Review Article
A Review on Clinical Pharmacist Care Services on Prevention and Management of Tuberculosis associated Burden in Health Care Practice
1Department of Pharmaceutics, SRM College of Pharmacy, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu, India
2Department of Pharmacy Practice, SRM College of Pharmacy, SRM Institute of Science and Technology, Kattankulathur, Tamil Nadu, India
3Department of Cardiology, Gleneagles Global Hospital, Perumbakkam, Chennai, India
4Department of Cardiology, SRM Medical College Hospital and Research Centre, Kattankulathur, Tamil Nadu, India
*Corresponding author: MS Umashankar, Department of Pharmaceutics SRM College of Pharmacy, SRM Institute of Science and Technology, Tamil Nadu, India, Tel: 91-9840333269, E-mail: umashankarms269@gmail.com
Received: December 16, 2019 Accepted: December 23, 2019 Published: December 30, 2019
Citation: Umashankar MS, Kumar AB, Kumar GB, Sriram V. A Review on Clinical Pharmacist Care Services on Prevention and Management of Tuberculosis associated Burden in Health Care Practice. Madridge J Intern Emerg Med. 2019; 4(1): 152-157. doi: 10.18689/mjiem-1000134
Copyright: © 2019 The Author(s). This work is licensed under a Creative Commons Attribution 4.0 International License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Tuberculosis is caused by a highly transmissible microorganism, and Mycobacterium tuberculosis spreads infection through air due to coughing, sneezing, spiting and talking imposes major health problems in the community. Every year more than 2 million people die with tuberculosis from Africa, China, and South East Asia having higher incidences. Around 2,80,000 people get affected by tuberculosis in India, the second highest drug resistance tuberculosis affected country in the world. The tuberculosis patients suffer with weight loss, loss of appetite, fever, sticky blood in sputum, chest pain and fatigue. Drug resistant tuberculosis often encountered by bacteria that do not respond to anti-tubercular drugs like Isoniazid and Rifampicin. Drug resistance emerges due to premature treatment pattern, poor medication adherence, ineffective drug formulations and poor quality of medications, improper storage and act of self medications. Though effective treatments are provided by the physician, the prevention and management of tuberculosis is now called for intervention by health care team. Clinical pharmacist being one in health care team can be effectively positioned in the treatment care can initiate and implement health screening awareness programme, education on early diagnosis, infection control practices, drug resistance burden awareness and offering supportive care. Clinical pharmacist in all clinical setup and community health care centre can render meticulous services in the eradication of tuberculosis disease.
Keywords: Tuberculosis; Disease burden; Clinical pharmacist; Community practice.
Introduction
Tuberculosis is a communicable disease caused by the Mycobacterium tuberculosis. It damages the vital organs and spreads the infection to all parts of the body. The patients who do not suffer infection are known as latent tuberculosis. The World Health Organization predicted that the one third of the world population is infected with tuberculosis and more than 8 million new cases of active tuberculosis occur annually. The resistance to anti tuberculosis drugs, a serious problem was identified in the early days of the treatment era [1-3].
In 2015, the largest number of new tuberculosis cases was occurred in Asia with 61% of new cases, followed by Africa with 26% of new cases. Six countries accounted for 60% of the new tuberculosis cases includes China, Nigeria, Pakistan, Indonesia, India, and South Africa. In March 2017, the Government of India announced a new aim with related to tuberculosis in India, was eradicated by 2025. Drug resistance tuberculosis is characterized by the ability of bacteria to produce poor response to the drugs and the manner by which the resistance acquired. Bovine tuberculosis is a disease caused by Mycobacterium bovis and majorly it affects cattle but can also affect human population.
