Research Article
Extrusion Foaming of High Impact Polystyrene: Effects of Processing Parameters and Materials Composition
1Department of Metallurgical and Materials Engineering, Chemical and Metallurgical Engineering Faculty, Istanbul Technical University, Maslak, 34469 Istanbul, Turkey
2Arcelik A.S. Central R&D Department, Materials Technologies, Tuzla 34950 Istanbul, Turkey
*Corresponding author: Mohammadreza Nofar, Department of Metallurgical & Materials Engineering, Faculty of Chemical and Metallurgical Engineering, Istanbul Technical University, Maslak/Istanbul, 34469, Turkey, E-mail: nofar@itu.edu.tr
Received: March 29, 2018 Accepted: April 9, 2018 Published: April 16, 2018
Citation: Demirtaş E, Özkan H, Nofar M. Extrusion Foaming of High Impact Polystyrene: Effects of Processing Parameters and Materials Composition. Int J Mater Sci Res. 2018; 1(1): 9-15. doi: 10.18689/ijmsr-1000102
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study investigates the extrusion foaming behavior of high impact polystyrene (HIPS) through a twin-screw extruder using two various types of chemical blowing agents (CBA). The die temperature profile was firstly tailored during the foaming of HIPS for two different CBAs. Then the CBA content effect on the foaming behavior of HIPS was verified for both CBAs. The effect of screw speed (RPM) on the foaming behavior of HIPS was, subsequently, illustrated for both CBAs. It was found that the cell density and the void fraction of the HIPS foamed samples increased at the maximum CBA content of 5 wt% and the minimum screw RPM of 100. The HIPS extrusion foaming behavior was further investigated via blending it with general purpose PS (GPPS) at the blending ratios (wt%/wt%) of 75/25 and 50/50, as well as, via compounding HIPS with three different inorganic fillers (i.e., micro-lamellar talc, talc, and calcium carbonate) at three different contents (i.e., 1, 2, and 3 wt %). The increase in GPPS and inorganic filler contents increased the cell density and void fraction of HIPS foams while the inorganic filler type did not reveal much differences in the foaming results.
Keywords: HIPS; Extrusion; Foaming; Chemical Blowing Agent.
Introduction
Polystyrene (PS) is an amorphous thermoplastic with a glass transition temperature around 100°C and is widely being used in commodity applications due to its low-cost and reasonable physical and mechanical properties [1]. Among the PS products, the use of PS foams is also of a great interest in variety of commodity applications, such as packaging, cushioning, construction, and food application. This is due to the reduced weight and cost benefit of the foamed samples while not high mechanical properties are expected in most of the noted applications. PS foams are currently being manufactured in various structures such as extruded PS sheets (XPS), expanded bead foams (EPS), and three-dimensional complex geometries via such manufacturing technologies as extrusion foaming, bead foaming, and foam injection molding, respectively [2].Extrusion foaming is widely being used in manufacturing continuous simple twodimensional profiles through which various foam densities could be obtained. The foam morphology in extrusion foaming could be controlled via controlling several parameters such as die temperature profile [3-4], die geometry (i.e., L/D ratio) [5-10], die pressure and pressure drop rate [12-17], melt rheological properties [18-26], crystallization kinetics [27-30] of the polymer/gas mixture, and the type and content of blowing agents [31-34]. Among the blowing agents, despite their high solubility in polymer melts, the use of hydro-fluorocarbons(HFCs), hydro-chloro-fluoro-carbon (HCFCs) and hydrocarbons is banned due to their toxic and flammable features [35-38]. Therefore, the attempts are being made to use green blowing agents such as gas and supercritical CO2 and N2 in manufacturing of plastic foams [39]. Among these, however, CO2 reveals high solubility than N2 which causes the achievement of lower density foams when using CO2 [40-42]. On the other hand, N2 possesses higher cell nucleation power due to its high diffusivity [43]. During the extrusion foaming, the decrease in barrel temperature increases the solubility of the CO2 inside the polymer melts whereas the solubility of N2 increase with barrel temperature increase [44-47]. In this context, to manufacture continuous foam products, the use of CO2 or N2 as physical blowing agents (PBAs) are highly preferred than chemical blowing agents (CBAs) due to the achievements of foams with more uniform structure and the lower cost of the blowing agents [48]. On the other hand, CBAs do not require much of side accessories and equipment such as a continuous syringe pump and extruder modifications required when using PBAs. Therefore, a lot of industries might be interested in using CBAs in order to avoid the modification of their production line.
