Research Article
Evaluation of Cationic Surfactant Benzalkonium Chloride as inhibitor of Corrosion of Steel in presence of Hydrochloric Acid Solution
1Department of Medical Laboratory, Pharos University, Canal El Mahmodya, Smouha, Alexandria, Egypt
2Department of Chemistry, Faculty of Science, El-Mansoura University, El-Mansoura, Egypt
*Corresponding author: Rania Assem, Assistant Professor, Department of Medical Laboratory, Pharos University, Canal El Mahmodya, Smouha, Alexandria, Egypt, E-mail: rania.assem@pua.edu.eg
Received: February 2, 2019 Accepted: March 5, 2019 Published: March 18, 2019
Citation: Assem R, Fouda AS. Evaluation of Cationic Surfactant Benzalkonium Chloride as inhibitor of Corrosion of Steel in presence of Hydrochloric Acid Solution. Madridge J Mol Biol. 2019; 1(1): 14-22. doi: 10.18689/mjmb-1000103
Copyright: © 2019 The Author(s). This wor is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Cationic surfactant Benzalkonium chloride was evaluated as inhibitor for carbon steel in presence of 1.0 M HCl by different methods like Chemical Method (Weight loss) or Electrochemical Impedance Spectroscopy (EIS), Potentiodynamic polarization and Electrochemical Frequency Modulation (EFM). The effect of temperature on the corrosion rate was evaluated. Tafel polarization proofed that the surfactant act as mixed type inhibitor. Langmuirʼs adsorption isotherm was the preferable fitted isotherm. The results showed that the efficiency of the investigated surfactant increases with raising both concentration and temperature. The thermodynamic parameters of activation and adsorption were calculated and discussed. The surfactantʼs adsorption led to a reduction of the double layer capacitance (Cdl) and an increase in the charge transfer resistance (Rct). Quantum Chemical Technique has been employed to discuss the inhibition efficiency by effectiveness of molecular structure of investigated surfactant. The various techniques which used have inhibition efficiency (IE) with the same trend.
Keywords: Corrosion Inhibition; Carbon Steel; HCl; Cationic Surfactant; Benzalkonium Chloride.
Introduction
Carbon steel is a material which has several applications in various directions according to its advantages in low cost and availability. But the challenge which is faced by researchers is its ability to destructive (corroded) easily which costs high financial burden to avoid or treatment it in industry, according to its important role in national economic development [1-3]. Corrosion operation is natural and costly process which destruct the metal via several reasons either chemical, electrochemical or biological agents between the metal and its medium, such as corrosion of iron in presence of moist air, will oxidize into metal oxide, corrosion of zinc when reacts with dilute sulfuric acid, and corrosion of magnesium in the presence of alcohols [4]. Corrosion of metals occurs usually in presence of many factors such as oxygen, aggressive medium and moisture, where two electrochemical reactions occur oxidation at anodic site and hydrogen evolution at the cathodic site [5-7]. There are different forms of corrosion such as general (uniform), atmospheric, galvanic, differential aeration, pitting, stress-corrosion cracking, intergranular, and erosion corrosion [8-11]. Also there are factors that influence the corrosion of metal such as reactivity of the metal, nature of surrounding environment, existence of impurities, nature of oxide, presence of CO2 in nature water, aeration and pressure [12]. Methods of prevention based on metal, environment, coating and cathodic protection [13] from the effective protection methods for corrosion of metals is using surfactants anionic, cationic or non-anionic surfactants [14,15]. This category of organic corrosion inhibitors characterized by their high ability to absorb on the metal surface or interface which lead to its inhibition efficiency [16,17]. In our study, we investigate the effect of a novel cationic surfactant Benzalkonium chloride on the corrosion of carbon steel in presence of 1 M-HCl by several methods either chemical (weight loss) method, electrochemical method (Potentiodynamic Polarization, Electrochemical Impedance Spectroscopy (EIS) and Electrochemical Frequency Modulation (EFM), and also estimated it via quantum calculations, the adsorption isotherm of the investigated surfactant was appreciated.
Materials and Methods
Materials
Tests were performed on carbon steel of the following composition (weight %): 0.15–0.20% C, 0.60–0.90% Mn, 0.04% P, 0.05% S, and the remainder Fe.
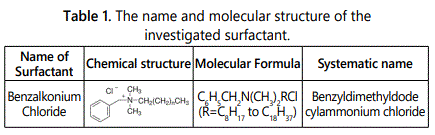
Surfactant: Surfactant which used in this research obtained from Fluka product of pure quality (97%), the chemical structure, molecular formula, and systematic name of the investigated surfactant are listed in table 1.
Solution: 1M hydrochloric acid solution used as corrosive medium which was prepared using double distillated water and was standardized by sodium carbonate. All chemicals used were of analytical grade reagents. All experiments were carried out under unstirred and aerated conditions.
