Research Article
Water Treatment using Electrospun PVC/PVP Nanofibers as Filter Medium
1Department of Mechanical and Industrial Engineering, College of Engineering, Majmaah University, Majmaah 11952, Kingdom of Saudi Arabia
2Department of Mechanical Engineering, Fahad Bin Sultan University, Tabuk, 71454, Saudi Arabia
3Department of Mechanical Engineering, Higher Colleges of Technology, Dubai, UAE
4Department of Mechanical Engineering, Wichita State University, 1845 Fairmount, Wichita, Kansas 67260, USA
*Corresponding author: Ramazan Asmatulu, Department of Mechanical Engineering, Wichita State University, 1845 Fairmount, Wichita, Kansas 67260, USA, E-mail: ramazan.asmatulu@wichita.edu
Received: July 24, 2018 Accepted: July 31, 2018 Published: August 5, 2018
Citation: Alarifi IM, Alharbi RA, Khan MN, Khan WS, Usta A, Asmatulu R. Water Treatment using Electrospun PVC/PVP Nanofibers as Filter Medium. Int J Mater Sci Res. 2018; 2(1): 43-49. doi: 10.18689/ijmsr-1000107
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
One of the prevailing problems afflicting everybody globally is the scarcity of clean and portable water supply. Water crisis constitutes a major issue to present status of world's water resources. Water pollution is now becoming a critical issue globally. Water filtration/purification with the latest technology is the urgent need in today's economy. Electrospun nanofibers with high filtration efficiency, small pore size, high permeability and low cost are materials of choice for many filtration applications. These outstanding properties are most suitable for filtration media. In this study, Polyvinyl chloride (PVC) was dissolved in N, N-Dimethylacetamide (DMAC) and the small weight percentages of Polyvinylpyrrolidone (PVP) was added in order to make the nanoporous membrane surface hydrophilic for escalating the filtration performance rate and stainability. Two different water samples (dam water and city wastewater) were used in this study. For dam water sample, the parameter such as, PH, turbidity, TDS, conductivity, Ca++, Mg++, hardness, sulfates, nitrates, fluoride, chloride, alkalinity and silica were measured and found out to be 7.5, 24.33 NTU, 2526 mg/l, 5049 μS/cm, 111 mg/l, 57.76 mg/l, 447 mg/l of CaCO3, 78.17 mg/l, 2.98 mg/l, 1.05 mg/l, 1862.96 mg/l, 137.7 mg/l and 85.27 mg/l, respectively. Similarly, for wastewater sample, the parameters such as, PH, turbidity, TDS, conductivity, TSS, COD, BOD5, phosphate, ammonia, oil-greases and DO were measured and found out to be 7.7, 7.7 NTU, 1507.33 mg/l, 30122 μS/cm, 6 mg/l, 38.33 mg/l, 7.67 mg/l, 36.03 mg/l, 18.33 mg/l, 0 mg/l and 6.85mg/l, respectively.
Keywords: Electrospinning, Dam Water, Wastewater Treatment, and Brane Technology.
Introduction
Background of Water treatment Technology
In this era of the water crisis, effective treatment of wastewater is indispensable for growing economy. It is imperative to develop and implement advanced wastewater treatment technologies having high efficiency and low capital investment. Among various treatment processes, recently advanced nanomaterials have become a focus of attention in filtration technology [1]. The application of nanomaterials in filtration technology has been exploited recently due to the high water quality standards. Nanofiltration is the widely used membrane processes for treating dam water, wastewater, air filtration and desalination [2]. Nanofiltration already substituted many filtration processes due to its distinctive features such as high flux and low energy consumption [2]. Membrane filtration is a pressure driven process in which membrane acts as a barrier to impede the flow of pollutants such as microorganisms, nutrients, organics, turbidity, metal ions, inorganics and other oxygen depleting contaminants, and permits relatively clean water to pass through [3]. With the recent advancement intechnology and rising standards of water quality, membrane technology has become an attractive solution to address water quality [4]. The traditional processes of water treatment are not capable to completely remove all contaminants and meet the laid down specifications [5]. Additionally, the existing water treatment processes have many drawbacks such as high energy requirement, incomplete removal of pollutants and production of toxic sludge [6]. Nanotechnology has outstanding potential in filtration applications due to its capability to create precise structural controlled materials. Electrospun Nanofibrous Membranes (ENMs) are cutting edge membrane technology that offers substantial high flux and high rejection rate compared to the conventional membrane. ENMs present a breakthrough in wastewater and water treatment by offering a lightweight, cost-effective, and less energy consuming process than conventional membranes. ENMs possess high porosity of approximately 80%, whereas conventional membranes possess around 35% porosity.
