Research Article
Synergistic Effects of Chain Extender and Nanoclay on the Crystallization Behaviour of Polylactide
Department of Metallurgical & Materials Engineering, Faculty of Chemical and Metallurgical Engineering, Istanbul Technical University, Maslak/Istanbul, 34469, Turkey
*Corresponding author: Mohammadreza Nofar, Department of Metallurgical & Materials Engineering, Faculty of Chemical and Metallurgical Engineering, Istanbul Technical University, Maslak/Istanbul, 34469, Turkey, E-mail: nofar@itu.edu.tr
Received: March 29, 2018 Accepted: April 9, 2018 Published: April 16, 2018
Citation: Nofar M. Synergistic Effects of Chain Extender and Nanoclay on the Crystallization Behavior of Polylactide. Int J Mater Sci Res. 2018; 1(1): 1-8. doi: 10.18689/ijmsr-1000101
Copyright: © 2018 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The crystallization behavior of polylactide (PLA) with various branching degrees and nanoclay contents were investigated using a differential scanning calorimeter (DSC). The dispersion quality of nanoclay within PLA and reactivity of chain extender with PLA molecules were analyzed using X-ray diffraction (XRD) and Fourier transform infrared spectroscopy test (FT-IR), respectively.
The results showed that chain branching in PLA produced higher crystallinity and fastened its slow crystallization kinetics. Also, the effects of processing (i.e., the same compounding process through a twin-screw extruder as for the PLA nanocomposites) on the crystallinity of "as received" PLAs were investigated to better compare them with the PLA nanocomposites. The results showed that the addition of nanoclay in different loadings did not have a significant effect on the melt crystallization of linear PLA. However, as the clay loading increased in half-branched and fully branched PLA's the reduced molecular mobility started to hinder the crystallization started to be hindered due to the reduced molecular mobility. This threshold occurred at lower clay loadings in fully branched PLA as the mobility started to be limited earlier. On the other hand, clay could improve even the linear PLA's crystallinity during isothermal treatments. On the other hand, branched PLA's processing significantly improved crystallinity due to the completion of chain-branching reactions during the processing. The effects of chain branching and nanoclay on PLA's isothermal melting crystallization kinetics at different temperatures were also studied using Avrami analysis.
Keywords: Polylactide; PLA; Branching; Chain extender; Nanoclay; Crystallization.
Introduction
Most current polymers and plastics are derived from fossil fuels. After being consumed, these polymer products become waste material in the environment, where they do not degrade. Consequently, global efforts are being made to create green and biodegradable polymers. Newly, biodegradable and biocompatible polymers that have reasonable properties are receiving increasing attention from researchers with biomedical and environmental points of view. Due to its renewable sources and biocompatibility, polylactide (PLA) is one of the polymers that have received much attention. PLA could be a promising candidate for many applications such as packaging, and can be used in place of many other non-degradable polymers [1-6].
Currently, PLA's slow crystallization behavior and low melt strength are of its most challenging drawbacks that limits its processing. Even in isothermal treatment, which PLA may encounter in extrusion and injection molding processes, it is difficult to achieve sufficient crystallinity [7-11]. This structural parameter is paramount in such polymer processes as foaming. Generally, in foaming, an optimal degree of crystallization is needed to achieve a desirable fine cell structure and a reasonable expansion ratio [6, 12]. Various studies have been done on PLA foaming using various fillers, D-content, PLA blends, branching degree, strain induction, and gas contents. All of these investigations show foaming technology is a well-established process while expansion and dissolved blowing agent could improve PLA's crystallization rate and its degree of crystallinity [6-24].
It has been shown that the amount of branched or linear chain structure has a significant effect on the degree of crystallization and its structure [16, 21-22]. In fact, increasing the molecular weight that occurs in branching will affect crystallinity behavior and improve such other properties as the polymer melt viscosity, the melt elasticity, and mechanical properties, which are crucial to the foaming process. To develop foam ability, which is dependent on melt viscosity, chain extender improves PLA's rheological properties by making the PLA end groups connect to each other to create long-branched chains [22]. In fact, a chain extender is used to create more branched structures, which increase the molecular weight and polydispersity index (PDI). The presence of such functional groups as hydroxyl, amine, anhydride, epoxy, and carboxylic acid at the two end groups of the linear branches with low molecular weight, results in branched structures with high molecular weight. The effect of branching on crystallinity could hinder the chains' mobility and flexibility. But, the effect could be positive if the chains' end groups functioned as crystal nucleation sites. In these instances, each effect can suppress the other and crystallinity may be reduced or may increase [16, 21-22, 25-28].
The chain branching effect becomes more complicated when filler is also added to the system [21-22, 25-29]. In Pilla's work [28], the addition of chain extender increased the melt viscosity and, consequently, the foam ability; it also decreased the degree of crystallinity in the presence of talc, which acted as a crystal nucleation agent in PLA [30]. Due to its nucleation power, the chain extender also increased the foam cell density and promoted the polymer's melt viscosity [22]. Talc is one of the available additives that have been used to explore PLA's crystallinity behavior. In a recent research [16, 21], it was seen that talc's nucleation effect is more pronounced in linear PLA than in branched PLA. This is so because chain branching in PLA dominates talc's crystallization efficiency due to decreased chain mobility.