Weight loss experiments carried out by Seven rectangular specimens bar of carbon steel of dimensions 2 cm × 2 cm × 2 cm were abraded using successive grades of sand papers (80-1200) before each test, then degreased using acetone, rinsed by double distilled water, dried through two filter papers and precisely weighed, then were totally immersed in blank and different concentrations (50,100,150,200,250,300) ppm. After equal time intervals, the samples were taken out of the solutions and washed out with double distillated water, dried through filter papers and reweighed, then average weight loss at a certain time was determined. The inhibition efficiency (IE) and degree of surface coverage (?) can be calculated from Eq.1 [18].
- %IE=[1-(ΔWinh/ΔWfree)] × 100=θ × 100 Eq.1
- Where ΔWinh, ΔWfree are weight losses in presence an bsence of inhibitor, respectively.
Electrochemical methods
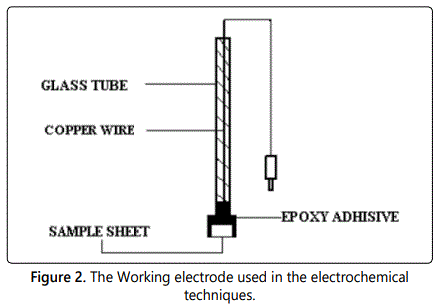
Glass cell consists of three electrodes used (Figure 1), the working electrode made of squared carbon steel (10 × 10) mm cut from the original bar used in weight loss method, the exposed surface area to the test solution was 1.0 cm2 (Figure 2). The reference electrode was a saturated calomel electrode (SCE) and the counter electrode used was platinum sheet (1 cm2). Luggin capillary is connected to a reference electrode to diminish the effect of potential drop (IR drop) and the tip of Luggin capillary to be very close to the surface of the electrode. All potential values were evaluated versus saturated calomel electrode (SCE), in open air at 25°C ± 1°C. The electrode must be prepared before every experiment as well as weight loss method, and then leave it for 30 min before starting measurements to stabilization; the measurements were carried out using Gamry instrument PC14G750 potentiostat/Galvanostat/ZRA which includes Gamry frame work system which based on ESA400. Gamry applications contain DC105 software for DC corrosion measurements, EIS 300 Software for electrochemical impedance spectroscopy and EFM140 software for electrochemical frequency modulation measurements a long with a computer for collecting data Echem analyst 6.03 software was utilized for plotting, graphing and fitting data [19].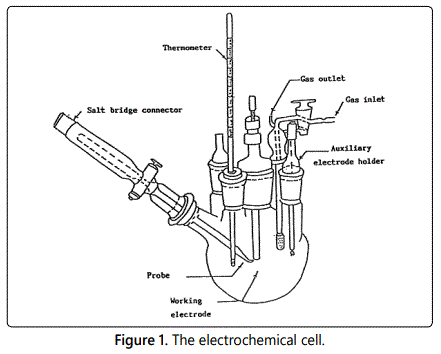
Potentiodynamic polarization: The electrode potential scanning from (-1000 to 0.0 mVSCE) was done spontaneously to get Tafel polarization graphs at open circuit potential at scan rate of 1 mVS-1. By using Stern-Geary method corrosion current was calculated via extrapolation of anodic and cathodic Tafel lines of charge transfer controlled corrosion reactions to a point which gives log icorr and the corrosion potential (Ecorr) for the free acid and for each concentration of surfactant from which surface coverage (?) and inhibition efficiency (%IE) can be calculated [20].
%IE=[1-(icorr(inh)/icorr(free)] × 100=? × 100 Eq.2
Where icorr(free) and icorr (inh) are the corrosion current densities in absence and presence of surfactant, respectively.
Electrochemical impedance spectroscopy (EIS): These measurements were carried out using ac signals at open circuit potential in the frequency range 10 kHz to 0.5 kHz with amplitude of 5 mV peak to peak. The main parameters obtained from the analysis of Nyquist diagram are the charge transfer resistance Rct (diameter of high frequency loop) and double layer capacity Cdl which calculated as the following
Cdl= 1/(2Π fmax Rct) Eq.3
Where fmax is maximum frequency. So the surface coverage can be calculated from eq.4.
% IE=1-(R1ct-Rct) × 100=θ × 100 Eq. 4
Where Rct1 and Rct are the value of charge transfer resistance in absence and presence of inhibitor surfactant [21].
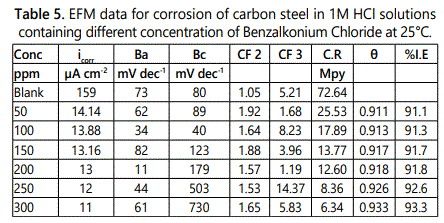
Electrochemical frequency modulation (EFM): By applying two various frequencies 2 and 5 Hz in electrochemical frequency modulation technique. The base frequency was set to be 0.1 Hz, so that one second is elapsed time for the current wave form. The designated current responses for harmonically and inter modulation current peaks can be obtained from the intermodulation spectra. Via larger peaks, the corrosion current density (icorr), was calculated the Tafel slope (βc and βa) and the causality factors CF2 & CF3 [22].