When the polymer melts along the extruder, with the addition of CBA or injecting the PBA into the melt, polymer/gas mixture starts to be generated under higher pressure and the mixture will be flowing along the extruder. However, the generated pressure must stay beyond the solubility limit of polymer/gas in order to avoid having undissolved gas within the melt along the extruder [49-50]. The die pressure and the pressure drop rate could be controlled by the die temperature, die geometry, and melt viscosity of the polymer/gas mixture. The sudden pressure drop at the die nozzle creates thermodynamic instability and causes cell nucleation and growth during the foaming step [51-52]. When the die temperature is too high, the die pressure drops and the gas loss through the hot skin layer of the polymer foam is more likely. Due to the increased gas loss and the low melt strength of the polymer, the cell coalescence would also be more probable. On the other hand, if the die temperature is too low, the polymer melt becomes too stiff to be expanded although the cell nucleation could be promoted with the increased die pressure and pressure drop rate.
Moreover, in order to improve the cell nucleation and to control the growth, and hence the final foams properties, inorganic fillers could be used as cell nucleating agents during foaming. The stress variations around the rigid fillers will generate pressure variation which causes heterogeneous cell nucleation around these solid particles. In the case of low melt strength polymers, the existence of these fillers could also improve the melt strength during the foaming and thereby the cell coalescence could be hindered while more expansion could be obtained.
In 1993, Park et al. [53-54] illustrated the microcellular foaming of HIPS through extrusion foaming using CO2 as physical blowing agent. They showed that the cell size reduction is highly dependent on the pressure drop rate and the L/D ratio of the filamentary die. They also observed that increasing the die pressure and the melt strength by lowering the die temperature profile profoundly increases the cell nucleation. Park et al. [55-56] also showed that the increase in CO2 content increases the cell density of HIPS foams. This was also shown by Shimbo et al.during the extrusion foaming of PS when using CO2 as the physical blowing agent [57].
In this study, we investigated how the processing parameters and materials modification influence the extrusion foaming behavior of HIPS when using chemical blowing agent. Moreover, the variations of foam morphology were explored when using HIPS blends with GPPS and composites by adding inorganic fillers.
Experimental
Materials
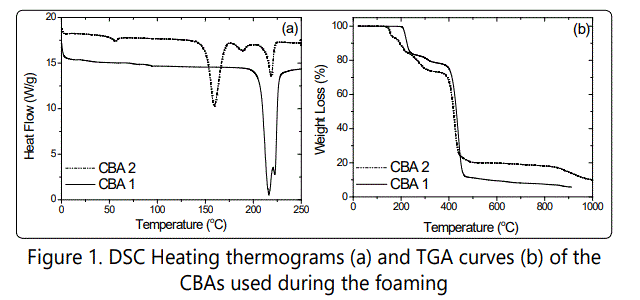
HIPS was supplied from Versalis SPA. This HIPS shows the environmental stress cracking resistant (ESCR) behavior. General Purpose PS(GPPS) was also supplied from Dow Chemicals.The melt flow indext (MFI) of HIPS and GPPS were measured as3.5 g/10 min and 4.5 g/10min, respectively. The specific gravity (Mettler A1201 density measurement device) of HIPS and GPPS are also 1.04 g/cm3 and 1.05 g/cm3 respectively. The CO2 based endothermic chemical blowing agents (CBA) used in these experiments were also Hydrocerol products produced by Clariant Inc. Two different grades of Hydrocerol that have different decomposition temperatures are named as CBA 1 and 2. Both CBAs were used at the contents of 1, 3 and 5 wt%. Figure 1 shows the DSC and TGA curves of these chemical blowing agents, respectively. In HIPS composites, the used inorganic fillers as cell nucleating agents were calcium carbonate (Omya 5-Gz), five micro-sized talc (Omya) and one micro-sized micro-lamellar talc (Imerys). In HIPS blends and composites foamingexperiments, 5% wt. of CBA was used.