Nanoclay is another additive that has attracted the attentions. Much varied research has been carried out on how nanoclay affects PLA crystallization. In a work that was implemented on PLA and its nanocomposites with different nanoclay contents using a compatible clay type (Cloisite 30B) [26], it was shown that the addition of nanoclay up to 5 wt% increased the crystallinity rather than hindering the polymer chains' mobility. However, the addition of 10 wt% nanoclay produced a strong interaction with the polymer matrix. Due to the interaction between the organically modified platelets of nanoclay and the functional groups of PLA chains, the polymer chains that interacted with the nanoclay hindered the chain's mobility and decreased their flexibility. Consequently, crystallization was also decreased. Krikorian et al. [25]. showed that the addition of nanoclay (Cloisite 30B) decreased the crystallinity behavior of Poly-L- Lactide (PLLA) compared with that of pure PLLA. In fact, the nanoclay, due to its platelet shape, mainly hindered the chains' mobility and flexibility. Therefore, the better the nanoclay dispersion, the lower the degree of crystallization [25]. On the other hand, Nofar et al. [19] illustrated that nanoclay improves the crystal nucleation and hence the crystallization rate. The large number of nucleated crystals together with the well dispersed clay platelets indeed limit the PLA's molecular mobility. This will eventually reduce the final crystallinity despite the expedited crystallization kinetics of PLA. Therefore, the addition of nanoparticles has a challenging influence on PLA's crystallization.
In this study, to investigate the synergistic effects of chain extender and nanoclay on PLA's crystallinity, the melt crystallization behavior of PLA, both with and without a chain extender, and its nanocomposites with four different nanoclay contents were examined using a differential scanning calorimeter (DSC). Non-isothermal and isothermal melt crystallization experiments were implemented to explore the crystallization kinetics and Avrami model was utilized to explore the crystallization kinetics of PLA. The dispersion quality of nanoclay within PLA and reactivity of chain extender with PLA molecules were also analyzed using x-ray diffraction (XRD) and Fourier transform infrared spectroscopy test (FTIR), respectively.
Experimental Procedure
Materials
Three types of PLAs in pellet form were supplied by NatureWorks® LLC in three formulations with similar D-lactide molar contents (4.5-4.6 mol %). The first PLA type was the commercially available Ingeo™ 8051D linear PLA with 0% of chain extender (L-PLA). The other two PLA types were produced by reactive extrusion with an epoxy-based multi-functional oligomeric chain extender (Joncryl ADR-4368C, BASF Inc.) based on 8051D with 0.35 and 0.7wt% CE, and were referred to as half branched PLA (HB-PLA) and fully branched PLA (FB-PLA), respectively. The molecular weights of linear, half branched, and fully branched PLAs were reported as 90,111, 98,592, 112,944 g/mol, respectively. The molecular structure and chain extension mechanism of this chain extender has been reported elsewhere [27]. Nanoclay montmorillonite, modified with a quaternary ammonium salt (Cloisite 30B), and supplied by Southern Clay Products Inc. was also used in four different contents.
Sample Preparation
All neat PLAs were used "as received" for the differential scanning calorimeter (DSC). To compound PLA nanocomposites with different nanoclay contents (0.2, 0.4, 1, and 2wt %) a Minilab twin-screw extruder was used at 180°C, with a screw speed of 40 rpm, for 5 minutes. To decrease the moisture level of the PLA pellets prior to compounding, they were vacuum oven-dried at 65°C for at 6 h. After compounding, the nanocomposite strips were pelletized by scissors. To check the effect of processing in the minilab twin-screw extruder on the crystallinity of the "as received" PLAs, the neat PLAs were also passed through the extruder as well to better compare them with the nanocomposite samples. Then, the "as received" PLAs, "processed" PLAs, and PLA nanocomposites were weighted (typically 15mg for each), punched, and loaded into aluminum pans for the differential scanning calorimeter (DSC) experiments.
XRD analysis was conducted to explore the clay dispersion in the polymer. The samples were prepared for XRD by compression molding at 180°C in a circle-shaped film of 1 mm thickness. XRD spectra were collected on a Siemens D5000 diffractometer equipped with a Kevex solid-state detector using a Cu Kα radiation source with a wavelength of 15.4 nm. Measurements were performed at 50 kV and 35 mA. The data were recorded in the reflection mode in a slow-step scan mode (ss: 0.02o/t: 1.3 s per step) at a range of 2θ = 1-10°.
Crystallization Behavior Study
A DSC Q2000 (TA Instruments) was used to investigate the crystallization behavior of "as received" and "processed" PLAs with three different CE contents (0, 0.35, 0.7wt %) and PLA nanocomposites. First, the non-isothermal melting crystallization of both neat PLAs ("as received" and "processed") and the PLA-nanocomposite materials were investigated. Samples were heated from room temperature to 200°C at a heating rate of 20°C/min. and then equilibrated at 200°C for 10 min. to remove all previous thermal and stress histories [21]. Then the samples were cooled to 25°C at a rate of 2°C/min. to allow sufficient diffusion time for the crystallization process [21].