Quantum calculations
Molecules investigated by using DMol3 module in materials studio version 7.0. Inside DMol3 module, where a basis set of double number polarization (DNP) plus the exchange correlation functions of Becke one parameter (BOP) with generalized gradient approximation (GGA) were performed and the solvent effects were treated using COSMO controls [23].Results and Discussion
Weight loss measurements
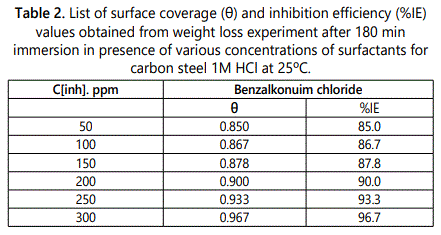
Weight loss measurement is effective method to measure the attitude of inhibitor on corrosion of different metals in aqueous environment by using aggressive electrolyte and different time of exposure. From which corrosion rate can be estimated according to Eq.1. The effect of addition of different surfactants on the dissolution rate of carbon steel in 1 M HCl was studied at 25–40°C by plotting the weight loss (mg cm–2) versus time (min). It can been seen from the resultant linear curves shown in figure 3, which represent that the weight loss curves in case of the solutions containing investigated surfactant, curve fall below in case of the free acid, which can be explained by formation of insoluble layer resulting from the adsorption of surfactant compounds on the carbon surface. In the investigated surfactant the inhibition efficiency increases with increasing its concentration reach to 96.7% at 300 ppm at 25°C the results of surface coverage and inhibition efficiency of the concentrations of studied surfactant at 25°C are listed in table 2, which result from the fact that the adsorption of surfactant on mild steel increases with increase of this surfactant. Thus the carbon steel surface is separated from the surrounding by high efficiency via formation of a film on its surface.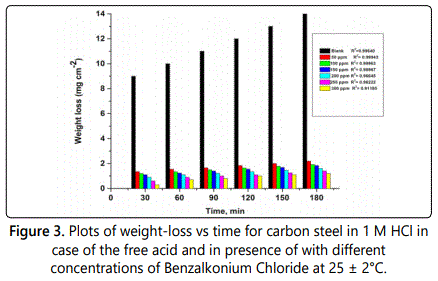
Potentiodynamic polarization measurements
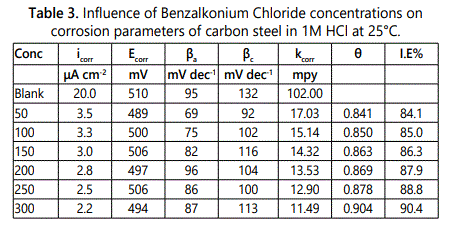
Electrochemical corrosion measurements are very useful method for measuring the corrosion of metals in corrosive mediums. A plot of current potential of an electrode can be measured quickly in a few minutes and accurately. In immersing a carbon steel electrode in a corrosive acid medium, redox reaction will take place on its surface. The carbon steel oxidizes (corroded) and the medium is reduced. So the hydrogen ions are reduced. The electrode of carbon steel (working electrode) could act as both anode and cathode, and both anodic and cathodic currents flow on its surface. The electrode considers as a potential (relative reference electrode) termed as the corrosion potential (Ecorr) when immersed in a corrosive medium without connection to any instrumentation. The carbon steel electrode at Ecorr has both anodic and cathodic currents occurs on its surface when these currents are exactly equal in magnitude so there is no net current to be measured hence the carbon steel electrode is at equilibrium with medium. So the definition of Ecorr is the potential at which the rate of oxidation is exactly equal to the rate of reduction. By plotting the external current response as a function of the applied potential the polarization characteristics can be measured experimentally. So the calculated current can differ over several orders of magnitude, the log current function is plotted vs. potential on a semi–log chart which called potentiodynamic polarization plot, in which Tafel plot was used and can provide a direct measure of corrosion current and corrosion rate. Figure 4 represents the polarization curve for corrosion of carbon steel in 1 M HCl in the absence and present of surfactant A at 25°C which indicated that the presence of surfactants shift the anodic polarization to more positive and the cathodic polarization to more negative values. By using the potentiodynamic polarization measurements, the values of the surface coverage (?) and inhibition efficiency (%IE) can be calculated by using Eq.2, The values of the electrochemical polarization parameters and the inhibition efficiency (% IE) percentage are given in table 3 from which deduced that; the resultant anodic and cathodic curves apply to Tafel type behavior. In