Experimental
Materials
Polyvinyl chloride (PVC), Poly (vinylpyrrolidone) (PVP), N, N-Dimethylacetamide (DMAC) were purchased from SigmaAldrich and used without any alterations.
Method
Synthesis of PVC/PVP Nano membrane Polyvinyl chloride (PVC) was dissolved in N, N-Dimethylacetamide (DMAC) at a 15: 85 % weight ratios. Then, 0, 2 and 5 wt. % of PVP was added to PVC/DMAC solution. The polymeric solution containing both PVC and PVP was mechanically stirred on a hot plate at 75°C (500 rpm) for one hour in order to make a homogeneous blend of the polymeric solution.
Fabrication and Electrospun Nanofibers Techniques
The experimental setup of an electrospinning process is depicted in Figure 1. This solution was then transferred to a 10 ml plastic syringe with an inside diameter of 0.5 mm. The syringe was then mounted on a syringe pump, and the mass flow rate was adjusted at 1 ml/hr and distance between the tip of the plastic syringe and collector screen was adjusted to 25 cm. A copper electrode (0.25 mm diameter) was attached to the DC power supply, and the other end of the electrode was inserted into the plastic syringe in order to charge the polymeric solution. The electrospinning process was then carried out under ambient conditions. The electrospun nanofibers were first dried in air for 12 hours then placed in an oven at 60 °C for one hour to remove all remaining solvent. The fibrous membrane thus fabricated via electrospinning process, possess flexibility, high surface area and porous structure which allow much higher sites for separation processes, are generally referred to as electrospun nanofibrous membranes (ENMs).
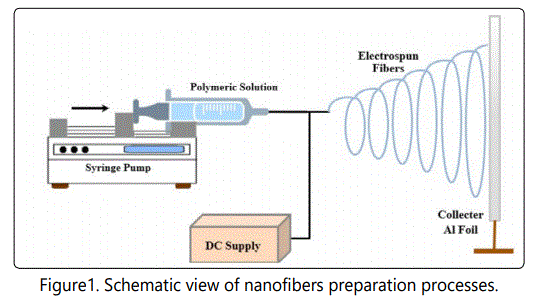
Electrospinning is the easiest, straightforward and costeffective process of producing nano and micro-sized fibers. Generally, electrospinning is used to produce high surface area submicron and nanosized fibers from a polymeric solution or melt. This process applies to all naturally occurring and synthetic polymers [7-8]. Electrospinning is a process in which a high voltage (electrostatic field) is used to fabricate nanofibers. Polymeric solution or melt that has to be electrospun is forced by means of a syringe pump through a spinneret to form a pendant drop of the polymeric solution at the tip of the capillary tube (spinneret). The tip of the capillary is connected to an electrode, and the other end of electrode is connected to a high DC supply. The repulsive forces induced by an electrostatic field cause stretching or plastic deformation of the jet. When the applied electrical forces surpass the surface tension of the polymeric solution, a cone type of structure is formed, which is appropriately called "Taylor cone," and a jet is initiated from the cone. The jet travels linearly for some distance and then undergoes whipping or curling motion before depositing on a collector screen. There are two types of electrostatic forces involved in electrospinning: the repulsive forces between the charges embedded in different jet segments and the guiding electrostatic forces applied by the external filed [9-11]. The interaction between these two forces causes bending instability. During jet motion from the syringe to the collector, most of the solvent evaporates thereby resulting in a long thin fiber deposited on the collector [12-15]. The thinning of the jet is caused by the curling motion, which is termed as "bending instability," thereby resulting in ultrafine fibers having a diameter in nano range [16-19]. Finally, the fibers are collectors on a collector screen, dried in an oven at 60°C for 1 hr.
Vacuum Filter Techniques
Figure 2 shows the filter setup used in this study. The filter consists of porcelain Buchner funnel with an inside diameter of 0.90 cm, and a rubber stopper with a hole to accumulate filtered water having a capacity of 1000 ml. The flask connected to pressure pump with two gauges. The vacuum gauge has a pressure range from 0 to 35 in Hg, while the pressure gauge has ranged from 0 to 11 bars.