Isothermal melting crystallization experiments of PLAs and their nanocomposites were also conducted after eliminating their thermal and stress histories as in the nonisothermal experiments. The samples were then cooled at a rate of 20°C/min. to various isothermal temperatures (90°C, 100°C, 110°C, and 120°C) and equilibrated at each temperature until full crystallization.
During all processes, the DSC curves were recorded and analyzed using TA Universal software. The initial degree of crystallinity, 𝛘 , i.e., the crystallinity of samples was calculated by using the following Equation:

Where ΔHc is the crystallization enthalpy and 93.6 is the melting enthalpy in J/g of 100% crystalline PLA [31]. The kinetics of isothermal melt crystallization at different isothermal temperatures was analyzed using the Avrami equation [32]:
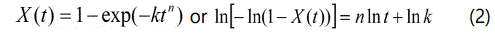
Where X(t) is the relative crystallinity at crystallization time t, k is the crystallization kinetic constant for nucleation and growth rate, and n is the Avrami exponent reflecting the mechanisms of crystal nucleation and growth. By plotting ln[-ln(1-X(t))] versus ln(t), the Avrami exponent, n, and the logarithm of kinetic constant, lnk, were determined.
Results and Discussion
Nanocomposites' Structure
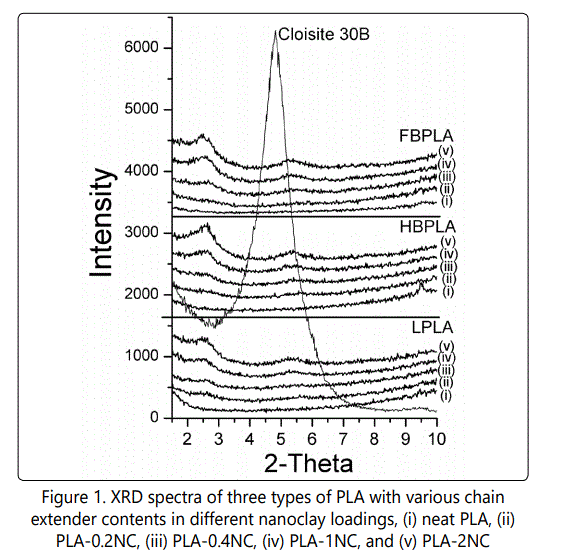
As mentioned previously, nanocomposites with different nanoclay contents (0.2, 0.4, 1, and 2 wt %) were produced by compounding. The main challenge with nanocomposites is the degree of intercalation and exfoliation of nanoparticles within the polymer. Figure 1 shows X-ray diffraction results in the range of 2θ=1-10° for PLA/nanoclay samples with different chain extenders and nanoclay contents. The characteristic peak of neat Cloisite 30B that corresponds to the interlayer spacing, d(001) = 18.5A° is shifted to a maximum d spacing of 33.04A° for 0.2 and 0.4wt% nanoclay contents and 34.03Ao for 1 and 2wt%, for all PLA types. The results show that the nanoclay is well intercalated and dispersed within the polymer matrix.
Non-Isothermal Melt Crystallization Behavior
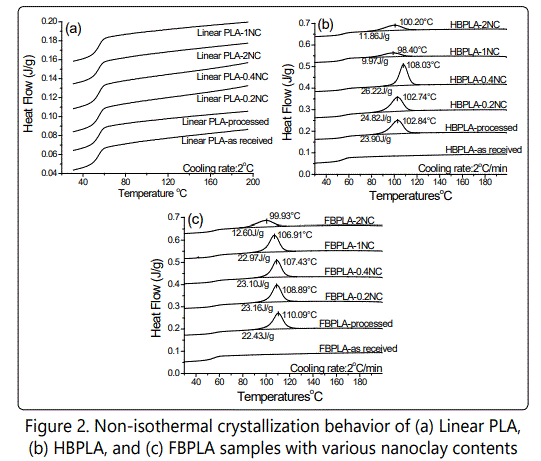
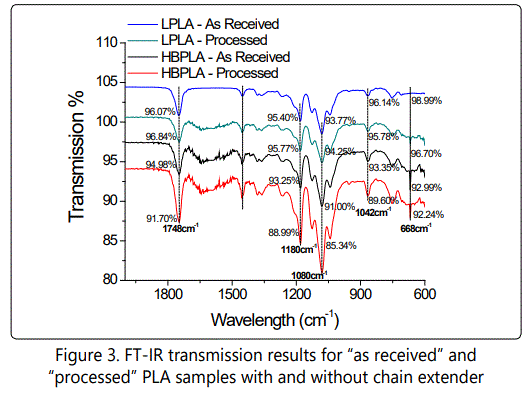
Samples with different compositions were cooled from 200°C to 25°C at a rate of 2 C°/min. As Figure 2 shows, the neat linear PLA, half branched, and fully branched "as received" PLAs depicted no exothermic peak as a consequence of melt crystallization even at a slow cooling rate of 2°C/min. However, the processed PLAs that have passed through the Minilab extruder showed that for L-PLA there is no detectable crystallization peak whereas for processed HB-PLA and FB-PLA, 24% and 23% of crystallinity has formed, respectively, during this cooling process. Later on also proved by fourier transform infrared spectroscopy test (FT-IR), it is illustrated that the branching effect of the chain extender was uncompleted when it was directly received from the supplier and hence it completed through the extruder process. Therefore, after processing, the end groups of PLA chains that were connected through the chain extender started to actively act as crystal nucleating sites. In other words, in "as received" HB-PLA and FB-PLAs the reactions between the chain extender and the PLA chains was not yet completed and this occurred through the process in the Minilab extruder at elevated temperature, a fact that was confirmed by the PLAs' supplier (NatureWorks). To verify the interactions between the L-PLA chains and the chain extender as noted earlier, an FT-IR was implemented to obtain an infrared spectrum of absorption by various bonds. The experiments were done on L-PLA and HB-PLA ("as received" and "processed") samples. As Figure 3 shows, different bonds have varying absorptions. The reaction between the PLA chains and chain extender occured at the PLA chains' end group, which is an alcohol group (–OH). As a result of the reaction between PLA chains' end groups and chain extender, additional ester compounds form. The ester compounds are derived by reacting an oxoacid such as epoxy group that exists in chain extender with a hydroxyl compound such as an alcohol which exists in PLA's chains' end groups. The absorption by ester and alcohol groups takes place at wavelengths of 1748 and 1180 cm-1 and 1080 and 1042 cm-1, respectively. As shown, the concentration of these bonds, which is represented by the peak's transmission intensity, is