a number of different methods in recent years. But, MA appears to be a versatile method to produce nanocrystalline powders in a variety of metals and alloys. The process is relatively simple, easy to upgrade it to a pilot plant/commercial scale, and a variety of metastable phases can also be synthesized. The three major issues the powders processed this way are the cost, consolidation, and contamination. Powder processing is inherently expensive and therefore there is not much that can be done to alleviate this difficulty. But, the possibility of producing near-net shape products with tailored properties will certainly offset this concern. The second one is consolidation. As discussed in the text, it is imperative that the consolidated product is fully dense and should not contain any porosity. This is a challenging problem and unless this is achieved, the integrity of the product is likely to be compromised. But, novel and innovative methods are being currently employed to achieve this objective and it is likely that great strides will be made in the near future. But, if the milled powder can be used without consolidation, for example, for catalytic purposes, MA will be an ideal process to produce such powders. The last concern is about contamination. This is a ubiquitous problem. Even though remedies have been suggested to avoid or minimize contamination in the milled powders, these appear to be expensive and perhaps, not easy to achieve on a commercial scale, without undue expenses. If the milled powders could be used in non-critical applications, where some amount of contamination could be tolerated, it is likely that this process will prove highly beneficial and attractive.
References
- Suryanarayana C. Non-Equilibrium Processing of Materials. 1st edition. Pergamon: Elsevier; 1999.
- Gleiter H. Nanocrystalline materials. Prog. Mater. Sci. 1989; 33(4): 223-315. doi: 10.1016/0079-6425(89)90001-7
- Suryanarayana C. Nanocrystalline materials. Internat. Mater. Rev. 1995; 40(2): 41-64. doi: 10.1179/imr.1995.40.2.41
- Gleiter H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000; 48(1): 1-29. doi: 10.1016/S1359-6454(99)00285-2
- Suryanarayana C. Recent developments in nanostructured materials. Adv. Eng. Mater. 2005; 7(11): 983-992. doi: 10.1002/adem.200500135
- Koch CC. Nanostructued Materials. Processing, Properties, and Applications. 2nd edition. Norwich, NY: William Andrew Publishing; 2007.
- Suryanarayana C. Mechanical alloying and milling. Prog. Mater. Sci. 2001; 46(1-2): 1-184. doi: 10.1016/S0079-6425(99)00010-9
- Suryanarayana C. Mechanical Alloying and Milling. New York, NY: Marcel Dekker; 2004.
- Mukhopadhyay DK, Suryanarayana C, and Froes FH. Extended solid solutions in Cd-Zn alloys by mechanical alloying. Scripta Metall. Mater.1994; 30(1): 133-137. doi: 10.1016/0956-716X(94)90372-7
- Suryanarayana C, Ivanov E, Boldyrev VV. The science and technology of mechanical alloying. Mater. Sci. & Eng. A. 2001; A304-306: 151-158. doi: 10.1016/S0921-5093(00)01465-9
- Al-Joubori A, Suryanarayana C. Synthesis of stable and metastable phases in the Ni-Si system by mechanical alloying. Powder Technology. 2016; 302: 8-14. doi: 10.1016/j.powtec.2016.08.033
- Suryanarayana C, Jones H. Formation and characteristics of quasicrystalline phases: A review. Internat. J. Rapid Solidification. 1988; 3(4): 253-293.
- Al-Joubori A, Suryanarayana C. Synthesis of metastable NiGe2 by mechanical alloying. Mater. & Design. 2015; 87: 520-526. doi: 10.1016/j.matdes.2015.08.051
- Suryanarayana C, Inoue A. Bulk Metallic Glasses. 2nd edition. Boca Raton, FL: CRC Press &Taylor & Francis, 2018.
- Sharma S, Vaidyanathan R, Suryanarayana C. Criterion for predicting the glass-forming ability of alloys. Appl. Phys. Lett. 2007; 90: 111915. doi: 10.1063/1.2713867
- Patil U, Hong SJ, Suryanarayana C. An unusual phase transformation during mechanical alloying of an Fe-based bulk metallic glass composition. J. Alloys & Compounds. 2005; 389(1-2): 121-126. doi: 10.1016/j.jallcom.2004.08.020
- Suryanarayana C, Klassen T, Ivanov E. Synthesis of nanocomposites and amorphous alloys by mechanical alloying. J. Mater. Sci. 2011; 46(19): 6301-6315. doi: 10.1007/s10853-011-5287-0
- Kuyama J, Inui H, Imaoka S, Nasu S, Ishihara KN, Shingu PH. Nanometersized crystals formed by the mechanical alloying in the Ag-Fe system. Jap. J. Appl. Phys. 1991; 30, Part 2(5A): L854-L856. doi: 10.1143/JJAP.30.L854
- Prabhu B, Suryanarayana C, An L, Vaidyanathan R. Synthesis and characterization of high volume fraction Al-Al2O3 nanocomposite powders by high-energy milling. Mater. Sci. & Eng. A. 2006; 425(1-2): 192-200. doi: 10.1016/j.msea.2006.03.066
- Suryanarayana C. Recent advances in the synthesis of alloy phases by mechanical alloying/milling. Metals & Materials. 1996; 2(4): 195-209. doi: 10.1007/BF03026094
- Karch J, Birringer R, Gleiter H. Ceramics ductile at low temperature. Nature. 1987; 330: 556-558.
- Groza JR. Nanostructured Materials: Processing, Properties, and Applications. Norwich, NY: William Andrew Publishing; 2007: 173-233.