presence of surfactants there is an increase in both cathodic and anodic over voltage and caused parallel displacement to the more negative and positive values, respectively. The parallel cathodic and anodic Tafel curves confirmed the evolution of hydrogen gas is activation controlled and the reduction and dissolution mechanism experienced constant in presence of surfactant. From which it can conclude that they behave as mixed mode inhibitors for corrosion of carbon steel in 1 M HCl solutions, as it may be adsorbed on the cathodic sites of carbon steel and reduce the evolution of hydrogen. Also these surfactants adsorbed on the anodic sites through the lone pairs of electrons of hetero atoms which could reduce the anodic dissolution of carbon steel. The value of corrosion current density (icorr) decreases as the surfactant concentration increases, so the dissolution rate of carbon steel in 1 M HCl solution decreases in investigated surfactant and the inhibition efficiency is dependent on the concentration and the type of surfactant present, while no definite trend was observed in the shift of Ecorr values, but the Tafel slopes (βa, βc) are approximately constant indicating that the retardation of the two reactions (cathodic hydrogen reduction and anodic metal dissolution) were affected without changing the dissolution mechanism. By using this method the inhibition efficiency of the studied surfactant reached to 90.4% at 300 ppm.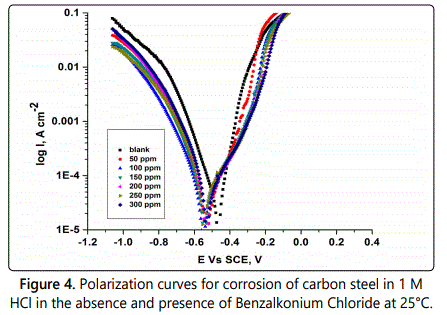
Electrochemical impedance spectroscopy (eis) measurements
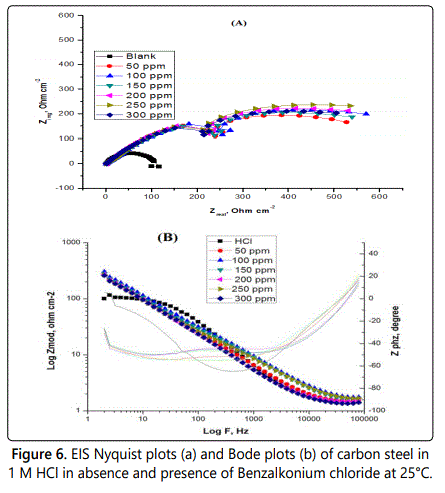
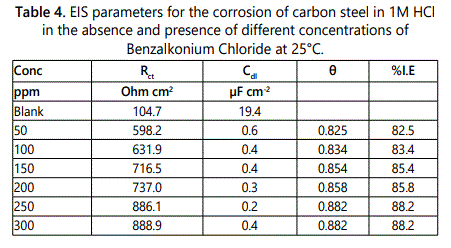
Electrochemical impedance spectroscopy (EIS) or AC impedance method is a measure of the ability of a circle to resist the flow of electrical current, which is measured by applying an AC potential to an electrochemical cell and to evaluate the current through the cell. The AC impedance instrument can record the real (resistance) and imaginary (Capacitance and inductance) components of the impedance response of the system. This method can give information which cannot be obtained from DC techniques also can be used in multiple electrochemical reactions so it had seen wide increase in last recent years. Electrochemical impedance can be analyzed by measuring an equivalent circuit model as shown in figure 5 for carbon steel, which consists of solution resistance Rs and soluble layer capacitance Cdl which is placed in parallel to the charge transfer resistance. According to the shape of the EIS spectrum, a circuit model or circuit description code and initial circuit parameters are evaluated and input by the operator. The type of electrical components in the circuit model and their interconnections control are the factors which govern the shape of the modelʼs impedance spectrums but the modelʼs parameter (resistance value of resistor) effect on the size of each feature in the spectrum both of these factors influence on the degree to which the modelʼs impedance spectrum confirm with the measured EIS spectrum. By using EIS method the corrosion behavior of carbon steel in 1 M HCl solution in absence and presence of different concentrations of studied surfactants are investigated at 25°C. For example figure 6 shows the Nyquist plots (A) and Bode plots (B) for corrosion of carbon steel for investigated surfactant. The Bode diagrams characterized by two time constants consisting of large capacitive loop at high to medium frequency and inductive loop at low frequency. The high frequencies of capacitive loop represent the double layer electric layer. The large capacitive loop makes an angle with the real axis and its interaction gives a resistance of solution (Rs) between the working electrode and the counter electrode a relaxation process obtained by adsorption species onto the electrode surface may be attributed by the presence of inductive loop. In 1 M HCl with the presence of different concentrations of inhibitors (surfactants), the impedance diagrams showed the same trend (one capacitive loop), but the diameter of this capacitive loop increases with increasing