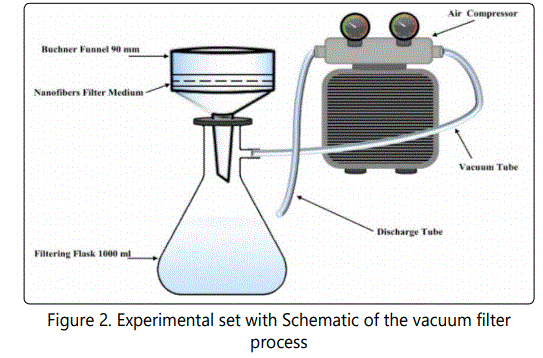
Samples Selection Technique
Two different samples of water, i.e., dam water and wastewater were selected for this experiment. The properties of dam water to be measured are total dissolved solids (TDS), pH, turbidity, conductivity, calcium (Ca2+), magnesium (Mg2+), total hardness, sulfates (SO42-), nitrates (NO3-), fluoride (F-), chloride (Cl-), bromate (BrO3-), total alkalinity, silica (SiO2) and bromate. On the other hand, the most important properties of wastewater to be measured are pH, turbidity, Total Dissolved Solids (TDS), conductivity, total suspended solids (TSS), Chemical Oxygen Demand (COD), Biochemical Oxygen Demand (BOD5), phosphate (PO43-), ammonia (NH3-N), oil and grease, and Dissolved Oxygen (DO).
Waste Water Cleaning and Dam Water Technology
A sample of wastewater was taken from Tabuk Sewage Treatment Plant (Tabuk STP) shown in Figure 3. It located in Tabuk city, Saudi Arabia. Tabuk STP has a design capacity of 100,000 m3/day of raw wastewater. Tabuk STP consists of three major stages: primary treatment, secondary treatment, and tertiary treatment. During primary treatment, mechanical screening is used to trap solids and floating objects, smaller particles such as grit and sand. Degreasing unit is used to separate and remove oil and grease from wastewater. The secondary treatment contains large aeration tank with eight aerators to feed the bacteria for their own growth and reproduction. Four sedimentations are also available, where more solids settle to the floor to finally produce clean treated water at the top. The tertiary treatment stage includes the sand filter, which has been designed with 50 % efficiency to remove most of the remaining impurities that were left in secondary treatment.
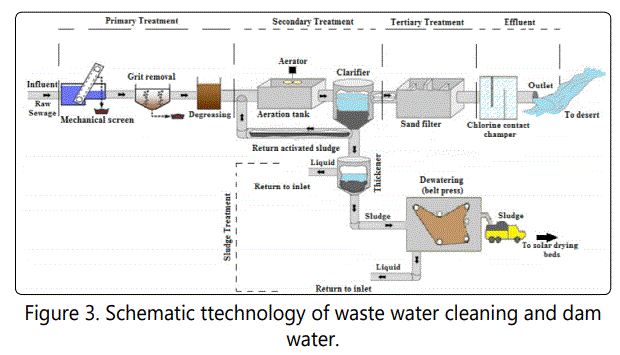
Disinfection such as chlorine addition is the final stage before discharge of the effluent. Finally, the sludge treatment process which includes: two thickener tanks to allow the sludge to settle to the bottom and separate from the water for up to 1 day usage, dewatering unit (belt press) for further sludge treatment to make it safer for the environment [20]. Table 1 shows the water properties before and after each treatment stage in Tabuk STP.
In the present study, PVC electrospun nanofibers incorporated with varying concentration of PVP were used as filter media (membrane). These membranes are appropriately called as an electrospun nanofibrous membrane, are generally used between secondary and tertiary treatment. The wastewater from the sewage after secondary treatment was allowed to pass through the ENM before tertiary treatment.