Experimental Setup and Procedure
Foaming was conducted in a twin screw extruder (PRISM TSE-24-HC) having an L/D ratio of 26 at a processing temperature range of 200-230 ° C. The filamentary die having, respectively, 2 mm diameter and 4 mm length was used in order to produce foamed filaments. Five different die temperatures were set as 130, 150, 160, 170, and 180 ° C. After determination of the most proper die temperature, the CBA type and content (i.e., 1,3 and 5 %wt.) were investigated. The RPM effect (i.e., 100, 150, 200 and 250 rpm) on the foaming behavior of HIPS was explored using both CBA 1 and CBA 2. In order to investigate the HIPS extrusion foaming behavior via blending with GPPS, the HIPS/ GPPA blends were foamed at the blending weight ratios of 75w/25w and 50w/50w. Moreover, the HIPS foaming behavior was analyzed via compounding HIPS with three different inorganic fillers (i.e., microlamellar talc, talc, and calcium carbonate) at three different contents (i.e., 1, 2, and 3 wt %). CBA1 at the ratio of 5 wt% was fixed for all foaming experiments of blends and composites. The die temperature and the screw RPM were also fixed at 150 ° C and 100, respectively.
Foam characterization
The foams' densities were measured according to Archimedes principle using Mettler A1201 density measurement device based on ASTM D792-00. Void fraction values were then calculated using Equation 1. Cell density and cell distributions of samples were observed using ZEISS scanning electron microscopy (SEM). The cell density of samples was measured using Equation 2.
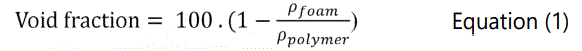
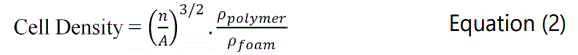
In these equations, n is the number of cells and A is the area of image. ρfoam and ρpolymer is the density of the foam and unfoamed polymer.
Rheological Properties
The rheological behavior of the HIPS and HIPS/GPPS blends were measured with two methods: an oscillatory rheometer (Anton Paar, Rheo Compass.), and the melt flow index.Circular samples were hot pressed and prepared with a diameter of 25 mm and a gap size of 1mm for rheological measurements.
Results and Discussions
Polybutylene (PB) size determination in PS matrix
Figure 2 compares the PB orientations of neat HIPS samples before and after extrusion process. It is seen that, PB orientations are similar in both situations. The diameters of PBs are as large as 20 μm.

Melt behavior of the HIPS, GPPS and their blends
Figure 3 shows the MFI results of HIPS, GPPS and their blends at 200 °C and 5 kg. It is seen that, melt flow index values of HIPS, GPPS and their blends are very similar. This shows that; HIPS, GPPS and their blends have similar melt properties. GPPS has the highest MFI value (4,5), while HIPS has 3,5 and increasing the content of GPPS increases the MFI of blends.
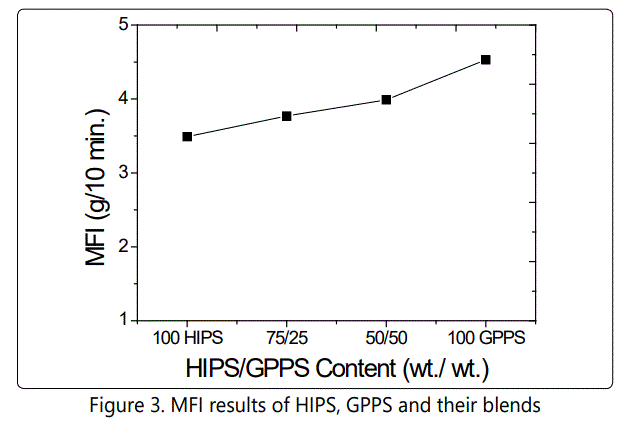
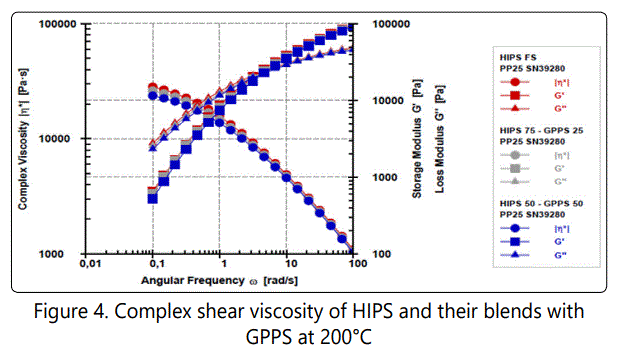
Figure 4 also compares the frequency sweep short amplitude oscillation shearing (SAOS) behavior of the noted samples. As seen the complex shear viscosities and the moduli of HIPS and their blends with GPPS are pretty similar within the while frequency range. Therefore, the melt properties of HIPS, GPPS and their blends will have a similar effect on the corresponding foaming behavior of the samples. The very slight decrease of the viscosity and moduli of the HIPS blends with GPPS is due to the slightly higher MFI of GPPS compared to that of HIPS.