Types of Tuberculosis
Latent tuberculosis:
- Tuberculosis bacteria are asleep in body
- Patients do not have symptoms
- Infection canʼt spread to others
- It can be detected through a TB skin test
- It is treated with medications over three to six months
Active tuberculosis:
- Tuberculosis bacteria is in active stage
- The patient suffer from tuberculosis symptoms
- Infection can spreads to the others
- It can be diagnosed using chest x-ray, tuberculin skin test
- Effective treatment implementation for six months
Pathogenesis of Tuberculosis
The bacterial species carry into the lungs through inhalation of infectious species deposits in the sub pleural air spaces of the lower lobes of the lungs. Initiation of the infection the bacterial species ingested by the macrophages and causes the phagocytosis process and invades the lungs. This situation makes the patientʼs immune system to be weak condition. The release of inflammatory cells precedes the inflammation and causes the inflammation in the lungs. It is known as early stage of infection. The invaded lungs having the highly transmissible infectious droplet nuclei continuously produce infectious conditions. The infected cells reach the lymphatic cells and migrate into the blood stream and circulate various viral organs and slowly it will alter the vital organ functions. These process will suppress the immune system function and intense to develop the infection. The tuberculosis bacilli can withstands prolong period of time in the body depends on the patients effective immune system. The patients with good immune function less prone to develop the infection. Healthy people affected with tuberculosis least chances to developing infection in future. The impairment of immune function status raise the chances of re-activation of tuberculosis process called re-activation tuberculosis. This process of the tuberculosis bacilli damage of various organs depends on the delayed type hypersensitivity reaction [4-8]. The damage of pleural spaces of the lungs causes emphysema. The bacterial proliferation in the lungs depends on the virulence of bacteria and host defensive mechanism in the body. The development of hypersensitivity reactions develops the infection progression and invades the lungs leads to cause tuberculosis condition in the body. The M. tuberculosis complex group of species which includes: M. bovis, M. africanum etc (Figure 1).
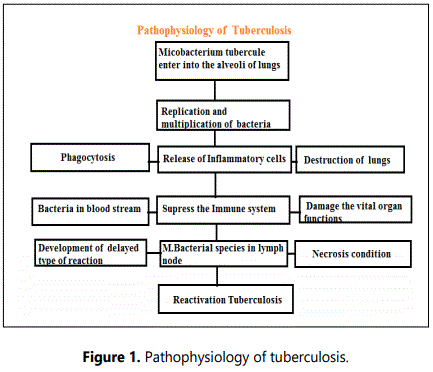
Causative Factors
Causative factors for tuberculosis infections include
- HIV infections
- Malnutrition
- Poverty
- Lung disease
- Silicosis
- Smoking, Alcoholic nature
- People suffering with chronic diseases
Symptoms of Tuberculosis
It includes following symptoms:
- Cough, chills
- Blood vomiting
- Fatigue
- Loss of appetite
- Fever
- Weight loss
- Night sweats
Diagnosis of Tuberculosis
- Blood test
- Sputum culture test
- Microscopic examination of sputum
- Nucleic acid amplification test
- Ziehl Nielsen staining examination
- Tuberculin skin test
- Gama interferon assay
- Microbiological culture of body fluids
Risk group for developing tuberculosis include:
- People infected within the previous two years
- Infants and children aged less than 4 years of age
- People infected with HIV infections
- People who have poor immune function
- People with diabetes, chronic renal failure people
Pharmacotherapy for Tuberculosis
First line drugs
- INH (Isonizid)
- Rifampicin
- Pyrizinamide
- Ethambutol
- Streptomycin
- Thiacetazone
- Second line drugs
- Amikacin
- Clarithromycin
- Azithromycin
- Kanamycin
- Rifabutin
- Capreomycin
- Viomycin
- Ethionamide
- Para amino salicylic acid
- Fluoroquinolones drugs include (ciprofloxacin, ofloxacin, sparfloxacin)
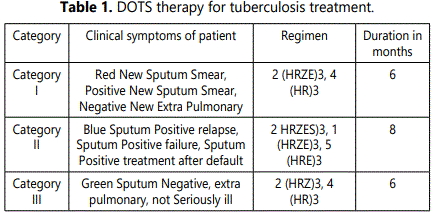
DOTS therapy for tuberculosis treatment is described in table 1.
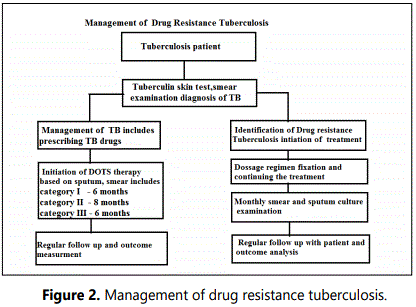
Basis for development of Drug resistant Tuberculosis
The drug-resistance was identified earlier after its introduction in the 1940s and investigated through treatment failure with first few months of therapy in patients being treated with streptomycin. The term drug-resistant TB refers to M. tuberculosis that is resistant to one of the first line treatment options, including ethambutol, isoniazid, pyrazinamide, rifampin or streptomycin. Multidrug-resistant tuberculosis refers to an isolate of M. tuberculosis that is resistant to at least isoniazid and rifampin, the two most effective first-line medications (Figure 2).