Results and Discussion
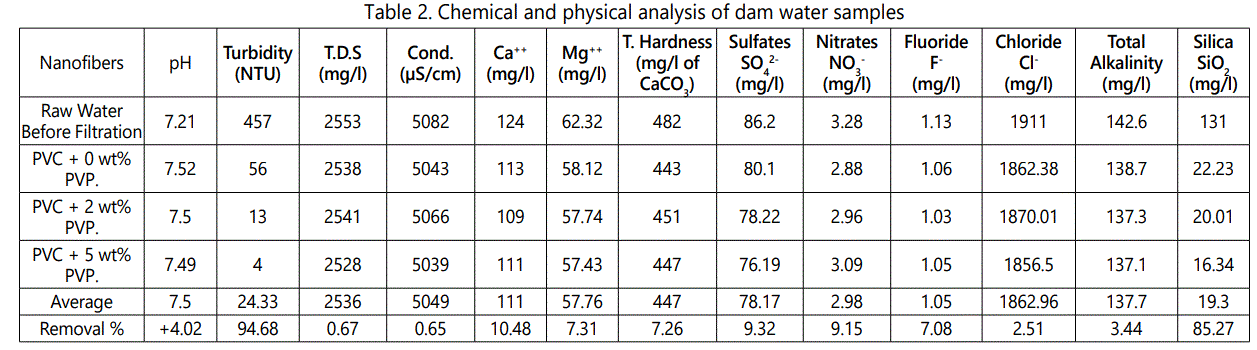
Chemical and Physical Analysis of Dam Water Sample
Table 2 shows the results of the chemical and physical analyses of filtered dam water sample from Alzraib dam. pH is potential of hydrogen in a solution, and it is used specify the acidity or basicity of an aqueous solution. pH is a measure of the hydrogen ions concentration in a solution. Solutions with a high hydrogen concentration have low pH and vice-versa. The pH is related to electrical conduction and total conduction, as well. In this study, the average pH value was found to be 7.5 after filtration of dam water. The average turbidity of water after filtration was found to be 24.33 nephelometric turbidity units (NTUs). The turbidity of PVC sample with 5 wt. % PVP was measured as 4 NTUs, which is promising, since many studies have shown the turbidity to be approximately 4 NTUs, after filtration. The average value of TDS was measured as 2,536 mg/l. The average electrical conductivity was determined as 5,049 μS/cm. As is known that pure water is not a good conductor of electricity. The conductivity ranges between 0.005 to 0.05 S/m for drinking water. The average hardness was determined as 447 mg/l. The sulfate concentration was found to be very low (78 mg/l) compared to IS 10500) specifications (200 mg/l). The acceptable limits for Ca++ in drinking water are 75 mg/l, according to IS10500 standards, and the present study showed a Ca++ concentration of 111 mg/l. The permissible limits for nitrates (NO3-) according to IS10500 standards is 45 mg/l, and present study showed a much lower value of nitrates (NO3-), i.e., 2.98. According to IS10500 standards, the fluoride concentration should be approximately 1 mg/l; our study showed 1.5 mg/l, which is in close agreement with the prescribed limits. The Chloride (Cl-) concentration was found to be 1862.96 mg/l, and total Alkalinity was found to be 137.7 mg/l, which is much lower than the acceptable limit of 200 mg/l.
Chemical and Physical Analysis of Wastewater Sample
Table 3 shows the chemical and physical analysis of filtered wastewater samples. In the present study, the average pH was found to be 7.7. The average turbidity after filtration of wastewater was determined as 7.7 NTUs. The TDS was found to be 1503.33 mg/l. The average electrical conductivity was found to be 3,012 μS/cm.
