Research Article
Preparation and Characterization of Gd1-xSrxAlO3 Cathode for Solid Oxide Fuel Cell
Advanced Energy Research Lab, PG & Research Department of Chemistry, Government Arts College, Dharmapuri, India
*Corresponding author: Mari Rajasekhar, Advanced Energy Research Lab, PG & Research Department of Chemistry, Government Arts College, Dharmapuri, India, E-mail: drmrchem@gmail.com
Received: July 23, 2018 Accepted: September 20, 2018 Published: September 26, 2018
Citation: Rajasekhar M, Kalaivani N. Preparation and Characterization of Gd1-xSrxAlO3 cathode for Solid Oxide Fuel Cell. Madridge J Nanotechnol Nanosci. 2018; 3(2): 112-115. doi: 10.18689/mjnn-1000121
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Gd1-xSrxAlO3 (0 ≤ x ≤ 0.5) cathode materials are synthesized with Gd(NO3)3, Sr(NO3)2,Al(NO3)3, and aspartic acid (fuel) by assisted combustion method with heating at 550°C for 6 hours. The surface morphology of the synthesized crystalline powder is characterized by Scanning Electron Microscopy (SEM). Thus, particle size and porosity were determined. The synthesis and crystallization are followed by thermochemical techniques (TGA/DTA) studies. The synthesized materials showed reasonable electrical conductivity. These results indicate that assisted combustion method is a promising method to prepare nanocrystalline Gd1-xSrxAlO3 for solid oxide fuel cell.
Keywords: Ionic conductivity; Scanning Electron Microscopy; Transmission Electron Microscope; Thermal Analysis.
Introduction
Fuel cells are electrochemical device that converts the chemical energy of a directly into electricalenergy. The challenge is to develop material with good performance at intermediate temperature (500-800°C) for SOFCs allowing to reduce the cost of the cell and increasing the long-term stability.For the fuel cell, the most commonly used materials are lanthanum strontium manganite (LSM) was used for the cathode, YSZ for the electrolyte and Ni–YSZ cermet for the anode. It is essential that the chosen interconnect material have highest chemical stability, the highest oxidation resistance as well as the highest electrical conductivity.
The permanent increment of human population is accompanied by increase of energy demand and more restrictive environmental regulations. In that instance, the Solid Oxide Fuel Cells (SOFC) technology has emerged as an efficient substitution of the presently existing energy devices. Fuel cells are important type of electrochemical devices which converts chemical energy into electrical energy in aclean and calm way. Assisted combustion synthesis (ACS) or self-propagating high-temperature synthesis (SHS) is an effective, low-cost method for production of various industrially useful materials. They have wide range of potential applications ranging from providing power for portable devices (eg. Mobile phones, laptop computers) and transport applications to small and large scale stationary power applications.
The assisted combustion method is a novel method in the production of ultra-fine ceramic powders with a small particle size and high porosity [1-2]. Fuel cells have many advantages compared to conventional electric power generation systems such as high conversion efficiency which is relatively independent of size as well as environmental compatibility.
Experimental Studies
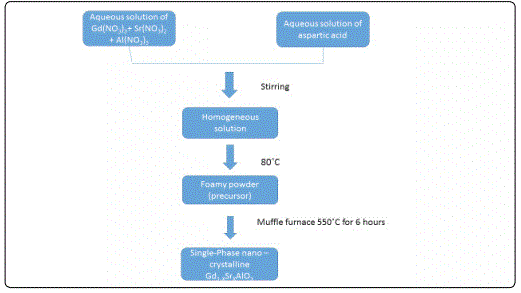
The nanocrystalline Gd1-xSrxAlO3 powder was synthesized by assisted combustion method using high purity of Gd(NO3)3, Sr(NO3)2, Al(NO3)3 and aspartic acid as fuel. All the reagents were purchased from Sigma Aldrich, >99.9%), the required stoichiometric amounts of the starting materials were dissolved in double distilled deionized water in order to obtain a homogeneous solution. This solution was kept at constant heating at 80°C to obtain the foamy powders of Gd1-xSrxAlO3 is show in AS Figure 1. For Calcination, the foamy powder was carried out in a muffle furnace at 550°C for six hours [3-4].
Characterization analysis
X-ray powder diffraction (XRD) data were collected at room temperature with a diffractometer (Model: Philips Xʼ Pert MPHR) with Cu Kα radiation. The data were recorded in the 2θ range of 10-70 with a 0.02° steps. The particle size and morphology of the produced powderwas analysed with a JEOL scanning electron microscopy SEM (Model: JSM-840A) equipment with INCA was used to determine the morphology of samples.
The thermal decomposition of the polymeric precursors was characterized by Perkin-Elmer TG/DTA thermal analysis (Model; Pyris Diamond). The TGA is a process which relies on measuring the change in physical and chemical properties of a sample as a function of temperature (with constant heating rate) or as a function of time (with constant temperature). It is predominantly used for determining the features of a material that exhibit either mass loss or gain due to decomposition, oxidation or loss of volatiles. Differential thermal analysis is a technique in which the temperature of a sample is compared with an inert reference material during the programmed change of temperature [5].