Effect of Die Temperature
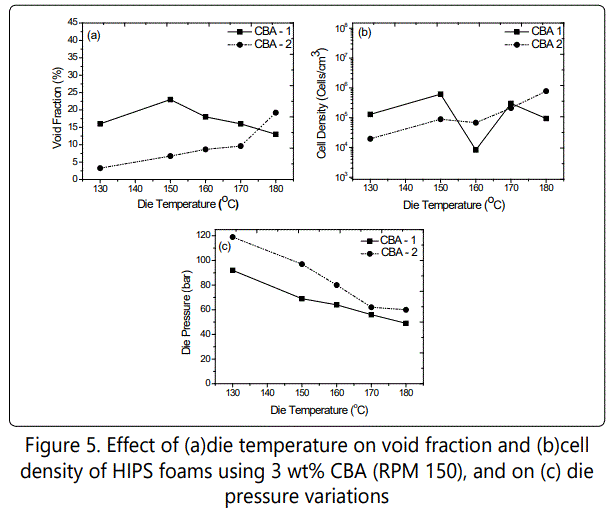
Figure 5 shows the effect of die temperature on void fraction and cell density values of the foamed samples as well as on the die pressure variations when using both CBAs. In foamed samples with CBA 1, the highest void fraction (~ 22%) was obtained at the die temperature of 150 ° which also possessed the highest cell density (~6 × 105 cells/cm3). This might be because the melt strength of polymer was proper enough for better cell nucleation and expansion at 150 ° die temperature. As expected, the decrease in die temperature increased the die pressure. When using CBA 2 however, in foamed samples, despite the increased die pressure with the decrease in die temperature, the void fraction and cell density decreased with the decrease in die temperature.This could have been due to lack of solubility of CBA in melt at lower temperature as well as too high a stiff polymer melt for expansion at lower die temperatures. Figure 6 shows the SEM images of how various die temperatures influenced the cell morphology of the foamed HIPS with different CBAs.

Effect of cba content
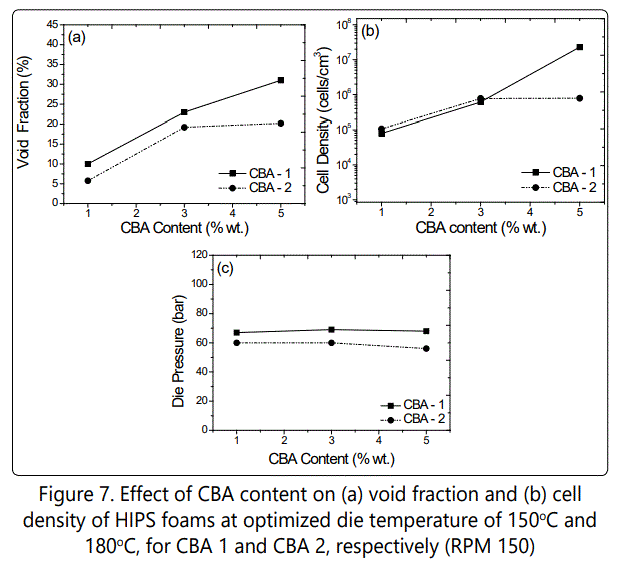
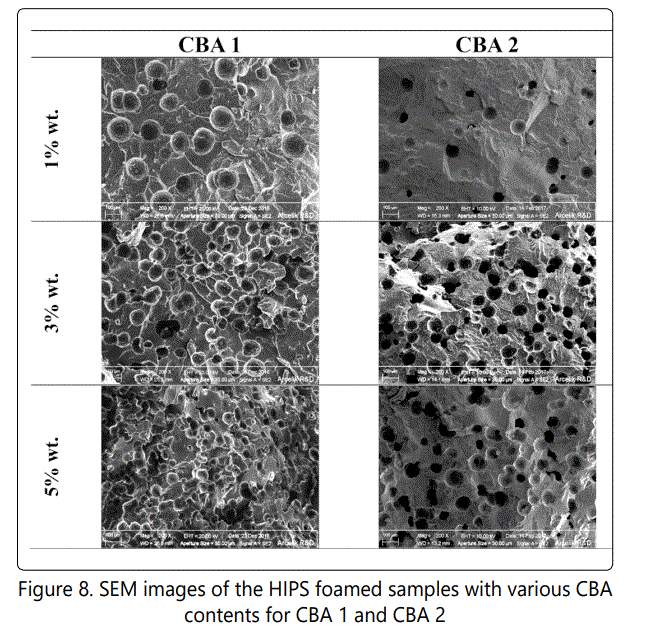
Figure 7 shows the effect of CBA content on the void fraction and cell density values of the foamed samples. It is shown that increasing the CBA content from 1 to 5 wt. % increased the void fraction and cell density in samples foamed with both CBA 1 and CBA 2. The highest void fraction (~32%) and the highest cell density (~2 × 107 cells/cm3) were obtained for samples foamed with 5 % wt. of CBA 1. On the other hand, the highest void fraction (~21%) and the highest cell density (~8 × 105 cells/cm3) were obtained for samples foamed with 5 % wt. of CBA 2. It should be noted that the cell density increased with increasing the CBA content from 1 to 5%wt due to the increase of the internal system pressure. Figure 8 shows the SEM images of how increasing CBA content influenced the cell morphology of the foamed HIPS with different CBAs.
Effect of RPM
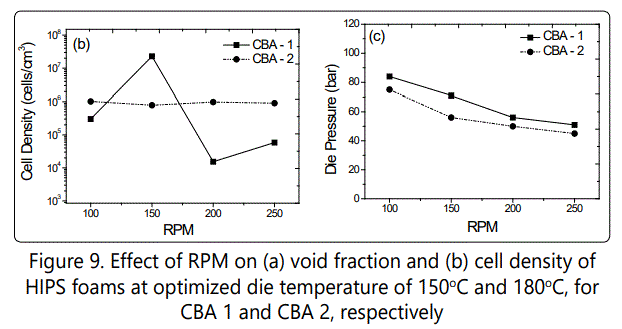
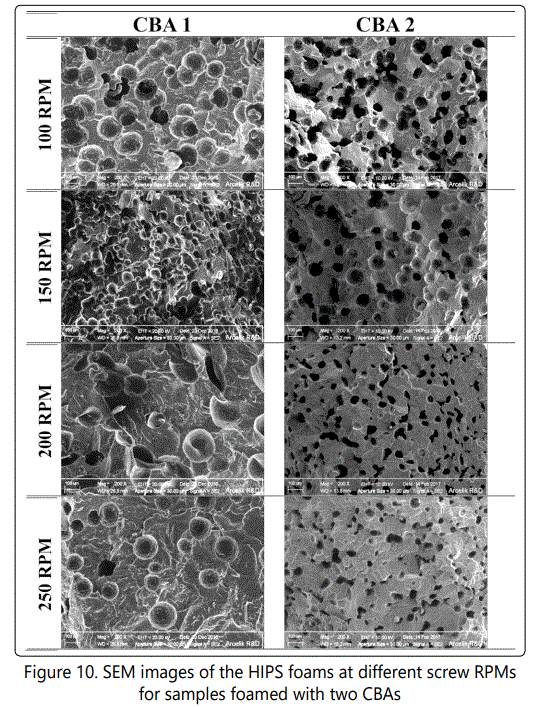
Figure 9 shows the effect of screw RPM variations on the void fraction and cell density of the foamed samples. As the RPM decreased from around 250 to 100, the die pressure and consequently the void fraction of the foamed samples increased and the maximum void fraction was achieved ~30 % for samples blown with both CBAs. On the other hand, the highest cell densities were obtained at 150 RPM and at 100 RPM for samples foamed with CBA 1(~ 2 × 107 cells/cm3) and CBA 2(~ 106 cells/cm3), respectively. The cell density and void fraction increase at lower RPMs were likely due to the better mixing of polymer melt and CBA due to the increased viscosity of polymer melt at lower RPM (lower frequencies as Figure 4 shows) and higher residual time, while the die pressures showed the highest values for both CBAs. Figure 10also shows the SEM images of how various RPM values influenced the cell morphology of the samples foamed with different CBAs.