concentration. Nyquist plots include various electrochemical parameters such as; the diameter of high frequency loop (charge transfer resistance Rct), the double layer capacity Cdl which can be calculated by using Eq.3. The inhibition efficiency and surface the values of impedance data in table 4, it can be conclude that; Rct directly proportional to the concentration of surfactant used but inversely proportional to the corrosion rate, by increasing the concentration of surfactant, the value of Cdl be very small, due to formation of a thin protective layer on the metal surface which lead to decrease in the local dielectric constant of the metal solution interface. The %IE obtained from EIS close to the results obtained from the potentio dynamic measurements for this surfactant reached to 88.2% at 300 ppm.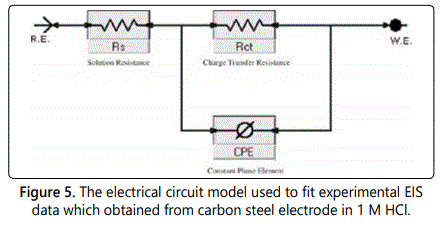
Electrochemical frequency modulation (EFM)
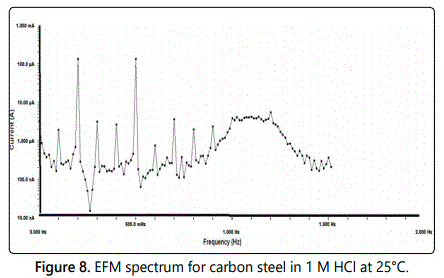
EFM or electrochemical frequency modulation can be defined as non–destructive corrosion method which directly gives values of corrosion current without prior knowledge of Tafel constants, by applying one or more sine waves a potential perturbation occurs and the current responses at higher frequencies than the frequency of the applied signal such as at zero harmonic and intermodulation frequencies. By using the resultant spectrum of current responses, EFM measurements can be explained as current response considered as a function of frequency [intermodulation spectrum] and examples for the effect of addition of various concentrations of surfactant (50–300 ppm) to 1 M HCl solution for carbon steel are shown in figure 7, also the EFM spectrum for carbon steel in 1 M HCl at 25°C is shown in figure 8. The spectrum includes current responses refers to harmonically and intermodulation current peaks, the corrosion current density (icorr) calculated from the larger peaks the Tafel slopes (βc and βa) and the causality factors (CF–2 and CF–3), all these electrochemical parameters were determined and listed in table 5, which showed that the corrosion current densities of carbon steel decrease with increasing of the investigated surfactants, and the highest value of current density was recorded in the free acid.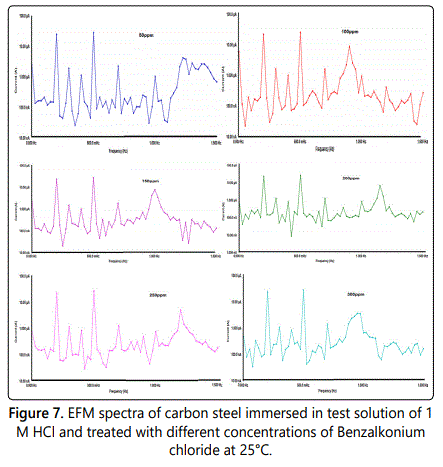
Adsorption isotherm
Addition of inhibitors to corrosive solutions, they can form protective layer on the metalʼs surface (metal/solution) interface. Adsorption of inhibitor molecules can be categorized into four classes; Electrostatic attraction between charged inhibitor molecules and changed metal surface, Interaction of unshared electron pairs on the inhibitor molecules in the vacant orbital of metal, Interaction of electrons with the metal, Combination of them.In the present work, the adsorption process obeys Langmuir adsorption isotherm;
(Cinh/θ) = (1/Kads) + Cinh (Eq. 5)
Where Cinh is the inhibitor concentration, θ is the surface coverage fraction, Kads is modified adsorption equilibrium constant.
Kads = [1/Csolvent] exp [–ΔG°ads/RT] (Eq. 6)
Where Csolvent is the molar concentration of solvent which in the case of the water 55.5 mol.L–1.
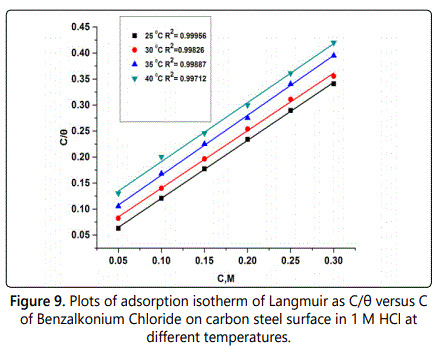
Graphical representation of (C/θ) as a function of the concentration of surfactant are shown in figure 9, from which ΔG°ads can be calculated from equation (Eq.6).
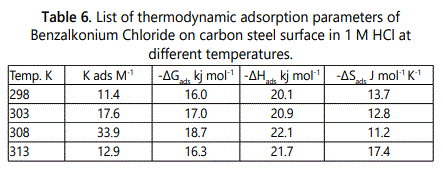
The degree of surface coverage (θ) was calculated from weight loss experiments by using Eq. 5. The regression coefficient R2 is greater than 0.9 which apply to the obtained results in table 6 which show the values of Kads and ΔG°ads obtained from Langmuir isotherm and all results thermodynamic parameters, the heat of adsorption (ΔH°ads) can be calculated by using Vanʼt Hoff equation.
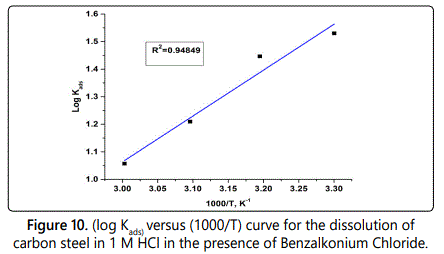
Log Kads=(–ΔH°ads/2.303RT)+Constant (Eq. 7)
To calculate the value of (ΔH°ads), log Kads was plotted against 1/T as shown in figure 10, the slope of the obtained straight lines equivalent to (–H°ads/ 2.303R).
Hence, (ΔS°ads) the entropy of adsorption can be evaluated at all temperatures by using equation.