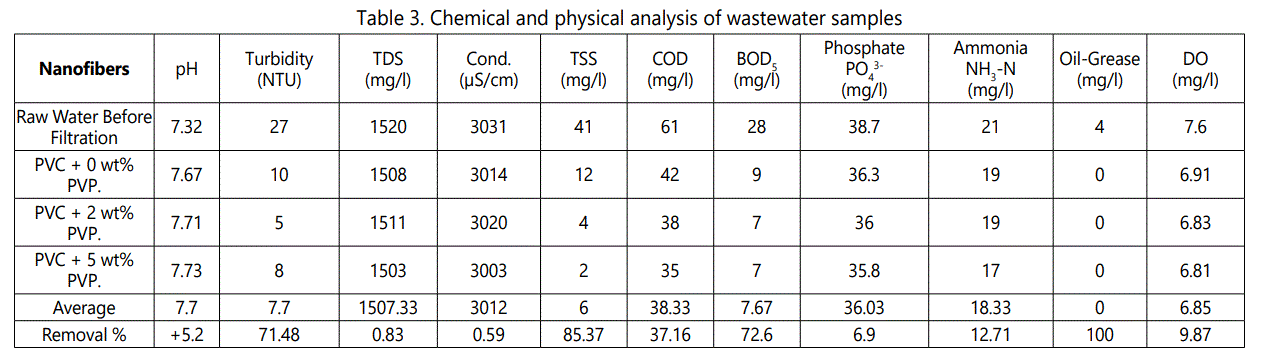
No grease was found in wastewater sample. The acceptable limits for dissolved oxygen are 5 mg/l. This study showed slightly high value, i.e., 6.85 mg/l. The importantparameters that are usually examined in measuring the quality of wastewater are turbidity, TSS, COD, and BOD5. By applying ENMs, turbidity, TSS, COD, and BOD5 were reduced by 71%, 85%, 37% and 72% respectively. Oil and greases contain the harmful substance. Therefore, it is important to remove them [21]. As is seen in Table 3, oil and grease were completely removed by ENM.
Wettability of Nano-membrane
Figure 4, 5 and 6 show the static contact angle of PVC fibers, PVC with 2 wt. % PVP fibers and PVC with 5 wt. % PVP fibers, respectively. PVP was added to PVC in order to make membrane slightly hydrophilic. The addition of PVP changed the contact angles values of fibers but not to the extent that it was expected initially. The membrane remained hydrophobic even after adding 5 wt. % PVP. The excessive amount of PVP was not added intentionally because PVP is soluble in water. The static water contact angle is an indication of hydrophobicity or hydrophilicity of a solid surface. Superhydrophobicity means that the solid surface is extremely hydrophobic (waterfearing), and the static water contact angle values usually between 150° and 180°[22]. When the contact angle is less than 10°, the surface is said to be superhydrophilic.
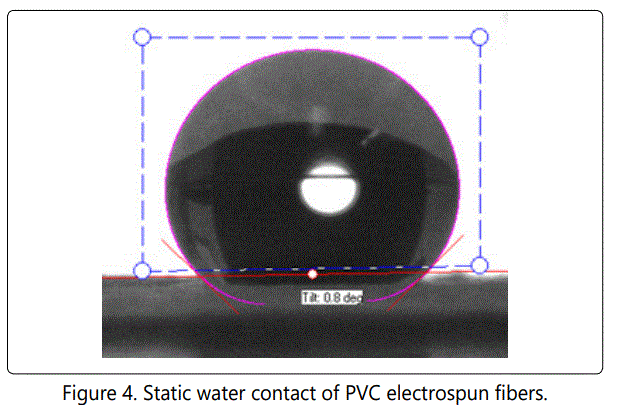
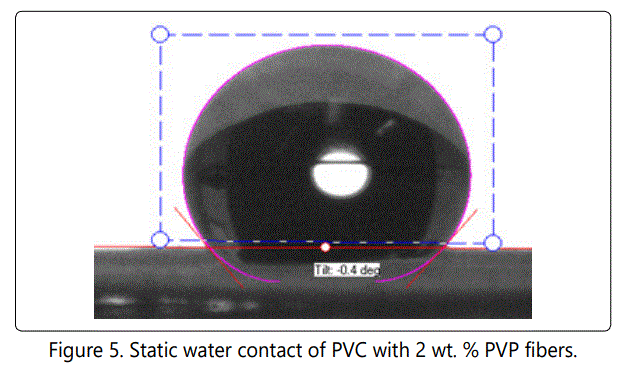
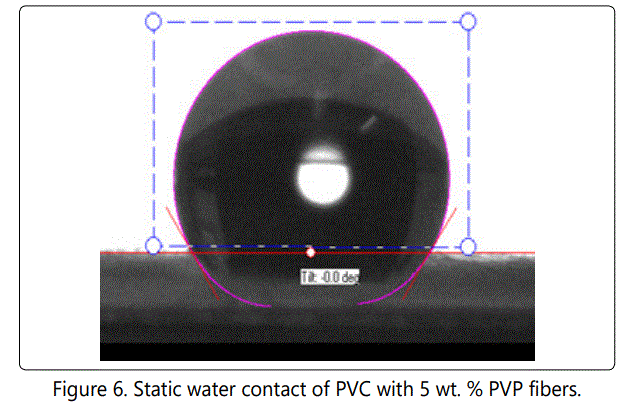
The contact angle value for pure PVC fibers was measured as 135o and for PVC with 2 wt. % PVP it was measured as 129o. The addition of 5 wt. % PVP reduced contact angle values to 123o. We did not add PVP beyond 5 wt. %, since PVP is a highly hydrophilic polymer and it is soluble in water, as well. Hydrophobic or "water- fearing" membranes, generally do not get wet unless water entry pressure is exceeded. They can be wetted by low surface tension fluid. Water can pass through a hydrophobic membrane, once the breakthrough pressure is reached. PTEE is a hydrophobic polymer, and it is used excessively in membrane production. PTEE has considerable applications in water filtration and separation.