the same for "as received" and "processed" L-PLA. However, when one compares the "as received" L-PLA and the HB-PLA, the bonds' absorption increases due to the interactions that occurred between the PLA chains end groups and the chain extender. When the HB-PLA is processed through the extruder, the absorptions increase much more effectively. This is due to the increased interaction between the PLA chains and the chain extender. In fact, when the HB-PLA and, consequently, the FBPLA pass through the extruder and are processed further at a high temperature the branching is completed. However, in "as received" samples, the branching was only partially completed.
As Figure 2 shows, with the addition of 0.35% of chain extender, crystallinity increased in HB-PLA as compared with that of "processed" L-PLA. By increasing the chain extender to 0.7% for FB-PLA, crystallization occurs faster and also increased in degree. By increasing the chain extender amount to 0.7wt% and thus creating more branched structures and connections between the chains, these interconnected chains and branched structures became potential crystal nucleation sites. The results in this case show that the crystallization behavior of FB-PLA improved more significantly. In addition, since there is no chain extender in L-PLA, no crystallinity occurs in it even when processed.
Figure 2 also shows that the addition of nanoclay does not create any crystallinity in the L-PLA samples even at a slow cooling rate of 2°C/min. In L-PLA, the hypothesis that no crystallization occurs is based on the idea that nano-layers couldn't actively behave as crystal nucleation agents. Considering the branched PLAs, since the crystallinity of the neat HB-PLA and FB-PLA samples was changed by passing through the extruder, the results of nanocomposite PLAs should be compared with the processed HB-PLAs and FB-PLAs to eliminate the effect of processing on the degree of the crystallinity. As shown, by adding nanoclay to HB-PLA up to 0.4wt%, crystallinity occur faster and the melt crystallization temperature increases from 102°C to 108°C. This may be due to the crystal nucleation effect that nanoclay might have created in addition to the crystal nucleation that occurs due to the branching effect. However, by increasing the nanoclay content up to 2wt% the degree of crystallinity decreased. Although the addition of nanoclay can expedite the crystallization rate, it also hinders the molecular mobility [19]. In the case of FB-PLA, crystallinity occurred faster and in a shorter period of time as there was a greater degree of branching in the FB-PLA. However, the addition of nanoclay to FBPLA further decreased the amount of crystallinity, causing it to occur over a longer period of time. This result is also due to the much lower mobility of the PLA chains that reduced the final crystallinity.
As shown in this work and in a previous study [16, 21], the addition of chain extender created crystal nucleation sites in PLA and expedite its crystallization kinetics. It was also shown that the synergy of chain branching and nanoclay could differently influence the PLA's crystallization. For HB-PLA and FB-PLA, it was shown that the processing had a significant effect on the degree of crystallinity and the chain extender could complete its reaction after the processing. Therefore, it is important to consider the effect of additional processes on any sample and the consequent results. For example, when compounding a polymer with a filler, such as nanoparticles, to investigate the filler's effect, it would be the best to maintain the samples' manufacturing consistency, even those using no filler. Thus, purely the effects of the filler could be explored.
Isothermal Melt Crystallization Kinetics
To better understand the effect of chain branching and nanoclay on crystallization behavior of PLA, isothermal crystallization experiments were conducted. To study the effect of different nanoclay contents in PLA with different chain extender contents, the isothermal temperature of 100°C was selected. To investigate the effect of different isothermal temperatures on crystallization kinetics, nanocomposites with 0.4wt% nanoclay were selected in a temperature range from 90°C to 120°C at an interval of 5°C. For linear PLA, only two isothermal conditions (100°C and 120°C) were selected due to the length of time for complete crystallization.