ΔG°ads = ΔH°ads– TΔS°ads (Eq. 8)
The negative values of ?G°ads represented in indicated that the adsorption of surfactants are spontaneously proceed on the surface of carbon steel, as when the values of ΔG°ads of order –20 kjmol-1 or less indicates that the adsorption is physicorption but on the other hand, when the values of ΔG°ads reaches –40 kjmol-1 or greater this indicates to be chemisorption.
In this study as listed in table 6, ΔG°ads has negative sign and less than –20 kjmol-1, which indicated that the mechanism of adsorption of surfactants on carbon steel in 1M HCl at the studied temperatures may proceed by electrostatic attraction (physisorption), by increasing temperature of the test solution, the free energy change (ΔG°ads) become less negative and the inhibitor molecules desorbed from the metal surface as the reaction temperature increases.
The negative sign of ΔH°ads suggests that, the adsorption of surfactants accompanies by exothermic process but exothermic adsorption process can be physisorption or chemisorption, while endothermic process is typical to chemisorption. So the value of ΔH°ads must be calculated to differentiate between physisorption and chemisorption as if the value of ΔH°ads up to 41.9 kj mol–1, is correlated to the electrostatic attraction between the charged molecules and charged metal (physisorption), but if the values of ΔH°ads approach or greater than 100 kj mol–1 are correlated to be (chemisorption). In case of studied surfactants, the obtained values of enthalpy ΔH°ads is correlated to physisorption mechanism. The negative values of (ΔS°ads) indicate the ordered of inhibitor molecules on carbon steel surface in presence of investigated surfactants.
Effect of temperature
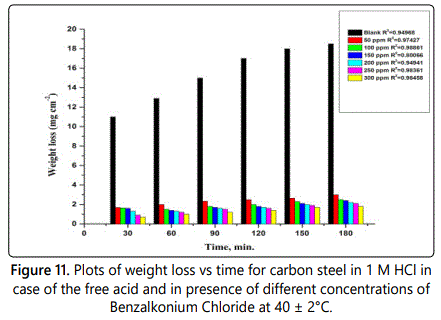
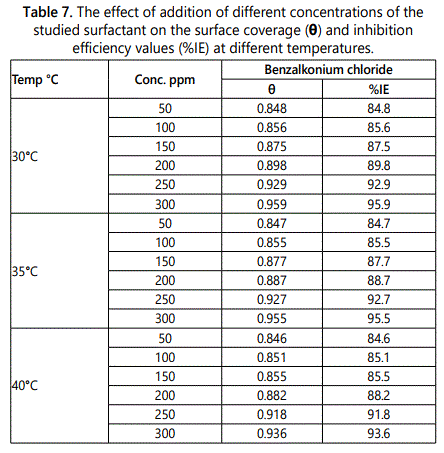
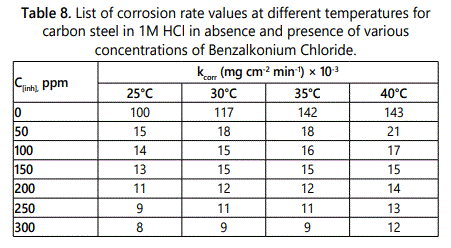
The effect of temperature on inhibition efficiency of this surfactant on carbon steel in 1M HCl was studied firstly by using weight loss methods on range (25-40)°C, the results showed that the inhibition efficiency of the surfactant decreases by increase the temperature. Figure 11 shows the wt-loss and time relation plot of the studied surfactant at 40°C as example. The values of inhibition efficiency at different temperatures of the three surfactants are listed in table 7. From which the corrosion rate can be measured at different temperatures according to Eq.9, the results listed in table 8 indicated that the corrosion rate increase by increasing the temperature.kcorr = (W1–W2)/a.t. (Eq.9)
Where W1 and W2 are initial and final weight value of the specimens, respectively, a is the exposed surface area of carbon steel specimen (cm2) and t is time immersion (min) (Table 6). List of corrosion rate values at different temperatures for carbon steel in 1 M HCl in the absence and presence of various concentrations of the three surfactants.
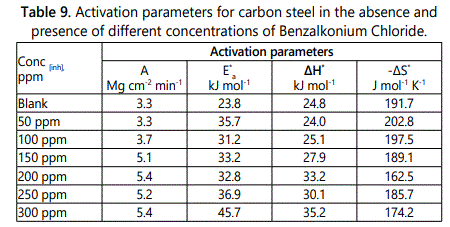
Activation parameters of corrosion process
Activation parameters of corrosion process, activation energy E*a, enthalpy of activation ΔH* and entropy of activation ΔS* can be calculated for carbon steel corrosion in HCl solution in absence and presence of different concentrations of surfactants by using equation (Arrhenius (Eq. 11).Rate (kcorr)=Aexp (–E*a/RT) (Eq. 10)
Transition state equation (3.8)
Rate (kcorr) = [RT/Nhexp (ΔS*/R) exp (–ΔH*/RT) (Eq. 11)
Where A is the frequency factor, h is Planckʼs constant and N is Avogadroʼs number.