Effect of GPPS
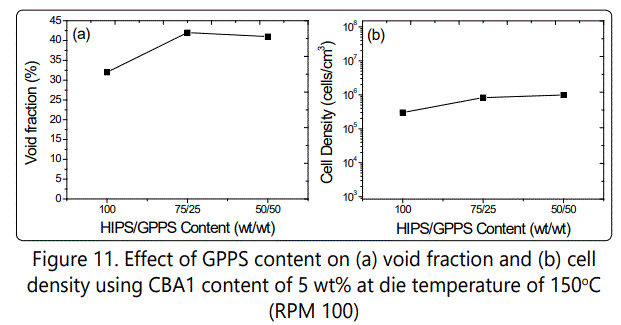
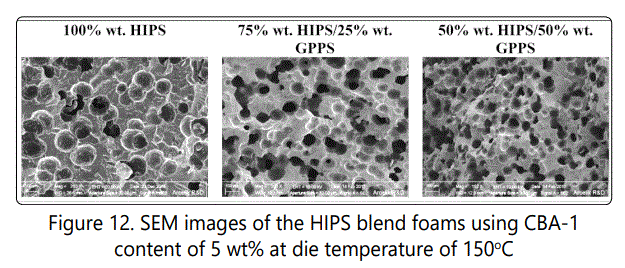
Figure 11 shows how the blending HIPS with GPPS could affect the void fraction and cell density of the foamed samples. The increase in GPPS content increased the cell densities of HIPS/GPPS blends to around 106 cells/cm3 with more of open celled structure. The SEM images of the blend samples (Figure 12) also confirms how the increase in GPPS increases the void fraction and cell density with more rigid but open-cell structure.
Effect of inorganic fillers
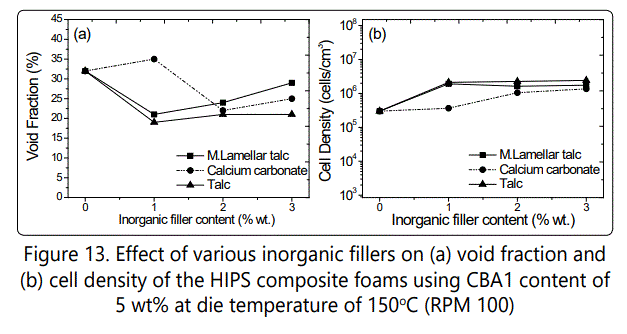
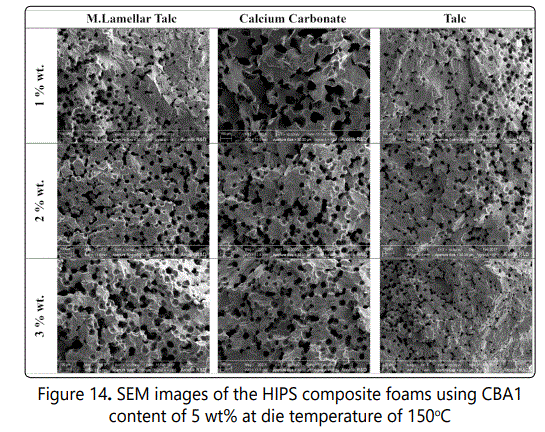
Figure 13 also shows the effect of various inorganic fillers as cell nucleating agents on void fraction and cell density variations of the HIPS foamed samples. Increasing the inorganic filler content from 1 to 3 wt %, increased the cell density for all fillers. Among these fillers, the highest cell density (~ 2 × 106cells/cm3) was obtained in composites with 3 wt % of talc. Figure 14 also shows the SEM images of the foamed HIPS composites and how various fillers influenced the cell morphology. The void fraction decreased with increasing the inorganic filler content from 1 to 3%wt most probably due to the increased rigidity of the foamed samples.