The particle size of the synthesized powder was observed by means of a JOEL transmission electron microscopy (Model: 1200 EX). The synthesized powder was analysed with FTIR spectrometry. (Agilent Cary 630 FTIR spectrometer) and which scanned in a region of about 4000-400cm-1. The ionic conductivity of the sintered pellets were measured by a dc-four probe method in which temperatures range 200-700°C in air.
Results and Discussion
Analysis of Crystalline Structure
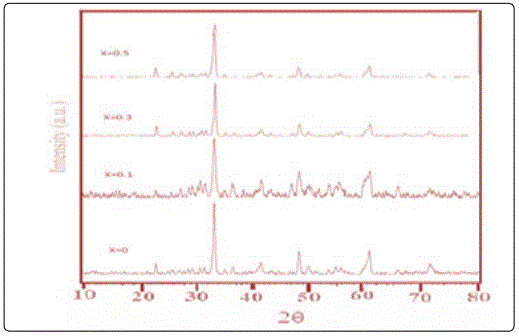
The powder XRD analysis was performed on the prepared Gd1-xSrxAlO3 nanocrystalline powders at 550°C for 6 hours. It was used for identifying the crystallite size of Gd1-xSrxAlO3 powder. The perovskite phase has existed in the resulting powder but the impurity phase has exist clearly as well. In general, all the diffracted peaks are broader than usually observed for highly crystalline powder. The broadening in the diffracted peaks is attributed to the superfine crystalline nature of composites. The size of the particles were calculated by Scherrer equation, it was 30 nm [6-8] as shown in Figure 2.
SEM analysis:
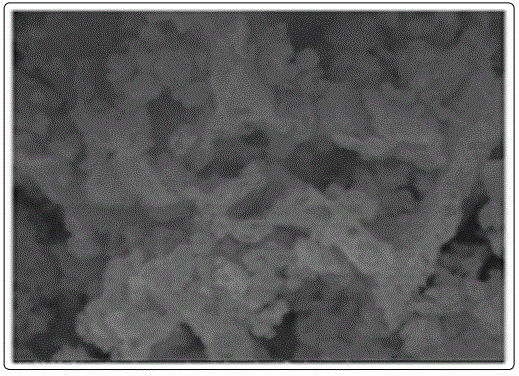
Figure 3. shows the SEM microstructure of Gd0.7Sr0.3AlO3 powder obtained at 550°C for 6 hours. The surface morphology of the synthesized product is different pores and grains. Further, the SEM image indicates that the particles are agglomeration. All the samples are relatively dense and do not show much difference in density. However Sr doping significantly improves the grain growth. The average grain size of the doped samples is between 4 and 12 μm. The average crystallite size is 30 nm. The particles are uniformly distributed. There is agglomeration of the particles. The particles of the synthesized products are in nanorange [9].
Thermal Analysis of TGA/DTA
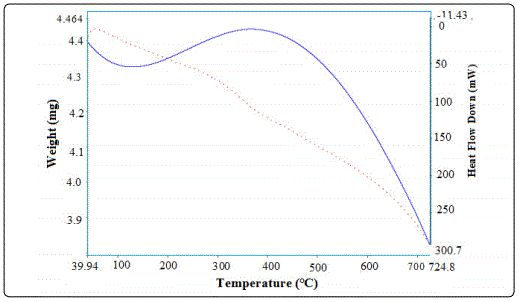
Figure 4. shows that the TGA/DTA pattern obtained on Gd1-xSrxAlO3 powder. The sample heating from 100°C-800°C which shows slight weight loss of about 0.035mg/min. Again the sample shows a weight increase from 105.3°C -386.39°C of 0.070mg/min. The weight gain and weight loss indicated that the Gd0.7Sr0.3AlO3 powder exhibited easy reversible absorbtiondesorbtion of oxygen from air. The weight loss is minimum because of the removal of residual H2O and different gases. The chemical decomposition with an increases of temperature was examined through DTA and it appeared as the endothermic and exothermic peaks. From the above TGA/DTA data, know the Gd0.7Sr0.3AlO3 gradually absorbs the oxygen from air with temperature [10].