Effect of nanoclay and processing on isothermal melt crystallization at 100°C
To study the PLA's (i.e., "as received" and "processed") and PLA-nanocomposites' isothermal melt crystallization at 100°C, their relative melt crystallinity versus time is plotted in Figure 4 (a, c, e). Their corresponding Avrami double-log plots are shown in Figure 4 (b, d, f). Avrami parameters (n, lnk, k) that are derived from Avrami plots are also shown in Table 1. It seems that the crystallization rate is significantly influenced by molecular structure. Avrami exponent n also does not change much, and for all samples it is between 2 and 3, indicating that mainly homogenous nucleation and twodimensional spherulitic growth occurred for neat PLAs and PLA nanocomposites. This shows that the addition of nanoclay as filler did not lead to heterogeneous crystal nucleation and three-dimensional spherulitic growth. However, talc is a wellknown efficient nucleating agent for PLA, and causes having n value above 3, indicating three dimensional and heterogeneous crystal nucleation and growth [21].
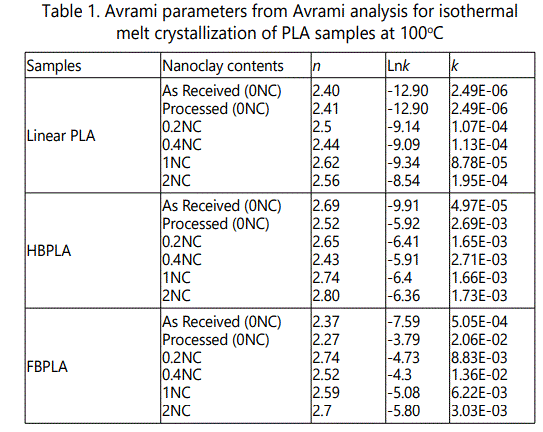
Figure 5 also shows the full crystallization half-time at 100°C for PLA samples with different chain extenders ("as received" and "processed") and their nanocomposites with different nanoclay contents. For L-PLA "as received" and "processed" samples, more than 3h half-time is needed for crystal formation. The "as received" and "processed" LPLA crystallization behavior s are very similar. However, the addition of nanoclay decreases the half-time crystallization to less than 30 min. This is because the linear chains in LPLA are free and possess extremely high mobility. This high mobility does not, however, create crystal clusters as the addition of nanoclay somewhat restricts their mobility and starts to behave actively as crystal nucleating sites. Therefore, during isothermal treatment, the nanoclay exerts control over the chains' mobility, and crystal clusters can form much easier. Therefore, nanoclay could act as crystal nucleation agent and improved the crystal formation.
In HBPLA and FBPLA "as received" samples, the crystallization time significantly decreased. As noted, this is due to the crystal nucleation behavior of the interconnected chains' end groups through the chain extender although the reaction might have not fully completed yet. This also demonstrates that, although the addition of the chain extender increased the samples' molecular weight and viscosity, the role of the end groups as nucleation sites is dominant in expediting crystallization. As previously explained, processing the PLA samples with the chain extender in the extruder has an important effect on crystallinity behavior. Figure 5 shows that processed HBPLA and FBPLA samples have a much faster crystallization half-time compared with the "as received" samples. Again, since the chain branching effect is undergoing completion while the samples pass through the extruder, branching degree increases and hence more crystal nucleating sites could be provided for crystallization. "Processed" HBPLA and FBPLA samples showed almost five times faster crystallization rate than the "as received" PLA samples. Therefore, comparing "processed" HBPLA and FBPLA with LPLA samples, it is seen that the isothermal crystallization half-time takes place much faster with added chain extender. Consequently, the crystallization half-time of FBPLA samples was smaller than for HBPLA.
On the other hand, compared with "processed" samples, the addition of nanoclay to HBPLA and FBPLA samples does not have a significant effect on crystallization behavior, but it does somehow increase the crystallization half-time and negatively affects expeditious crystallization. This is the opposite of what happens with LPLA nanocomposites. Although the movement of the chains is restricted by the branching in half-branched and fully branched PLA samples, the chains' end groups serve as crystal nucleation agents. However, the addition of nanoclay restricts the crystallization effect that can be introduced by the branching effect. It can be an issue that the addition of nanoclay improves the crystallization behavior of LPLA by exerting more control on the chains' mobility. However, the addition of nanoclay to HBPLA and FBPLA restricts the chains' movements even more, so that the chain branching can scarcely improve crystallinity.