Fourier Transform Infrared Radiation Analysis
Fourier transformation infrared radiation (FTIR) analysis is a helpful tool for determining the chemical interactions of material. With the use of FTIR spectra, it is possible to understand the chemical changes, molecular components, and structures. Figures 7 and 8 shows the FTIR spectra of PVC and PVP electrospun nanofibers, respectively.
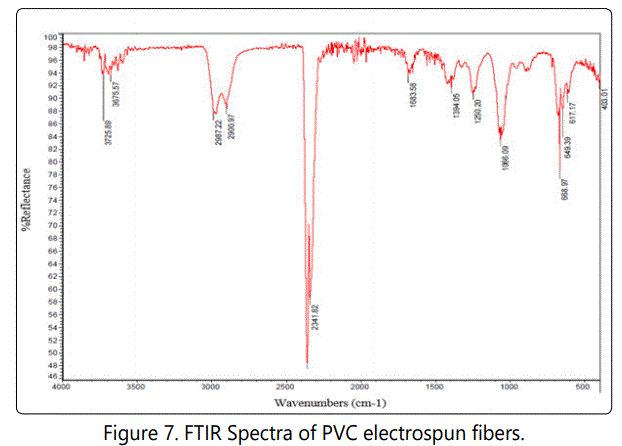
As is seen in Figure 7 the peaks corresponding to 666.97, 649.39 and 617.17 cm-1 are due to C-Cl stretching mode [23]. Generally, the characteristic bands of PVC can be classified into three regions. The first region is commonly called the C-Cl stretching region in the range of 600-700 cm-1.The second region can be attributed to C-C stretching in the range of 900-1200 cm-1.The third region is from 1250 -2970 cm-1 due to many C-H modes [24]. The bands at 2987.22 cm-1 are due to C-H stretching from CH-Cl and at 2900.97 cm-1 is due to C-H stretching from CH2. The band at 1394.05 cm-1 is due to wagging of CH2 [24].
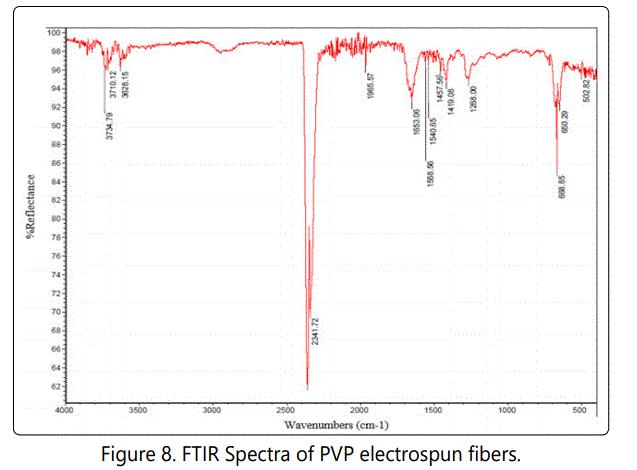
The band around 3749.79 – 3628.15 cm-1 can be attributed to O-H stretching of the hydroxyl group. The presence of hetero atomic molecules and carbonyl groups in the pyrrolidone ring of PVP can be attributed to a peak at 1653.06 cm-1 as the signature of C=O stretching. The peak at 1268 cm-1 is due to CN stretching of the amide group. The peak at 1419 cm-1 is due to C-H deformation of CH2 group [25]. From the FTIR spectra of PVP electrospun nanofibers, several peaks at1653, 1540, 1457, 1419, 1268 and 668 cm-1 are observed, which can be attributed to the vibration of carbonyl (C=O), carboxylic (O-H), lactone structure, amide 111(-C≡N, stretching), (O-H Stretching), and CH2¸respectively [26]. Figure 9 shows the FTIR spectra of PVC with different wt. % of PVP electrospun nanofibers before filtration.