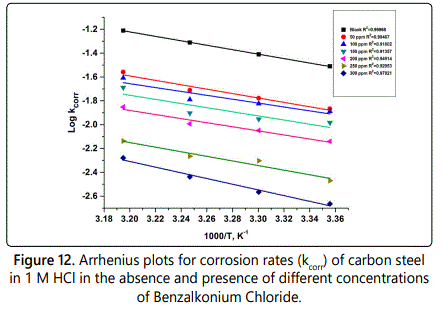
A plot of (log kcorr) versus (1/T) was plotted which give straight lines with slope (–E*a/2.303R) and another plot between log (Kcorr/T) versus (1/T) which gave straight line with slope of (–ΔH*/2.303), the intercepts will be A and (log R/Nh + ΔS*/2.303R) for Arrhenius and transition state equations, respectively.
Figure 12 represent log rate vs 1/T plot and figure 13 represent log (kcorr/T) vs 1/T curves. Also in table 9 the values of activation energy E*a, activation enthalpy ΔH* and activation entropy S* were listed. The values of activation energy increases when the surfactants are present in corrosive solution. This caused by high energy barrier occurs as a consequence of the adsorption of surfactant on surface of metal. The variation in the apparent activation energy is observed to be analogous to the pre–exponential factor (A). The results in table 9 indicated that the activation enthalpy increases with increasing of the concentration of surfactant, which confirm with the inhibition mechanism deduced from the similar variation of the activation energy values. The values of activation entropy (ΔS*), explained that in the rate determining step of the adsorption process in the activated complex suggests an association take places rather than dissociation.
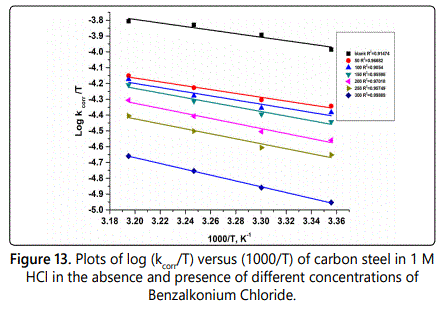
Quantum calculation
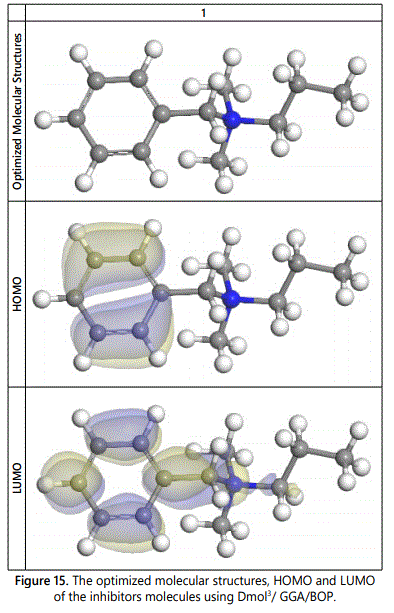
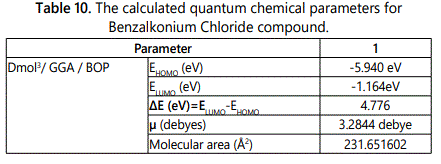
The chemical structure of the molecule which used in quantum calculation and the optimized molecular structures, HOMO and LUMO of Benzalkonuim Chloride molecules using Dmol3/GGA/BOP are shown in figures 14 and 15 respectively, also all the calculted quantum chemical parameters for the investigated surfactant are listed in table 10 which confirms with all results of experimental methods used.
Conclusion
So all measurments experimental [chemical method (weight loss) or electrochemical methods (potentiodynamic polarization, electrochemical impedance spectroscopy (EIS), or electrochemical frequency modulation (EFM) ) and theoritical quantum calculation proofed that the studied cationic surfactant (Benzalkonuim Chloride) act as good inhibtor for corrosion of steel in presence of strong corrosive meduim 1 M HCl.