Effect of various temperatures on isothermal melt crystallization of PLA and PLA-0.4NC
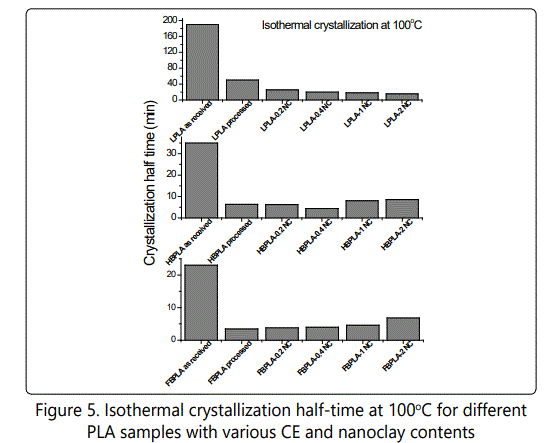
Figures 6-8 show the effect of different isothermal temperatures on the crystallization behavior of PLA with various chain extender contents ("as received" and "processed") and their nanocomposites with 0.4wt% nanoclay. By increasing the chain extender, crystallization occurs faster and this happens more significantly for the "as received" HBPLA and FBPLA samples when they are processed in the extruder. However, as shown earlier, the addition of 0.4wt% nanoclay does not improve the crystallization behavior of HBPLA and FBPLA. But it does improve the LPLA's crystallization. Figures 6-8 illustrate that isothermal treatment temperatures between 100°C and 110°C expedite crystallization much more effectively than other isothermal temperatures for HBPLA and FBPLA samples and their nanocomposites. Considering the enthalpy values in the isothermal results, it can be seen that the addition of 0.4wt% nanoclay to LPLA, HBPLA, and FBPLA samples increased the crystallinity more significantly for linear PLA than for neat branched polymers. This as noted was due to the chains' greater degree of regularity compared with those in branched PLAs, regardless of the restrictions caused by the nanoclay.
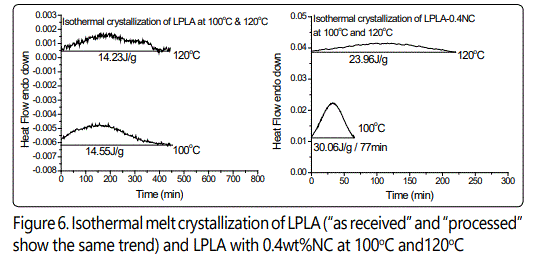
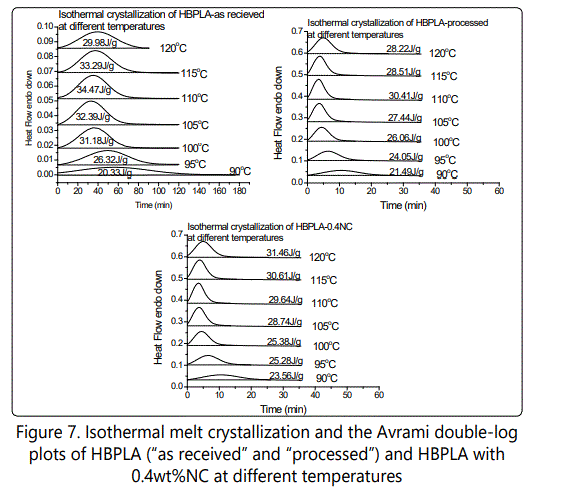
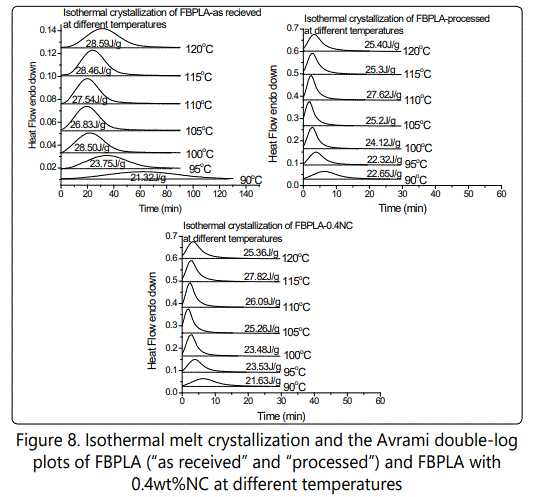
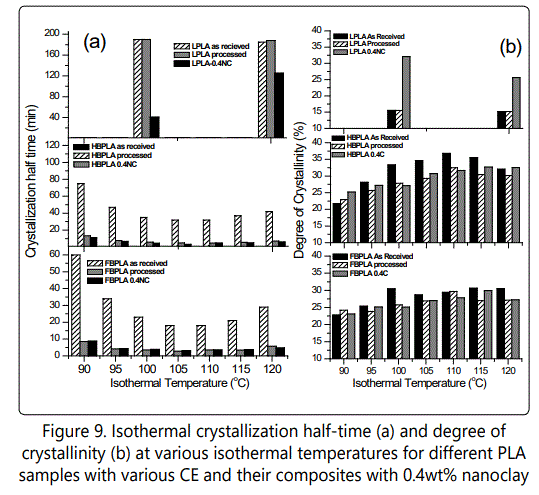
Figure 9(a). Plots the crystallization half-time, which is the time corresponding to 50% of the final crystallinity, and which is derived from the integration curves of the DSC isothermal curves. It can be seen that linear PLA's crystallization half-time at both 100°C and 120°C is very long. With the presence of branched chain structures, crystallization completion time dramatically decreased because branched points acted as nucleation sites for linear segments, which had a higher local order. However, this reduction in crystallinity half-time becomes much more significant when the neat HBPLA and FBPLA samples undergo the same process as their nanocomposites go through the twin-screw extruder. With the completion of the HBPLA and FBPLA "processed" samples' branching processes, their more densely branched structures led to crystallinity occur much faster due to their higher number of crystal nucleation sites. By comparing the crystallization half-time of two branched PLAs shown in Figure 9a, it is clear that the crystallization rate of fully branched PLA is also faster than half-branched PLA. By comparing processed PLAs with their nanocomposites, it also can be seen that the addition of nanoclay improves LPLA's crystallization behavior, and it becomes less effective with the addition of CE and with the presence of more branched structures. This is due to LPLA's higher degree of chain regularity. The isothermal melting crystallization study results may be tentatively interpreted to mean that branched chains act as nucleating agents that accelerate crystallization to a certain degree, but that also decrease the final crystallinity. However, depending on the type of filler compounded with PLA, the effect of branching on the crystallization nucleation rate can be highlighted more when compared with linear structures.