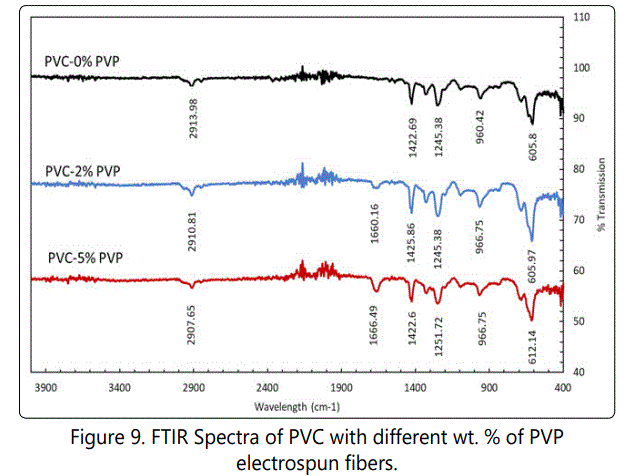
As is seen in Figure 9, the band at 2913.96 cm-1 is due to CH-CL stretching in PVC-0% PVP curve, which can also be seen at 2987.22 cm-1 in Figure 9. The band at 1422 cm-1 in PVC-0% PVP is due to wagging of CH2, which can be seen in Figure 9 at 1394.05 cm-1. The peak at 605.8 cm-1 (Figure 9, PVC- 0% PVP) corresponds to C-CL Stretching mode, which can be seen in Figure 9, as well. A peak at 2910.81cm-1 and at 2907.65 cm-1 for PVC-2% PVP and PVC-5%PVP, respectively correspond to CH-CL stretching, which is visible at 2987.22 cm-1 in Figure 9 for pure PVC spectra. The addition of PVP has shifted CH-CL band stretching to lower values. The addition of PVP in PVC did not show any profound effect, as can be seen in all curves in Figure 7. All the curves (Figure 9) displayed more or less the same behavior. The band at 605 cm-1 for PVC-0 % PVP, at 605 cm-1 for PVC-2 % PVP and at 612 cm-1 for PVC-5% PVP corresponds to C-CL stretching. The bands in the range of 1200 to 2900 cm-1 in all curves represents C-H modes [24].
Conclusion
The electrospun nanomembrane is a novel candidate in membrane technology, and it can be usedfor water and wastewater treatment in adding to other applications such as desalination, where its application is increasingly playing an important role to replace RO partially. The polymeric nanofibers produced via electrospinning have extremely high surface area, porous, flexibility and permeable structure with good pore-connectivity plus functionalities and surface chemistry, which make them ideal candidates for a range of advanced separation applications. Electrospun nanofibrous membranes (ENMs)are cutting edge membrane technology that offers substantial high flux and high rejection rate compared to the conventionalmembrane. ENMs present a breakthrough in water and wastewater treatment by offering a lightweight, cost-effective, and less energy consuming process, when compared to the conventionalmembranes. Additionally, the most important factors that must be evaluated for measuring the filtered water quality are turbidity, TSS, COD, and BOD5. These nanomembranes reduced all these factors to an acceptable level. The FTIR analysis was also carried out on PVC and PVP electrospun nanofibers to identify molecular components and structures.