- Suryanarayana C, Ivanov E. Mechano chemical synthesis of nanocrystalline metal powders. In: book: Advances in Powder Metallurgy. 2013; 42-68. doi: 10.1533/9780857098900.1.42
- Hellstern E, Fecht HJ, Garland C, Johnson WL. Mechanism of achieving nanocrystalline AlRu by ball milling. Mater. Res. Soc. 1989; 132: 137-142. doi: 10.1557/PROC-132-137
- Eckert J, Holzer JC, Krill III, CE, Johnson WL. Structural and thermodynamic properties of nanocrystalline FCC metals prepared by mechanical attrition. J. Mater. Res. 1992; 7(7): 1751-1761. doi: 10.1557/JMR.1992.1751
- Mohamed FA. A Dislocation model for the minimum grain size obtainable by milling. Acta Mater. 2003; 51(14): 4107-4119.doi: 10.1016/S1359-6454(03)00230-1
- Mohamed FA, Xun Y. On the minimum grain size produced by milling Zn22% Al. Mater. Sci.& Eng. 2003; 358(1): 178-185. doi: 10.1016/S0921-5093(03)00294-6
- Nieh TG, Wadsworth J. Hall-Petch relation in nanocrystalline solids. Scripta Metall Mater. 1991; 25(4): 955-958. doi: 10.1016/0956-716X(91)90256-Z
- Sun NX, Lu K. Grain size limit of polycrystalline materials. Phys. Rev. 1999; 59: 5987-5989. doi: 10.1103/PhysRevB.59.5987
- Froes FH, Suryanarayana C. Powder processing of titanium alloys. Rev. Particulate Mater. 1993; 1: 223-276.
- Miracle DB. Metal matrix composites – from science to technological significance. Compos. Sci. Technol. 2005; 65(15-16): 2526-2540. doi: 10.1016/j.compscitech.2005.05.027
- Clyne TW, Withers PJ. An Introduction to Metal Matrix Composites. 1st edition. Cambridge, UK: Cambridge University Press; 1995.
- Suryanarayana C, Al-Aqeeli N. Mechanically alloyed nanocomposites. Prog. Mater. Sci. 2013; 58(4): 383-502. doi: 10.1016/j.pmatsci.2012.10.001
- Suryanarayana C. Synthesis of nanocomposites by mechanical alloying. J. Alloys & Compounds. 2011; 509: S229-S234. doi: 10.1016/j.jallcom.2010.09.063
- Kim HM, Kim TS, Suryanarayana C, Chun BS. Microstructure and wear characteristics of rapidly solidified Al-Pb-Cu alloys. Mater. Sci. & Eng A. 2000; 287(1): 59-65. doi: 10.1016/S0921-5093(00)00813-3
- Son HT, Kim TS, Suryanarayana C, Chun BS. Homogeneous dispersion of graphite in a 6061 aluminum alloy by ball milling. Mater. Sci. & Eng. A. 2003; 348(1-2): 163-169. doi: 10.1016/S0921-5093(02)00749-9
- Hong SJ, Kim HM, Huh D, Suryanarayana C, Chun BS. Effect of clustering on the mechanical properties of SiC particulate-reinforced aluminum alloy 2024 metal matrix composites. Mater. Sci. & Eng. A. 2003; 347(1-2): 198-204. doi: 10.1016/S0921-5093(02)00593-2
- Liu JL, Suryanarayana C, Ghosh D, Subhash G, An L. Synthesis of Mg-Al2O3 nanocomposites by mechanical alloying. J. Alloys & Compounds. 2013; 563: 165-170. doi: 10.1016/j.jallcom.2013.01.113
- Al-Aqeeli N, Abdullahi K, Hakeem AS, Suryanarayana C, Laoui T, Nouari S. Synthesis, characterization and mechanical properties of SiC reinforced Al-based nanocomposites processed by MA and SPS. Powder Metall. 2013; 56(2): 149-157. doi: 10.1179/1743290112Y.0000000029
- Chen J, Bao CG, Wang Y, Liu JL, Suryanarayana C. Microstructure and lattice parameters of AlN particle-reinforced magnesium matrix composites fabricated by powder metallurgy. Acta Metall. Sinica. 2015; 28(11): 1354-1363. doi: 10.1007/s40195-015-0333-6
- Klassen T, Suryanarayana C, Bormann R. Low temperature superplasticity in ultrafine-grained Ti5Si3-TiAl composites. Scripta Mater. 2008; 59(4): 455-458. doi: 10.1016/j.scriptamat.2008.04.029
- Suryanarayana C, Behn R, Klassen T, Bormann R. Mechanical characterization of mechanically alloyed ultrafine-grained Ti5Si3 + 40 vol.% γ-TiAl composites. Mater. Sci. & Eng. A. 2013; 579: 18-25. doi: 10.1016/j.msea.2013.04.092
- Suryanarayana C. Structure and properties of ultrafine-grained MoSi2+Si3N4 composites synthesized by mechanical alloying. Mater. Sci. & Eng. A. 2008; 479(1-2): 23-30. doi: 10.1016/j.msea.2007.07.021
- Suryanarayana C, Ivanov E, Noufi R, Contreras MA, Moore JJ. Synthesis and processing of a Cu-In-Ga-Se sputtering target. J. Mater. Res. 1999; 14(2): 377-383. doi: 10.1557/JMR.1999.0055
- Seelam UMR, Barkhordarian G, Suryanarayana C. Is there a HCP → FCC allotropic transformation in mechanically milled Group IVB elements? J. Mater. Res. 2009; 24(11): 3454-3461. doi: 10.1557/jmr.2009.0423