The degree of crystallization is also shown in Figure 9b. The final crystallinity of linear PLA with 0.4wt% nanoclay is much higher than for the neat LPLA. This shows that the addition of nanoclay to LPLA, besides expediting the crystallization half-time, increases crystallinity due to its nucleating behavior in the LPLA sample with its greater chain regularity. However, in HBPLA and FBPLA samples the nanoclay effect is suppressed because the chains are interconnected and have low mobility. Also, in Figure 9a, at lower temperature ranges (from 90°C to 100°C) for HBPLA and FBPLAs ("as received", "processed", and nanocomposite) crystallization half-time decreases as temperature increases. When isothermal temperatures are 105°C and 110°C, the crystallization half-time decreased significantly and is more pronounced in the FBPLA sample. Although, the crystallization half-time decreases significantly when the isothermal temperature is increased to 105°C and 110°C, the degree of crystallization does not show a high sensitivity to the isothermal temperature. Figure 9a-b also shows that the degree of crystallinity and crystallization half-time are not significantly influenced by the inclusion of nanoclay compared with that of the "processed" neat PLAs, except for LPLA. Increasing the isothermal temperature from 110°C to 120°C causes an increase in crystallization half-time due to a higher chain mobility at higher temperatures that makes the creation of a crystalline structure more difficult.
Conclusions
A DSC instrument was used to investigate the melting crystallization of linear PLA and branched PLAs and their nanocomposites with different nanoclay and chain extender contents. Non-isothermal melt crystallization experimental results showed that the addition of chain extender increased the crystallization kinetics due to its role as a crystal nucleation agent. However, to facilitate comparison of the results with the composite samples, the "as received" samples passed through the same compounding process that was used for the PLA nanocomposites in the minilab twin-screw extruder.
The crystallinity of the processed PLAs with chain extender improved much more significantly due to the completion of chain branching reactions in the extrusion process. It was shown that the chain extender significantly improved crystallinity and that processing the "as received" PLAs more significantly affects the crystallinity behavior of HBPLA and FBPLA. The nanocomposite samples with nanoclay showed that LPLA crystallinity improves more during isothermal melt crystallization treatment since nanoclay exerts more control of the LPLA's chain mobility and can affect the formation of crystal clusters. However, in HBPLA and FBPLA the addition of nanoclay can even slightly restrict the chains' movement,so that chain branching can be less effective in improving crystallinity. Isothermal melting crystallization experiments showed that branched PLAs and their nanocomposites crystallized much faster than linear PLA.
Acknowledgements
The author would like to acknowledge the financial supports of Istanbul Technical University Scientific Research Projects (ITU-BAP) with the project numbers of 39415 and 40616.