Acknowledgment
I would specially like to thank Prof. Dr. Abd El Aziz El-Sayed Fouda, Prof. of physical chemistry, Department of Chemistry, Faculty of Science, University of Mansoura for his friendly careful supervision, for his patience and hard working.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
References
- Fouda AS, Rashwan SM, Rashwan SM, Shaban M, Ibrahim HE, Elbhrawy MF. Evaluation of a novel cationic surfactant based on 2-(2(dimethylamino) ethoxy)ethanol as corrosion inhibitor for carbon steel 1018 on 1.0 M HCl solution. Egyptian Journal of Petroleum. 2018; 10(3): 295-306. doi: 10.1016/J.ejpe.2017.05.001
- Sathiyanarynan S, Marikkanu C, Palaniswamy N. Corrosion Inhibition Effect of Tetra mines for Mild Steel in 1 M HCl. Appl Surf Sci. 2005; 241(3-4): 477-484. doi: 10.1016/j.apsusc.2004.07.050
- Craig BD. Fundamental Aspects of Corrosion Films in Corrosion Science. Springer US, New York. 1991. doi: 10.1007/978-1-4899-2557-2
- Kumar A. Corrosion Inhibition of Mild Steel in Hydrochloric Acid by Sodium Lauryl Sulfate. J Chem. 2008; 5(2): 275-280. doi: 10.1155/2008/574343
- Fouda AS, Elmorsi MA, Samy MS, Azazy O. Evaluation of N-(3-(dimethyl hexadecyl ammonio) propyl) palmitamide bromide as cationic surfactant corrosion inhibitor for API N80 steel in acidic environment. Egyptian Journal of Petroleum. 2018; 27(4): 683-694. doi: 10.1016/j.ejpe.2017.10.004
- Okafor PC, Zheng Y. Synergistic inhibition behaviour of methyl benzyl quaternary imidazoline derivative and iodide ions on mild steel in H2SO4solution. Corros Sci. 2009; 51(4): 850-859. doi: 10.1016/j.corsci.2009.01.027
- Omoren SA, Solomon MM. Synergistic corrosion inhibition effects of metal cations and mixtures of organic compounds. J Environ Chem Eng. 2017; 5(1): 426-273. doi: 10.1016/j.jece.2016.12.001
- Zhang B, He C, Wang C, Sun P, LI F, Lin Y. Synergistic corrosion inhibition of environment friendly inhibitors on the corrosion of carbon steel in soft water. Corros Sci. 2015; 94: 6-20. doi: 10.1016/j.corsci.2014.11.035
- Zarrouk A, Hammouti B, Lakhlifi T, Traisnel M, Venzin H, Bentiss F. New 1H-Pyrrole-2,5-dione derivatives as inhibitors of carbon steel corrosion in hydrochloric acid medium : Electrochemical, XPS and DFT studies. Corros Sci. 2015; 90: 572-584. doi: 10.1016/j.corsci.2014.10.052
- Bouanis M, Tourabi M, Yassi AN, Zarrouk A, Jama C, Bentiss F. Corrosion inhibition performance of 2,5-bi(4-dimethylaminophenyl)-1,3,4-oxadiazole for carbon steel in HCl solution. Appl Surf Sci. 2016; 389: 952-966. doi: 10.1016/j.apsusc.2016.07.115
- Bertolini L, Sener El, Pedeferri P, Redadaelli E, Polder RB, Wiely J. Corrosion of steel in concrete and its prevention in aggressive chloride bearing environments. 5th International Conference on Durability of Concrete Structures. China. 2016.
- Fanun M. The role of Colloidal Systems in Environmental Protection. 1st edition. Elsevier. 2014: 1-720.
- Mittal KL, Dinesh OS. Adsorption and Aggregation of Surfactants in solution. CRC Press, New York. 2002.
- Assem R, Fouda AS, Ibrahim AA, Saadawy M. Some Anionic surfactants as corrosion inhibitors for carbon steel in hydrochloric acid solution. Key Engineering Materials. 2018; 786: 134-148. doi: 10.4020/www.scientific.net/KEM.786.134
- Zhu Y, Free ML, Woollam R, Durnie W. A review of surfactants as corrosion inhibitors and associated modeling. Prog Mater Sci. 2017; 90: 159-223. doi: 10.1016/j.pmatsci.2017.07.006
- Shaban SM. Studying the effect of newly synthesized cationic surfactant on silver nanoparticles formation and their biological activity. J Mol Liq. 2016; 216: 137-145. doi: 10.1016/j.molliq.2015.12.098
- Shaban SM, Aiad I, El-Sukkary MM, Soliman EA, El-Awady MY. Evaluation of some cationic surfactants based on dimethylaminopropylamine as corrosion inhibitors. J Ind Eng Chem. 2015; 21:1029-1038. doi: 10.1016/j.jiec.2014.05.012
- Tawfik SM. Ionic Liquids based Gemini cationic surfactants as corrosion surfactants as corrosion inhibitors for carbon steel in hydrochloric acid solution. J Mol Liq. 2016; 216: 624-635. doi: 10.1016/j.molliq.2016.01.066
- Yesudass S, Olasunkanmi LO, Bahadur I, Kabanda MM, Obot IB, Ebenso EE. Experimental and theoretical studies on some selected ionic liquids with different cations/anions as corrosion inhibitors for mild steel in acidic medium. J Taiwan Inst Chem Eng. 2016; 64: 252-268. doi: 10.1016/j.jtice.2016.04.006
- Zhang X, Zhang S, Li W, gong M, Yin L. Experimental and theoretical studies of two imidazolium based ionic liquids as inhibitors for mild steel in sulfuric acid. Corros Sci. 2015; 95: 168-179. doi: 10.1016/j.corsci.2015.03.012
- Bayol E, Gurten AA, Dursun M, Kayakirilmaz K. Adsorption behavior and inhibition corrosion effect of sodium carboxymethyl cellulose on mild steel in acidic medium. Acta Physico-Chimica Sinica. 2008; 24: 2236-2243. doi: 10.1016/S1872-1508(08)60085-6
- Fouda AS, Fouad RR. New azonitrile derivative as inhibitors for copper in nitric solution. Cogent Chem. 2016; 2: doi: 10.1080/23312009.2016.1221174