Conclusion
In this study, the extrusion foaming of HIPS through a twin-screw extruder with two different chemical blowing agents was studied and the effects of die temperature profile and screw speed (RPM); chemical blowing agent type and content; various inorganic fillers (i.e., micro lamellar talc, talc, and calcium carbonate) at three different contents (i.e., 1, 2, and 3 wt %) and the foaming behavior of HIPS/GPPS blends at the blending ratios (wt%/wt%) of 75/25 and 50/50 were studied. According to results; when the CBA content was maximum (5 wt%) and the screw RPM was minimum (100), the cell density and the void fraction of the foamed HIPS increased. The increase in GPPS and inorganic filler contents increased the cell density and void fraction of HIPS foams while the type of inorganic fillers didn't reveal much differences from each other in the foaming results.
References
- Naguib HE, Park CB, Reichelt N. Fundamental Foaming Mechanisms GoverningVolume Expansion of Extruded PP Foams. J.of App.Polym Sci. 2004; 91(4): 2661-2668. doi: 10.1002/app.13448
- Lee JWS, Wang K, Park CB. Challenge to Manufacture of Low-Density Microcellular Polycarbonate Foams Using CO2. Ind. Eng. Chem. Res. 2004; 44(1): 92-99. doi: 10.1021/ie0400402
- Xu X, Xu D, Chen W, Pop-I liev R, Park CB, et al. Effects of the Die Geometry on Cell Nucleation of PS Foams Blown with CO2 . SPE Topical Conference, Montreal 2001.
- Xu D, Park CB, Fenton RG. Strategies for the Manufacture of LowDensity, Fine-Celled PBS Sheet Foams Blown with CO2 Using an Annular Die. Society of Plastics Engineers, Annual Technical Conference - ANTEC, Boston, Massachusetts, 2005.
- Lee PC, Kaewmesri W, Wang J, Park CB, Pumchusak J, et al. Effect of Die Geometry on Foaming Behaviors of High-Melt-Strength Polypropylene with CO2 . Society of Plastics Engineers, Annual Technical Conference -ANTEC, Cincinnati, Ohio, 2008; 109(5): 3122-3132. doi: 10.1002/app.28204
- Wang J, Park CB, James DF. Effect of Die Land Length on Die Pressure During Foam Extrusion-Part I Experimental Observations, Society of Plastics Engineers, Annual Technical Conference-ANTEC, Cincinnati, Ohio, 2007.
- Mark LH, Xu M, Park CB. Melt Fracture Behavior of PLA Foamed Using Different Die Entry Angles, Society of Plastics Engineers, Annual Technical Conference-ANTEC, Orlando, Florida, 2012.
- Park CB, Suh NP. Extrusion of Microcellular Polymers Using a Rapid Pressure Drop Device, Society of Plastics Engineers, Annual Technical Conference-ANTEC, New Orleans, 1993.
- Guo Q, Wang J, Park CB, Ohshima M. Design of a Foaming Simulation System with a High Pressure Drop Rate, Society of Plastics Engineers, Annual Technical Conference -ANTEC, Chicago, Illinois. 2004.
- Leung SN, Park CB. Effects of Gas Content and Pressure Drop Rate on Foaming, Annual Technical Conference-ANTEC, Charlotte, North Carolina. 2006.
- Leung SN, Wong A, Park CB. Pressure Drop Threshold for Nucleation of PS/CO2 Foaming, Society of Plastics Engineers, Annual Technical Conference -ANTEC, Cincinnati, Ohio, 2007.