FTIR Analysis
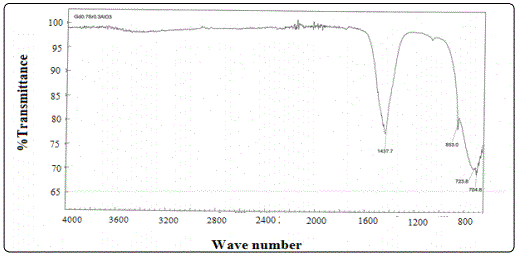
FTIR spectroscopy was used to confirm metal oxide bond formation in the crystal and is investigated their vibrational behavior in solid state of Gd1-xSrxAlO3 powder. It was recorded in the range of 4000 cm-1 to400 cm-1. The infrared spectrums of synthesized samples of Gd0.7Sr0.3AlO3 powder are shown in Figure 5. The broad band at 1437.7cm-1 is assigned to vibration mode of chemically bonded hydroxyl groups. The peak appeared at 853.0 cm-1 corresponds to the H-O-H bond mode confirming the presence of moisture in the sample. The peak appeared at 1437.7 cm-1 is due to the presence of CO2 in the sample. The Gd0.7Sr0.3AlO3 exhibited a low intensity peak at 704.8 cm-1 and the sample exhibited three peaks obtained between the wavelength regions 600-1000 cm-1 which observed at 853.0, 723.8, and 704.8cm-1. The peak appeared at 1437.7 cm-1 is related to the O-H stretching vibration of H2O in the sample. The broad band at 1437.7 cm-1 is assigned to vibration mode of chemically bonded hydroxyl groups.
Conductivity
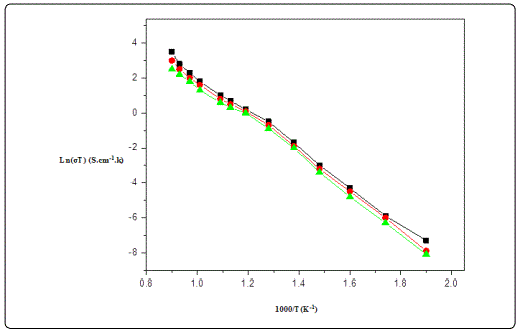
Figure 6. shows that the Arrhenius plots of conductivity for Gd1-xSrxAlO3 samples sintered at different temperature range. It can be seen from Figure 6, that the conductivity of the samples increases gradually with increasing the temperature. In this case, grains grow excessively, and the pores are trapped among the grains or grain boundaries, blocking oxygen ion migration leading to the decreases in the conductivity of the sample. The volatilization of gaseous SrO and O2 from Gd1-xSrxAlO3 at excessively high sintering temperature were detected by mass spectroscopy resulting in the sample volumes bloating and density reduction, so that the conductivity decreases.
Conclusion
The present investigation was carried out to improve the performance of Gd1-xSrxAlO3 by the synthesis method. The electrochemical behavior of Gd1-xSrxAlO3based on the method of synthesis and sintering temperature. The present work was mainly focused on synthesis, and ionic conductivity of Gd1-xSrxAlO3.
References
- Rajasekhar M, Subramania A, Muzhumathai S. Microwave Synthesis of Nd1-xCaxCoO3 Nanopowders as Cathode Material for Intermediate Temperature Solid Oxide Fuel Cells. European Journal of Applied Science and Technology. 2014; 1(2): 50-56.
- Kahlaoui M, Chifi S, Inoubla A, Madani A, Chefi C. Synthesis and electrical properties of co-doping with La3+, Nd3+, Y3+, and Eu3+ citric acid-nitrate prepared samarium-doped ceria ceramics. Ceramics International. 2013; 39(4): 3873-3879. doi: 10.1016/j.ceramint.2012.10.230
- Cabouro G, Caboche G, Chevalier S, Piccardo P. Opportunity of metallic interconnects for ITSOFC: Reactivity and electrical property. Journal of Power Sources. 2006; 156: 39-44. doi: 10.1016/j.jpowsour.2005.08.039
- Laishram K, Mann R, Sharma R, Bhardwaj D, Shakya S, Malhan N. Nd: GGG Nanopowders by Microwave Gel Combustion Route and Sinterability Studies. Defence science journal. 2014; 64(5): 490-494. doi: 10.14429/dsj.64.5409
- Caboura G, Caboche G, Chevalier S, Piccardo P. Opportunity of metallic interconnects for ITSOFC: Reactivity and electrical prorerty. Journal of power sources. 2006; 156(1): 39-44. doi: 10.1016/j.jpowsour.2005.08.039
- Simeonovl S, Kozhukharov S, Grenier JC, Machkova M. Kozhukharov V. Assessment of Nd2-XSrXNiO4-d as a cathodic material for solid oxide fuel cell applications. Journal of chemical technology and metallurgy. 2013; 48(1): 104-110.
- Hui S, Roller J, Yick S, et al. A brief review of the ionic conductivity enhancement for selected oxide electrolytes. J. Power Sources. 2007; 172(2): 493-502. doi: 10.1016/j.jpowsour.2007.07.071
- Aruna ST, Mukasyan AS. Combustion synthesis and nanomaterials. Current Option in Solid State and Materials Science. 2008; 12(3-4): 44-50. doi: 10.1016/j.cossms.2008.12.002
- Orera A, Slater PR. New chemical Systems for Solid Oxide Fuel Cells. chem. Mater. 2010; 22(3): 675-690. doi: 10.1021/cm902687z