Causes of drug resistance
Causes of drug resistance originated during the year 1944 improper treatment pattern in infections management and in 1950, Renee Dubos identified that bacteria would eventually develop resistance to antibiotics through normal mutations mechanism. Next two decades additional tuberculosis drugs were discovered includes amino salicylic acid, isoniazid, and rifampin, but as their longer usage increased eventually caused the appearance of tuberculosis strains showed resistant to isoniazid, rifampicin. In 1956 tuberculosis strains showed resistant to streptomycin, Para aminosalicylic acid and isoniazid (INH) were discovered in Britain [9-18].
The development of drug resistance by the following mechanisms which includes following steps
Resistance by exclusion
- Loss of drug accumulation mechanism
- Higher drug elimination
Resistance by metabolism
- Improper active forms of drug conversion
- Prolong period of drug change its nature to active form
Alterations in Drug target
- Removal of drug target
- Less affinity towards drug target affinity
- Excessive gene amplification
- Overproduction of drug target
- Over deposition of metabolites
Clinical factors promoting drug resistance [19]
- Improper diagnosis methods
- Inappropriate drug regimen prescribing
- Inappropriate course of treatment
- In effective treatment modifications
- Prescribing pattern of single drug to a failing drug regimen
- In effective use of chemoprophylaxis
- Poor medication adherence and quality of drugs
- Improper storage of drugs
- Less effective drug formulations
- Failure to meet the DOTS treatment, Mal absorption of drugs
Types of drug resistance tuberculosis
Multidrug-resistant tuberculosis: It is caused by tuberculosis a bacterium that is resistant to at least isoniazid and rifampin, the two most potent tuberculosis therapy.
Extensively drug-resistant tuberculosis: It is a rare type of multidrug resistant tuberculosis which is resistant to isoniazid and rifampin or any second-line drugs such as amikacin and kanamycin. Drug resistance is developed through spontaneous genetic mutation. Once a drug-resistant strain has developed and proliferates, it may attain resistance to drugs through the same process. The resistant strains may be transmitted from one person to another person [20-24]. A person infected with a drug-resistant strain from another person is chances to develop primary drug resistance. Tuberculosis cases not have been treated because of re infections problems and greater chances of transmission of drug-resistant.
Diagnosis test for drug resistance tuberculosis
- Genexpert test
- Geno type MTBDR
- Molecular based assays
- Genotypic test
- FAST Plaque-Response bacteriophage assay
Clinical Pharmacists Intervention Care on the Prevention of Tuberculosis
Clinical pharmacists have a wider knowledge in disease prevention and management. Clinical pharmacists can directly interact with patients and health care professionals to address the disease related burden in the community level. Regular establishment of the clinical pharmacist care services in the community will help the health care team to identify and investigate the diseases. The pharmaceutical care services based on individual patientʼs needs by resolving problems associated with the use of medications and to motivate the patients about tuberculosis prevention and management.
Early implementation of clinical pharmacy services in the community level can encourage the health care professionals to meet the tuberculosis burden and early to detect the tuberculosis affected people in the community. Rural areas lack health care services and less number of hospitals. Early implementation of clinical pharmacist services in the rural areas collaborated with multiple health care professionals can provide necessary tuberculosis testing and treatment services, screening of affected people and further treatment possibilities [25-31].
In rural areas the clinical pharmacist care services can needed. In rural areas the lack of health care facilities and medical care services and poor hygiene, sanitation, lack of financial facilities, education background and spending more health care expenditure currently in Indian scenario can affect the treatment burden.
Early placing the clinical pharmacist in the community level will reduce the tuberculosis associated health care burden in the community.
Clinical Pharmacist role in Patient Education about Tuberculosis
It includes
- Cause of tuberculosis
- Tuberculosis transmission routes
- Diagnosis of tuberculosis
- How to use the medication
- Side effects to medication
- Measures to prevent the spread of tuberculosis
Preventive measures to control drug resistant tuberculosis include
- Isolation and identification and curing the tuberculosis patients at the initial stages
- Provide rapid diagnosis
- Proper infection control practice
- Effective prescribing of second-line drugs to the patients
- Adhering to the standard tuberculosis treatment guidelines
- Avoiding exposure to drug resistant tuberculosis patients
- Good medication adherence practice
- Proper follow up care facilities
- Proper storage of drugs
- Improving health related quality of life
- Effective supplying the medications
Treatment of Drug Resistant Tuberculosis
It includes
- First-line oral anti-tuberculosis drugs isoniazid, ethambutol, rifampicin, and pyrazinamide.