References
- Sinclair RG. The case for polylactic acid as a commodity packaging plastic. J Macromol Sci Part A Pure Appl Chemi. 1996; 33(5): 585-597. doi: 10.1080/10601329608010880
- Grijpma DW, Pennings AJ. (Co)polymers of L-lactide, 2. Mechanical properties. Macromol Chem Phys. 1994; 195(5): 1649-1663. doi: 10.1002/macp.1994.021950516
- Auras R, Harte B, Selke S. An overview of polylactides as packaging materials. Macromol Biosci. 2004; 4(9): 835-864. doi: 10.1002/mabi.200400043
- Drumright RE, Gruber PR, Henton DE. Polylactic acid technology. Adv Mater. 2000; 12(23): 1841-1846. doi: 10.1002/1521-4095(200012)
- Nofar M, Park CB. Poly (lactic acid) foaming. Prog Polym Sci. 2014; 39(10): 1721-1741. doi: 10.1016/j.progpolymsci.2014.04.001
- Nofar M. Effects of nano-/micro-sized additives and the corresponding induced crystallinity on the extrusion foaming behavior of PLA using supercritical CO2 . Mater Design. 2016; 101: 24-34. doi: 10.1016/j.matdes.2016.03.147
- Keshtkar M, Nofar M, Park CB, Carreau PJ. Extruded PLA/Clay Nanocomposite Foams Blown with Supercritical CO2. Polymer. 2014; 55(16): 4077- 4090. doi: 10.1016/j.polymer.2014.06.059
- Ameli A, Jahani D, Nofar M, Jung PU, Park CB. Processing and Characterization of Solid and Foamed Injection-Molded Polylactide with Talc. J Cell Plast. 2013; 49(4): 351-374. doi: 10.1177/0021955X13481993
- Ameli A, Nofar M, Jahani D, Park CB. Development of high void fraction polylactide composite foams using injection molding: Mechanical and thermal insulation properties. Compos Sci Technol. 2014; 90: 88-95. doi: 10.1016/j.compscitech.2013.10.019
- Ameli A, Nofar M, Jahani D, Park CB. Development of High Void Fraction Polylactide Composite Foams Using Injection Molding: Crystallization and Foaming Behaviors. Chem Eng J. 2015; 262: 78-87. doi: 10.1016/j.cej.2014.09.087
- Nofar M, Ameli A, Park CB. Development of Polylactide Bead Foam with Double Crystal Melting Peak Structure. Polymer. 2015; 69: 83-94. doi: 10.1016/j.polymer.2015.05.048
- Nofar M, Ameli A, Park CB. A Novel Technology to Manufacture Biodegradable Polylactide Bead Foam Products. Mater Design. 2015; 83: 413-421. doi: 10.1016/j.matdes.2015.06.052
- Nofar M, Zhu W, Park CB. Effect of dissolved CO2 on the crystallization behavior of linear and branched PLA. Polymer. 2012; 53(15): 3341-3353. doi: 10.1016/j.polymer.2012.04.054
- Nofar M, Tabatabaei A, Ameli A, Park CB. Comparison of melting and crystallization behaviors of polylactide under high-pressure CO2, N2, and He. Polymer. 2013; (54): 6471-6478. doi: 10.1016/j.polymer.2012.04.054
- Nofar M, Ameli A, Park CB. The thermal behavior of polylactide with different D-lactide content in the presence of dissolved CO2 . Macromol Mater Eng. 2014; 299(10): 1232-1239. doi: 10.1002/mame.201300474
- Nofar M, Tabatabaei A, Park CB. Effects of nano-/micro-sized additives on the crystallization behaviors of PLA and PLA/CO2 mixtures. Polymer. 2013; 54(9): 2382-2391. doi: 10.1016/j.polymer.2013.02.049
- Najafi N, Heauzey MC, Carreau PJ, Therriault D, Park CB, et al. Rheological and foaming behavior of linear and branched polylactides. Rheol Acta. 2014; 53(10-11): 779-790. doi: 10.1007/s00397-014-0801-3
- Nofar M, Zhu W, Park CB, Randall J. Crystallization kinetics of linear and long-chain-branched polylactide. Ind Eng Chem Res. 2011; 50(24): 13789- 13798. doi: 10.1021/ie2011966
- Wang J, Zhu W, Zhang H, Park CB. Continuous processing of low-density, microcellular poly(lactic acid) foams with controlled cell morphology and crystallinity. Chem Eng Sci. 2012; 75: 390-399. doi: 10.1016/j.ces.2012.02.051
- Liao X, Nawaby AV. Solvent Free Generation of Open and Skinless Foam in Poly(L-lactic acid)/Poly(D,L-lactic acid) Blends Using Carbon Dioxide. Ind Eng Chem Res. 2012; 51(19): 6722-6730. doi: 10.1021/ie3000997
- Nofar M, Tabatabaei A, Sojoudi H, et al. Mechanical and bead foaming behavior of PLA-PBAT and PLA-PBSA blends with different morphologies. Eur Polym J. 2017; 90: 231-244. doi: 10.1016/j.eurpolymj.2017.03.031
- Krikorian V, Pochan D. J. Poly (l-Lactic Acid)/Layered Silicate Nanocomposite: Fabrication, Characterization, and Properties. Chem Mater. 2003; 15: 4317-4324. doi: 10.1021/cm034369
- Di Y, Iannace S, Di Maio E, Nicolais L. Poly(lactic acid)/organoclay nanocomposites: Thermal, rheological properties and foam processing. J Polym Sci: Part B: Polym Phys. 2005; 43(6): 689-698. doi: 10.1002/polb.20366
- Nam JY, Ray SS, Okamoto M. Crystallization Behavior and Morphology of Biodegradable Polylactide/Layered Silicate Nanocomposite. Macromolecules. 2003; 36(19): 7126-7131. doi: 10.1021/ma034623j
- Li H, Huneault MA. Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer. 2007; 48(23): 6855-6866. doi: 10.1016/j.polymer.2007.09.020
- Fischer E, Sterzel,Wegner G, Hans J, Collo K, et al. Investigation of the Structure Of Solution Grown Crystals of Lactide Copolymers by Means of Chemical Reaction. Polym. Sci. 1973; 251(11): 980-990. doi: 10.1007/ BF01498927