References
- Anjum Muzammil, Miandad R, Muhammad Waqas, Gehany F, Barakat MA, et al. Remediation of waste water using various nano-materials. Arabian J. of Chem. 2016. 10.1016/j.arabjc.2016.10.004
- Shannon Mark A, Paul Bohn W, Menachem Elimelech, John Georgiadis G, Benito Marinas J, et al. Science and Technology for water purification in the coming decades. Nature. 2008; 452: 301. doi: 10.1038/nature06599
- Qu X, Brame J, Li Q, Alvarez PJ. Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc Chem Res. 2013; 46: 834-843. doi: 10.1021/ar300029v
- Greiner A, Wendorff JH. Electrospinning: a fascinating method for the preparation of ultrathin fibers. Angewandte Chemie International Edition. 2007; 46(30): 5670-5703. doi: 10.1002/anie.200604646
- Khan WS, Asmatulu R., Ceylan M, Jabbarnia A. Recent progress on conventional and non-conventional electrospinning processes. Fibers and Polymers. 2013; 14(8): 1235-1247. doi: 10.1007/s12221-013-1235-8
- Reneker Darrell H, Iksoo Chun. Nanometre Diameter Fibres of Polymer, Produced by Electrospinning. Nanotechnology. 1996; 7(3): 216. doi: 10.1088/0957-4484/7/3/009
- Doshi Jayesh, Darrell H. Reneker. Electrospinning process and applications of electrospun fibers. Journal of electrostatics. 1995; 35(2-3): 151-160. doi: 10.1016/0304-3886(95)00041-8
- Bellan Leon M, Harold Craighead G. Nanomanufacturing Using Electrospinning. Journal of manufacturing science and engineering. 2009; 131(3): 034001. doi: 10.1115/1.3123342
- Zhou Feng-Lei, Rong-Hua Gong, Isaac Porat. Needle and needleless electrospinning for nanofibers. Journal of applied polymer science. 2010; 115(5): 2591-2598. doi: 10.1002/app.31282
- Wang, Tong, Satish Kumar. Electrospinning of polyacrylonitrile nanofibers. Journal of applied polymer science. 2006; 102(2): 1023-1029. doi:10.1002/ app.24132
- Alarifi Ibrahim M, Abdulaziz Alharbi, Waseem Khan S, Andrew Swindle, Ramazan Asmatulu. Thermal, Electrical and Surface Hydrophobic Properties of Electrospun Polyacrylonitrile Nanofibers for Structural Health Monitoring. Materials. 2015; 8(10): 7017-7031. doi: 10.3390/ ma8105356
- Waseem Khan S. Asmatulu R, Ahmed I, Ravigururajan TS. Thermal conductivities of electrospun PAN and PVP nanocomposite fibers incorporated with MWCNTs and NiZn ferrite nanoparticles. International Journal of Thermal Sciences. 2013; 71: 74-79. doi: 10.1016/j. ijthermalsci.2013.03.006
- Huang, Zheng-Ming, Zhang YZ, Kotaki M, Ramakrishna S, et al. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites science and technology. 2003; 63(15): 2223- 2253. doi: 10.1016/S0266-3538(03)00178-7
- Reneker Darrell H, Alexander L. Yarin Hao Fong, Sureeporn Koombhongse. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. Journal of applied physics. 2000; 87(9) 4531-4547. doi: 10.1063/1.373532
- Shin YM, Hohman MM, Brenner MP. Rutledge GC. Electrospinning: A whipping fluid jet generates submicron polymer fibers Rutledge. Applied physics letters. 2001; 78(8): 1149-1151. doi: 10.1063/1.1345798
- Alharbi Abdulaziz R, Ibrahim Alarifi M, Waseem Khan S, Ramazan Asmatulu. Highly Hydrophilic Electrospun Polyacrylonitrile/ Polyvinypyrrolidone Nanofibers Incorporated with Gentamicin as Filter Medium for Dam Water and Wastewater Treatment. Journal of Membrane and Separation Technology. 2016; 5; 38-56.
- Darmanin Thierry, Frédéric Guittard. Superhydrophobic and superoleophobic properties in nature. Materials Today. 2015; 18(5): 273-285. doi: 10.1016/j. mattod.2015.01.001
- Narita S, Ichinohe S, Enomoto S. Infrared spectrum of polyvinyl chloride. I. Journal of Polymer Science Part A. 1959; 37: 131: 273-280. doi:10.1002/ pol.1959.1203713121
- Reksamunandar RP, Edikresnha D, Munir MM, Damayanti S, Khairurrijal, et al. Encapsulation of β-carotene in poly (vinylpyrrolidone) (PVP) by electrospinning technique. Procedia Engineering. 2017; 170: 19-23. doi: 10.1016/j.proeng.2017.03.004
- Dong G, Xiao X, Liu X, Qian B, Ma Z et al. Preparation and characterization of Ag nanoparticle-embedded polymer electrospun nanofibers. Journal of Nanoparticles Research. 2010(12): 1319-1329. doi: 10.1007/s11051-009- 9665-3