- Injectable medications include kanamycin, streptomycin, amikacin, and capreomycin.
- Fluoroquinolones drugs include ofloxacin, moxifloxacin, levofloxacin, and gatifloxacin.
- The second-line anti-tuberculosis drugs like ethionamide, protionamide , cycloserine, terizidone, and para amino salicylic acid.
Newer Drugs to Treat Multi Drug Resistance Tuberculosis [32]
- Bedaquiline is the first drug in a new class of anti tuberculosis medications to be approved in more than 40 years by the US Food and Drug Administration.
- The current recommended dose of bed aquiline is 400 mg given orally with food.
A new anti-tubercular drug
Delamanid
- It is the first in a new class of tuberculosis drugs called nitro imidazoles.
- Delamanid used for the treatment of multi drug resistance tuberculosis.
- It is available as 50 mg tablets and the recommended dose is two tablets taken twice a day with food and it is continued for six months duration.
- Linezolid is has shown good activity against drug-resistant strains of Mycobacterium tuberculosis.
Conclusion
The rational use of drugs and meeting the patient care services in the community can be achieved by clinical pharmacist practices through working with multiple health care professionals. Clinical pharmacist applies the clinical knowledge on disease prevention and management, identification and solving drug related problems in the community. The drug resistant can be prevented through proper understanding of the mechanism of drug nature, early treatment initiation and good follow up patients for medication adherence, proper storage of drugs, effective infection control practices, early diagnosis and improving patientʼs health related quality of life will motivates the effective control of drug resistance problem [33,34]. Clinical pharmacist should positioned in the community care and initiation of awareness programmes on prevention and management meeting effective prescribing pattern of second line drugs can reduce the drug resistance problem in the community care.
References
- Myneedu VP, Visalakshi P, Verma AK, Behera D, Bhalla M. Prevalence of XDR TB cases--A retrospective study from a tertiary care TB hospital. Indian J Tuberc. 2011; 58(2): 54-59.
- Laszlo A, Rahman M, Espinal M, Raviglione M. Quality assurance programme for drug susceptibility testing of Mycobacterium tuberculosis in the WHO/IUATLD Supranational Reference Laboratory Network: five rounds of proficiency testing, 1994-1998. Int J Tuberc Lung Dis. 2002; 6(9): 748-756.
- Mitnick CD, Shin SS, Seung KJ, et al. Comprehensive treatment of extensively drug-resistant tuberculosis. N Engl J Med. 2008; 359(6): 563-574. doi: 10.1056/NEJMoa0800106
- Gandhi NR, Shah NS, Andrews JR, et al. HIV co infection in multidrugand extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010; 181(1): 80-86. doi: 10.1164/rccm.200907-0989OC
- James P, Christopher DJ, Balamugesh T, Gupta R. Death of a health care worker with nosocomial extensively drug-resistant tuberculosis in India. Int J Tuberc Lung Dis. 2009; 13(6): 795-796.
- John TJ, John SM. Paradigm shift for tuberculosis control in high prevalence countries. Trop Med Int Health. 2009; 14(12): 1428-1430. doi: 10.1111/j.1365-3156.2009.02392.x
- Zai S, Haroon T, Mehmood KT. Socioeconomic Factors Contributing to Multidrug-Resistant Tuberculosis (MDR-TB). J Biomed Sci Res. 2010; 2(4): 279-283.