- Park CB, Suh NP. Filamentary Extrusion of Microcellular Polymers Using a Rapid Decompressive Element. Polym. Eng. Sci. 1996; 36(1): 34-48. doi: 10.1002/pen.10382
- Park CB, Baldwin DF, Suh NP. Effect of Pressure Drop Rate on Cell Nucleation in Continuous Processing of Microcellular Polymers. Polym. Eng. Sci. 1995; 35(5): 432-440. doi: 10.1002/pen.760350509
- Han CD, Villazimar CA. Studies on Structural Foam Processing I. The Rheology of Foam Extrusion. Polym. Eng. Sci. 1978; 18(9): 687-698. doi: 10.1002/pen.760180904
- Blyler LL Jr, Kwei TK. Flow Behavior of Polyethylene Melts Containing Dissolved Gases. J. Polym. Sci. 1971; 35(1): 165-176. doi: 10.1002/polc.5070350113
- Fridman ML, Sabsai OY, Nikolaeva NE, Barshtein GR. Rheological Properties of Gas-Containing Thermoplastic Materials during Extrusion. J. Cell. Plast. 1989; 25(6): 574-595. doi: 10.1177/0021955X8902500610
- Kim KU, Kim BC, Hong SM, Park SK. Foam Processing with Rigid Polyvinylchloride. Int. Polym. Proc. 1989; 4(4): 225-231. doi: 10.3139/217.890225
- Nofar M, Park CB. Poly (lactic acid) foaming. Prog Polym Sci. 2014; 39(10): 1721-1741. doi: 10.1016/j.progpolymsci.2014.04.001
- Nofar M. Majithiya K, Kuboki T, Park CB. The foamability of low-meltstrength linear polypropylene with nanoclay and coupling agent. J. Cell. Plast. 2012; 48(3): 271-287. doi: 10.1177/0021955X12440271
- Kesht kar M, Nofar M, Park CB, Carreau PJ. Extruded PLA/Clay Nanocomposite Foams Blown with Supercritical CO2 . Polymer. 2014; 55(16): 4077-4090. doi: 10.1016/j.polymer.2014.06.059
- Li G, Wang J, Park CB. Measurement of N2 Solubility in Polypropylene and Ethene/Octene Copolymer. SAE Tech. Pap. 2006; 4: 3-6.
- Li YG, Hasan MM, Park CB. Determination of the Solubility of Blowing Agent in Polymer without Using any Equation of State, Society of Plastics Engineers, Annual Technical Conference -ANTEC, Chicago, Illinois, 2009.
- Hasan MM, Li G, Park CB, Chen P. PVT and Solubility Behaviors of CO2 / N2 Blends in PS Melts, Foams. 2010, Seattle, Washington, 2010.
- Sato Y, Fujiwara K, Takikawa T, Sumarno, Takishima S, Masuoka H,et al. Solubilities and diffusion coefficients of carbon dioxide and nitrogen in polypropylene, high-density polyethylene, and polystyrene under high pressures and temperatures. Fluid Phase Equilib. 1999; 162(1-2): 261- 276. doi: 10.1016/S0378-3812(99)00217-4
- Li G, Li H, Turng LS, Gong S, Zhang C, et al. Measurement of gas solubility and diffusivity in polylactide. Fluid Phase Equilib. 2006; 246(1-2): 158- 166. doi: 10.1016/j.fluid.2006.05.030
- Hasan MM, Li YG, Li G, Park CB, Chen P. Determination of Solubilities of CO2 in Linear and Branched Polypropylene Using a Magnetic Suspension Balance and a PVT Apparatus. J. Chem. Eng. Data. 2010; 55(11); 4885- 4895. doi: 10.1021/je100488v
- Naguib HE, Park CB, Lee PC. Effect of Talc Content on the Volume Expansion Ratio of Extruded PP Foams. J. of Cell. Plas. 2003; 39(6): 499- 511. doi: 10.1177/0021955X03039247
- Park CB, Baldwin DF, Suh NP. Effect of the Pressure Drop Rate on CellNucleation in Continuous Processing of Microcellular Polymers. Polym. Eng. And Sci. 1995; 35(5): 432-440. doi: 10.1002/pen.760350509
- Park CB, Behravesh AH, Venter RD. Extrusion of Low Density Microcellular HIPS Foams Using CO2 . Polym. Eng. Sci. 1998; 38(11):1812-1823. doi: 10.1002/pen.10351
- Park CB, Baldwin DF, Suh NP. Effect of the pressure drop rate on cell nucleation in continuous processing of microcellular polymers. Polym. Eng. and Sci.1995 ; 35(5) :432-440. doi: 10.1002/pen.760350509