- Cattamanchi A, Dantes RB, Metcalfe JZ, et al. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clin Infect Dis. 2009; 48(2): 179-185. doi: 10.1086/595689
- Dorman SE, Johnson JL, Goldberg S, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009; 180(3): 273-280. doi: 10.1164/rccm.200901-0078OC
- Nardell EA, Mitnick CD. Are second-line drugs necessary to control multidrug-resistant tuberculosis? J Infect Dis. 2006; 194(9): 1194-1196. doi: 10.1086/507910
- Dietze R, Hadad DJ, McGee B, et al. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am J Respir Crit Care Med. 2008; 178(11): 1180-1185. doi: 10.1164/rccm.200806-892OC
- Schecter GF, Scott C, True L, Raftery A, Flood J, Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2010; 50(1): 49-55. doi: 10.1086/648675
- Migliori GB, Eker B, Richardson MD, et al. A retrospective TBNET assessment of linezolid safety, tolerability and efficacy in multidrugresistant tuberculosis. Eur Respir J. 2009; 34(2): 387-393. doi: 10.1183/09031936.00009509
- Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997; 349(9064): 1513-1515. doi: 10.1016/S0140-6736(96)12273-X
- Chan ED, Laurel V, Strand MJ, et al. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2004; 169(10): 1103-1109. doi: 10.1164/rccm.200308-1159OC
- Burgos M, Gonzalez LC, Paz EA, et al. Treatment of multidrug-resistant tuberculosis in San Francisco: an outpatient-based approach. Clin Infect Dis. 2005; 40(7): 968-975. doi: 10.1086/428582
- Tahaoğlu K, Törün T, Sevim T, et al. The treatment of multidrugresistant tuberculosis in Turkey. N Engl J Med. 2001; 345(3): 170-174. doi: 10.1056/NEJM200107193450303
- Leimane V, Riekstina V, Holtz TH, et al. Clinical outcome of individualized treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005; 365(9456): 318-326. doi: 10.1016/S0140-6736(05)17786-1
- Nathanson E, Lambregts-van Weezenbeek C, Rich ML, et al. Multidrugresistant tuberculosis management in resource-limited settings. Emerg Infect Dis. 2006; 12(9): 1389-1397. doi: 10.3201/eid1209.051618
- Nathanson E, Nunn P, Uplekar M, et al. MDR tuberculosis--critical steps for prevention and control. N Engl J Med. 2010; 363(11): 1050-1058. doi: 10.1056/NEJMra0908076
- Forget EJ, Menzies D. Adverse reactions to first line anti tuberculosis drugs. Expert Opin Drug Saf. 2006; 5(2): 231-249. doi: 10.1517/14740338.5.2.231
- Centers for Disease Control and Prevention (CDC). Multidrug-resistant tuberculosis in Hmong refugees resettling from Thailand into the United States, 2004-2005. MMWR Morb Mortal Wkly Rep. 2005; 54(30): 741-744.
- Oeltmann JE, Varma JK, Ortega L, et al. Multidrug-resistant tuberculosis outbreak among US-bound Hmong refugees, Thailand, 2005. Emerg Infect Dis. 2008; 14(11): 1715-1721. doi: 10.3201/eid1411.071629
- Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrugresistant tuberculosis: the perfect storm. J Infect Dis. 2007; 196: S86-S107. doi: 10.1086/518665
- Zignol M, Hosseini MS, Wright A, et al. Global incidence of multidrugresistant tuberculosis. J Infect Dis. 2006; 194(4): 479-485. doi: 10.1086/505877
- Barbachyn MR, Hutchinson DK, Brickner SJ, et al. Identification of a novel oxazolidinone (U-100480) with potent anti mycobacterial activity. J Med Chem. 1996; 39(3): 680-685. doi: 10.1021/jm950956y
- Sargazi A, Gharebagh RA, Sargazi A, Aali H, Oskoee HO, Sepehri Z. Role of essential trace elements in tuberculosis infection: A review article. Indian J Tuberc. 2017; 64(4): 246-251. doi: 10.1016/j.ijtb.2017.03.003
- Chaudhari KS, Patel HM, Surana SJ. Pyridines: Multidrug-resistant tuberculosis (MDR-TB) inhibitors. Indian J Tuberc. 2017; 64(2): 119-128. doi: 10.1016/j.ijtb.2016.11.012
- Bedouch P, Allenet B, Labarere J, et al. [Diffusion of pharmacist interventions within the framework of clinical pharmacy activity in the clinical ward]. Thérapie. 2005; 60(5): 515-522. doi: 10.2515/therapie:2005015
- Tavitian SM, Spalek VH, Bailey RP. A pharmacist-managed clinic for treatment of latent tuberculosis infection in health care workers. Am J Health Syst Pharm. 2003; 60(18): 1856-1861. doi: 10.1093/ajhp/60.18.1856
- AlMatar M, AlMandeal H, Var I, Kayar B, Köksal F. New drugs for the treatment of Mycobacterium tuberculosis infection. Biomed Pharmacother. 2017; 91: 546-558. doi: 10.1016/j.biopha.2017.04.105
- Saha A, Vaidya PJ, Chavhan VB, et al. Factors affecting outcomes of individualized treatment for drug resistant tuberculosis in an endemic region. Indian J Tuberc. 2019; 66(2): 240-246. doi: 10.1016/j.ijtb.2017.04.001
- Quenard F, Fournier PE, Drancourt M, Brouqui P. Role of second-line injectable antituberculosis drugs in the treatment of MDR/XDR tuberculosis. Int J Antimicrob Agents. 2017; 50(2): 252-254. doi: 10.1016/j.ijantimicag.2017.01.042


